Open Journal of Pathology
Vol.3 No.2(2013), Article ID:30257,4 pages DOI:10.4236/ojpathology.2013.32012
Co-Orelation of Histological Dating and Glycogen Content by Histochemical Stain during Various Phases of Menstrual Cycle in Primary Infertility
![]()
1Department of Pathology, Institute of Human Behaviour & Allied Sciences (IHBAS), Delhi, India; 2Department of Pathology, Pt. B. D. Sharma University of Health Sciences Rohtak, India; 3Department of Obstetrics & Gynaecology, Pt. B. D. Sharma University of Health Sciences Rohtak, India.
Email: *dransh2002@yahoo.co.in
Copyright © 2013 Anshu Gupta et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received August 11th, 2012; revised September 11th, 2012; accepted October 9th, 2012
Keywords: Glycogen; Endometrium; Primary Infertility
ABSTRACT
Glycogen and its products of metabolism are considered to be the most important and direct source of nutrient for the early conceptus. The premenstrual biopsies were taken from 100 women with primary infertility whose male partners had normal semenograms. The biopsies were then subjected to Haematoxylin and Eosin (H & E) and Periodic Acid Schiff (PAS) staining with and without diastase to study histological and histochemical changes particularly glycogen content in the endometrium respectively. Histolomorphologic spectrum of endometrium on H & E stained sections revealed early proliferative phase (4%), late proliferative phase (7%) and early secretory phase (7%), mid secretory phase (33%) and late secretory phase (49%). PAS staining with or without diastase for glycogen was performed and glycogen content, as demonstrated by magenta color, was graded from + to ++++. Glycogen content was observed to be “+” to “++” in proliferative phase, where as in secretory phase, it varied from “++” to “+++”. Glycogen was confirmed by treatment with diastase enzyme, after that PAS stain gave negative staining, i.e. loss of magenta color as glycogen was digested by diastase. Glycogen deficiency was observed in 24.7% of secretory phase endometrium of infertile women. Depletion of glycogen results in inadequate preparation of endometrium at the time of implantation, and hence, may be one of the causes of infertility.
1. Introduction
Infertility incidence ranges from 5% to 15% [1]. It has been regarded as a disgrace and social stigma thrust upon healthy young adults [2]. Infertility results from physiological and pathological factors [1]. One of the pathological factors of sterility is poor quality endometrium that leads to death of ovum before or after implantation. Glycogen is believed to be the direct source of nutrients for the early conceptus from the time it enters the uterine cavity to the time it is actively supported by maternal blood stream. Glycogen shows cyclical changes under the influence of estrogen and particularly progesterone. The endometrium when fails to produce adequate amount of glycogen is termed as “Glycopenic uteri” [3].
The present study has been undertaken to determine histological and histochemical profile of endometrium during different phases of the menstrual cycle in primary infertile women to evaluate the role of glycogen in infertility.
2. Material and Methods
The study was conducted on endometrial biopsies received in the Department of Pathology, PGIMS, Rohtak. 100 women with primary infertility, whose male partners had normal semenograms, were selected from total 350 female patients attending Obstetrics and Gynecology Outpatient Department (OPD) in two years duration, on the basis of clinical history such as menorrghagia, secondary infertility, discharge etc., physical examination and semen analysis of their husbands. Their premenstrual endometrial biopsies were taken and subjected to routine paraffin sectioning. These sections were stained with Haemotoxylin and Eosin (H & E), Periodic Acid Schiff (PAS) and Periodic Acid Schiff with Diastase (PASD) for histochemical study of glycogen in the endometrium.
2.1. Procedure of Hematoxylin and Eosin (H & E) Staining
1) Deparaffinize and hydrate to water.
2) Harris,s hematoxylin for 10 minutes.
3) Differentiate by dipping 3 - 4 times in 1% acid alcohol after a quick rinse in water.
3) Wash in running tap water for 20 minutes.
4) Counterstain with eosin from 15 seconds to 2 minutes depending on the age of the eosin, and the depth of the counterstain desired.
5) Dehydrate in 95% and absolute alcohols, two changes of 2 minutes each or until excess eosin is removed. Check under microscope.
6) Clear in xylene, two changes of 2 minutes each and mount.
2.2. Results
Muscle deep pink
Basophilic cytoplasm pink
Nuclei blue
Erythrocytes cherry red
2.3. Procedure of Periodic Acid Schiff (PAS) Staining
1) Deparaffinize and hydrate to water.
2) Oxidize in 0.5% periodic acid solution for 5 minutes.
3) Rinse in distilled water.
4. Place in Schiff reagent for 15 minutes (Sections become light pink color during this step).
5) Wash in tap water for 5 minutes (Immediately sections turn dark pink color).
6) Counterstain in Mayer’s hematoxylin for 1 minute.
7) Wash in tap water for 5 minutes.
8) Dehydrate and cover slip using a mounting medium.
2.4. Results
Glycogen magenta
Nuclei blue
Erythrocytes Pale pink
Glycogen content was graded as:
0 Negative reactions
+ Very small granules
++ Coarse granules
+++ Small masses
++++ Large masses
2.5. Procedure of Periodic Acid Schiff Diastase (PASD) Staining
1) Deparaffinize and bring sections to distilled water.
2) Treat sections with diastase/amylase solution for 20 mins.
3) Wash well in running tap water.
4) Treat with 0.5% periodic acid for 5 mins.
5) Wash well in distilled water.
6) Stain with Schiff's reagent for 15 mins.
7) Wash in fast running tap water 3 - 5 mins.
8) Stain nuclei with haematoxylin 1 min.
9) Rinse in running tap water.
10) Differentiate with acid alcohol.
11) Wash in running tap water for 5 minutes.
12) Dehydrate, clear and mount.
2.6. Results
Glycogen is removed by diastase/amylase and is therefore unstained in a PASD.
3. Results
Medical data has been summarized in simple tabulation and contingency tabulation with frequency distribution. The age distribution in women of primary infertility was 18 to 32 years. The pattern of menstrual cycles was regular in 92% and irregular in 8%. Based on the morphological characteristics on H & E stained sections, endometrium turned out to be proliferative in 11% and secretory in 89% of cases. Distribution of various phases of endometrium is shown in Table 1. 82 endometria were histologically normal, the maturity corresponded with the clinical or chronological maturity expected of the endometrium, irrespective of the glycogen content.
Results of PAS staining in glands and stroma in secretory endometrium are depicted in Table 2. Glycogen content was “+++” to “++++” in most of the cases of secretory phase and is more in glands than in stroma.
Glycogen depletion was observed in 24.7% of secretory endometrium as shown in Table 3.
4. Discussion
The endometrium is the tissue of interest since it is the most favoured site for the implantation of the ovum [4]. Numerous morphological and biochemical changes take
Table 1. Various phases of endometrium in primary infertility.

Table 2. PAS staining in glands and stroma in secretory endometrium.
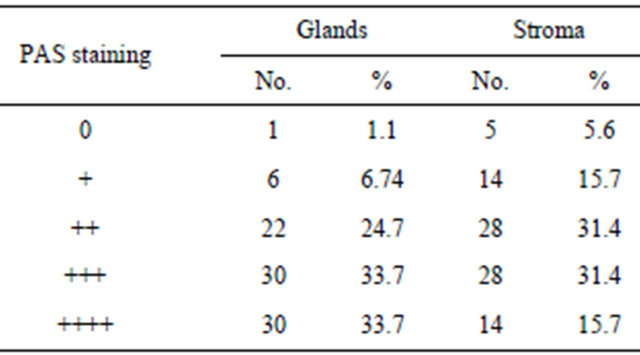
Table 3. Glycogen depletion in secretory endometrium.
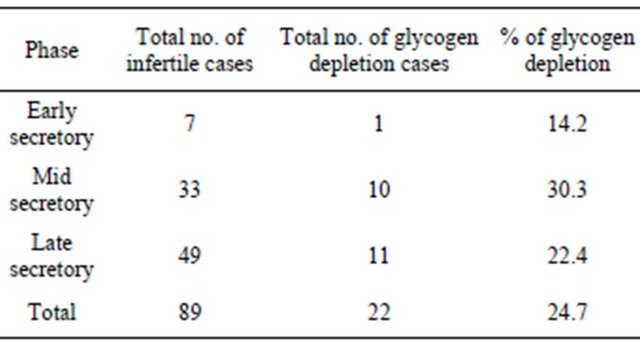
place in it within a relatively short period under the influence of oestrogen and progesterone [5]. The implantation depends upon the receptive condition of the endometrium, which is determined by glycogen. Glycogen is present in traces in the proliferative phase and increase progressively during the secretory phase [3].
The secretory phase is divided into three stages, the early secretory phase (2nd to 4th post ovulatory day), mid secretory phase (from 5th to 9th post ovulatory day) and the late secretory phase (from 10th to 14th post ovulatory day) [6].
In the present study, the premenstrual endometrial biopsies were histologically normal in 82% of the cases and immature in the form of proliferative or early secretory changes in 18% of cases. The histological immaturity was also observed by others [3,7-14].
Endometrial function was usually evaluated on the basis of morphological variation occurring during the various phases of a menstrual cycle and the histochemical reaction received little attention. In our study, increased concentration of glycogen was observed during secretory phase (Figure 1) as compared to the proliferative phase. Our finding corresponded to the studies carried out by other authors [15-17].
The increase in glycogen content in secretory phase could be possible because of shift from predominantly anaerobic glycolysis during proliferative phase to predominantly aerobic glycolysis during progestational phase [18]. Arronet and Latour laid much emphasis on the morphological picture of the endometrium rather than the glycogen contents [19].
Dockery et al. studied the fine structure of the human endometrial glandular epithelium in cases of unexplained infertility and observed abnormalities associated with intracellular deposits of glycogen—rich material in infertile women [20].
A synchronized development of embryo and endometrium is a prerequisite for a successful implantation. Pinopods which appear on the endometrial surface during implantation, play a role in implantation process. Considerable amount of glycogen is part of the content of pinopods, which suggest that the formation of pinopods is an energy consuming process [21].
In our study, glycogen depletion was noticed in secretory endometrium in 22 cases (24.71%) (Figure 2). The
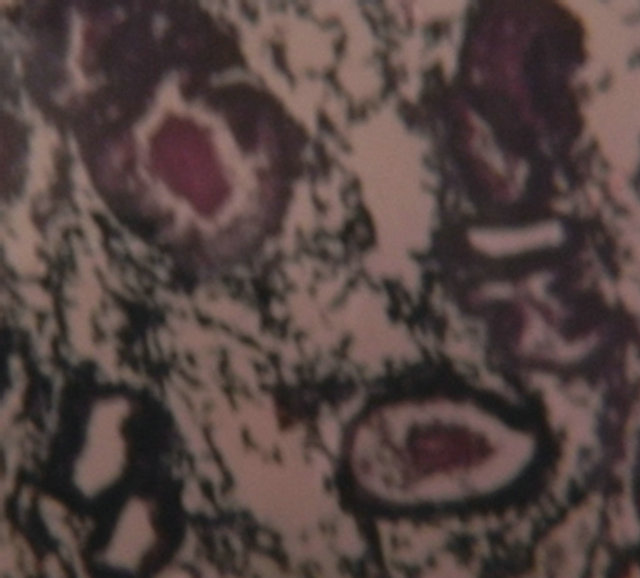
Figure 1. Photomicrograph showing +++ to ++++ glycogen in secretory phase (PAS, ×40).

Figure 2. Photomicrograph showing glycogen depletion in late secretory phase (PAS, ×40).
findings are consistent with the results obtained by Rohtagi et al. (22.5%) [3] but differs from Hughes et al. [8], Zower et al. (30%) [22]. Various factors responsible for glycogen depletion have been suggested. Low levels of alkaline phosphatase enzyme, defective oxidation and glycogen utilization make the environment unfavourable for the early embryo. Quantitative deficiency of hormones, pituitary and ovaries that control metabolism also leads to glycogen deficiency. Decreased glycogen deposition in secretory phase endometrium leads to improper nidation for ovum, resulting in sterility.
5. Conclusion
A comparative histopathological and histochemical study was carried out on endometria of primary infertile women to study histological dating and glycogen content. Women who show endometria in proliferative phase are not ovulating. In such cases, it is the histological immaturity which is responsible for infertility. Secretory phase endometrium means that the woman is ovulating and the defect lies somewhere else. Defect may be depletion of glycogen which results in inadequate preparation of endometrium at the time of implantation and hence, may be one of the causes of infertility.
REFERENCES
- V. Padubidri and S. N. Daftary, “The Pathology of Conception,” In: V. Padubidri, Ed., Shaw’s Textbook of Gynaecology, Churchill Livingston, Edinburgh, 1991, pp. 205-227.
- B. J. Mostyn, “Counselling the Infertile Patients,” In: B. J. Mostyn, Ed., Recent Advances in Obstetrics and Gynaecology, Churchill Livingston, Edinburgh, 1994, pp. 137- 151.
- K. Rohtagi, V. K. Singh and V. S. Rajvanshi, “Study of Endometrial Glycogen in Cases of Dysfunctional Uterine Hemorrhage and Subfertility,” Journal of Obstetrics & Gynaecology of India, Vol. 27, 1976, pp. 905-910.
- D. G. Mckay, A. T. Hertig and W. A. Bardawil, “Histochemical Observations on the Endometrium,” American Journal of Obstetrics & Gynecology, Vol. 8, No. 1, 1956, pp. 22-39.
- E. C. Hughes, A. W. Ness and C. W. Lloyd, “Relationship of the Endometrium to the Chorioplacental Development and Its Gonadotrophin Output,” American Journal of Obstetrics & Gynecology, Vol. 60, No. 3, 1950, pp. 575-585.
- C. H. Buckley and H. Fox, “The Anatomy and Histology of the Endometrium,” In: C. H. Buckley, Ed., Biopsy Pathology of the Endometrium, Chapmen and Hall Medical, London, 1989, pp. 11-29.
- E. C. Hughes, W. V. Ness and C. W. Lloyd, “The Nutritional Value of the Endometrium for Implantation and in Habitual Abortion,” American Journal of Obstetrics & Gynecology, Vol. 59, No. 6, 1950, pp. 1292-1302.
- E. C. Hughes and N. Y. Syracuse, “Relationship of Glycogen to Problems of Sterility and Ovular Life,” American Journal of Obstetrics & Gynecology, Vol. 49, No. 1, 1945, pp. 10-18.
- S. M. Shahani and B. N. Purandare, “Histochemical Changes in Human Endometrium and Their Importance in Diseased Condition,” Journal of Obstetrics & Gynaecology of India, Vol. 2, 1959, pp. 131-138.
- B. M. Venugopala, “Endometrium in Subfertility,” Journal of Obstetrics & Gynaecology of India, Vol. 10, 1959, pp. 139-142.
- S. P. Tyagi, N. Abbari and S. Hameed, “Study of Endometrial Glycogen in Case of Infertility,” Journal of Obstetrics & Gynaecology of India, Vol. 27, 1975, pp. 217-220.
- P. M. Sareen, R. Kalra and S. K. Lodha, “Significance of Endometrial Glycogen in Primary Sterility,” Journal of Obstetrics & Gynaecology of India, Vol. 34, 1984, pp. 877-880.
- S. G. Gupta, N. Khan and M. Trivedi, “Study of Glycogen and Its Significance in Endometrium in Cases of Primary Sterility,” Journal of Obstetrics & Gynaecology of India, Vol. 32, 1982, pp. 819-821.
- S. C. Sharma, A. K. Banerjee and M. L. Hasan, “Endometrial Glycogen in Sterility,” Journal of Obstetrics & Gynaecology of India, Vol. 34, 1984, pp. 1073-1076.
- D. D. Hagerman and C. A. Ville, “Effects of the Menstrual Cycle on the Metabolism of Human Endometrium,” Endocrinology, Vol. 53, No. 6, 1953, pp. 667-673. doi:10.1210/endo-53-6-667
- P. L. Gupta and M. Jethani, “Endometrial Glycogen—An Important Parameter of Infertility,” Journal of Obstetrics & Gynaecology of India, Vol. 80, 1994, pp. 4-7.
- V. E. Krishnamohan and B. Nair, “A Histopathological Study of Endometrium in Primary Sterility,” Journal of Obstetrics & Gynaecology of India, Vol. 43, 1993, pp. 580-584.
- R. J. Stein and V. M. Stuermer, “Cytodynamic Properties of the Human Endometrium,” American Journal of Obstetrics & Gynecology, Vol. 61, No. 2, 1951, pp. 414-417.
- G. H. Arronet and J. P. A. Latour, “Studies on endometrial Glycogen,” The Journal of Clinical Endocrinology & Metabolism, Vol. 17, No. 2, 1957, pp. 261-271. doi:10.1210/jcem-17-2-261
- P. Dockery, K. Pritchard and A. Taylor, “The Fine Structure of the Human Endometrial Glandular Epithelium in Cases of Unexplained Infertility: A Morphometric Study,” Human Reproduction, Vol. 8, No. 5, 1993, pp. 667- 673.
- C. Anneli and E. Staverns, “Characteristics and Possible Function of Pinopods Seen on the Surface of the Receptive Human Endometrium,” Middle East Fertility Society, Vol. 10, No. 1, 2005, pp. 22-28.
- M. P. Zowar, N. M. Deshpande and P. A. Gadgsl, “Histopathological Study of Endometrium in Infertility,” Indian Journal of Pathology and Microbiology, Vol. 46, No. 4, 2003, pp. 630-633.
NOTES
*Corresponding author.

