Open Journal of Gastroenterology
Vol.06 No.11(2016), Article ID:72379,10 pages
10.4236/ojgas.2016.611036
Changes in Serum Lipid Profile among Patients Suffering from Chronic Liver Disease Secondary to Hepatitis C
Fakhar Ali Qazi Arisar1, Shameem Behram Khan2, Anam Umar2, Noor ul Saba Shaikh2, Fizza Choudhry2
1Departement of Medicine, Section of Gastroenterology, The Aga Khan University Hospital, Karachi, Pakistan
2Departement of Medicine, Jinnah Postgraduate Medical Centre, Karachi, Pakistan

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: October 25, 2016; Accepted: November 26, 2016; Published: November 29, 2016
ABSTRACT
Objective: To find out the changes in lipid metabolism among patients suffering from chronic liver disease secondary to hepatitis C. Study Design: Hospital based observational study. Setting: Medical Unit-I, Ward?5, Jinnah Postgraduate Medical Centre, Karachi. Duration: July 2013 to December 2013. Patients and Methods: About 110 patients admitted in Medical Unit-I with a diagnosis of chronic liver disease were included in the study. Patients suffering from DM, HTN, CKD were excluded from the study. Fasting lipid profile was done in all cases. Results and Observations: There were 44 (40%) male and 66 (60%) female patients. Mean age of the patients was 50.18 (±11.7) years. Total cholesterol was decreased in 76 (69.09%) patients. Normal range was present in 34 (30.91%) patients. None of the patient had hypercholesterolemia. Serum triglyceride levels were low in 14 (12.72%) patients, normal in 82 (74.54%), borderline high in 7 (6.36%) and hypertriglyceridemia was seen in 7 (6.36%). HDL-c was below normal in 26 (23.63%) cases, normal in 78 (70.91%), and high in 6 (5.45%). LDL was near optimal/above optimal in only 5 (4.5%) patients. Mean TC/HDL ratio was 2.53 (±1.02). Mean LDL/HDL ratio was 1.23 (±0.73). Mean TC of HCV +ve patients was 130.5 mg/dl as compared to that of HCV ?ve patients which was 82.85 mg/dl (P-value: 0.011). Mean TGs of HCV +ve group was 151.5 mg/dl while that of HCV ?ve was 79.9 mg/dl (P-value: 0.025). Mean HDL & LDL levels were 43.67 mg/dl and 39.78 mg/dl in HCV group while 34.83 mg/dl & 64.67 mg/dl in the other group with P-value of 0.026 and 0.081 respectively. Conclusion: When it comes to its relationship with lipid metabolism, HCV is a remarkable virus. Its interaction with lipoproteins and its ability to induce massive steatosis are quite unique and idiosyncratic. Despite of causing hepatic steatosis, chronic HCV infection is associated with a paradoxically favorable lipid profile, although its reason cannot be enlightened precisely. There is a need for very well settled molecular and genetic studies to well understand HCV infection and lipid metabolism.
Keywords:
Dyslipidemia, Hepatitis C, Chronic Liver Disease

1. Introduction
Hepatitis C is a worldwide problem. The hepatitis C virus is a major cause of both acute and chronic hepatitis. An estimated population of more than 185 million worldwide was infected with hepatitis C virus in 2005, making a prevalence of 2.8% [1] [2] . Hepatitis C is divided into six distinct genotypes throughout the world with multiple subtypes in each genotype class. Among them, genotypes 1, 2, and 3 appear to have a worldwide distribution and their relative prevalence varies from one geographic area to another. HCV subtypes 1a and 1b are the most common genotypes in the United States [3] . These subtypes also are predominant in Europe. The predominant HCV genotype in Pakistan is type 3a followed by 3b and 1a [4] .
Many authors had made efforts to find out the interaction between chronic hepatitis C virus and lipid metabolism [5] [6] . Several important interactions were noticed. First, host serum lipid has a role in the entry of hepatitis C virion in liver cells and its circulation in blood. A fraction of circulating hepatitis C viral particles makes lipoviroparticles on assimilation with host lipoproteins [7] , and uses LDL receptors to get entered in hepatocytes [8] . After entering into the hepatocytes, the replication of HCV virion is again dependent on host lipid interactions. New hepatitis C virion formation requires viral binding to either an endoplasmic reticulum phospholipid membrane or an endoplasmic reticulum-associated membranous web [9] .
HCV reproduction may produce effects similar to those observed with HMGR inhibitors. Intrahepatic cholesterol synthesis is affected by HCV through two possible path- ways; first, it may shunt geranylpyrophosphate, out of the mevalonate pathway, decreasing the quantity of this necessary intermediate available for cholesterol synthesis. Second, it may divert cholesterol to the synthesis of intracellular membranes that are necessary for the viral replication complex. The net effect of these diversions is the decrease of available cholesterol for physiologic intracellular processes and for peripheral delivery via VLDL, ultimately resulting in decreased serum cholesterol levels. The decrease in available intracellular cholesterol may also lead to an increase in LDL receptors and intrahepatic LDL. This increase in LDL uptake may account for the decreased serum LDL levels in HCV infection [9] . Also the metabolic processes which are associated with viral replication may be associated with a drop in triglycerides levels [10] [11] .
2. Materials and Methods
This study was carried out in the Department of Medicine, Medical Unit-I, Ward-5, Jinnah Postgraduate Medical Centre, which is a tertiary care public sector hospital in Karachi, Pakistan with four medical units where around 4000 patients are admitted annually in each unit. About 110 patients were consecutively enrolled from July 2013 to December 2013. The patients included were suffering from chronic liver disease. Most of the patients belong to class B and C of Child Pugh’s Classification. Table 1 refers the child Pugh’s classification. Study was approved by research ethics committee (REC) of the institute and all patients gave informed written consent.
2.1. Exclusion Criteria
1. Patients with hepatitis B infection or any other chronic liver disease..
2. Patients consuming alcohol or on lipid lowering medications.
3. The body mass index of >30 kg/m2.
4. The patients suffering from concomitant illnesses like diabetes mellitus, hypertension, chronic kidney disease or thyroid problem.
All patients in this study were subjected to:
1. Full medical history and examination.
2. Liver function tests (total plasma proteins, serum albumins, SGOT, SGPT, total and direct serum bilirubin, alkaline phosphatase and prothrombin time).
3. Lipid profile: Fasting cholesterol, low density lipoproteins (LDL), high density lipoproteins (HDL), and triglycerides.
4. Kidney functions tests (serum urea and creatinine).
5. Random blood glucose level and electrolytes.
6. Complete blood count.
7. Anti HCV antibody.
8. Thyroid function test.
Table 1. Child Pugh’s classification.
Child Turcotte-Pugh class (obtained by adding score of each parameter) [12] [13] . Child A (5 - 6): Mild disease; Child B (7 - 9): Moderate disease; Child C (10 - 15): Severe disease.
9. Abdominal ultrasound.
10. Body mass index (BMI) (weight in kilogram/height in cubic meter).
2.2. Operational Definitions
Levels of triglycerides and total, LDL & HDL cholestrols were defined according to ATP III classification as shown in Table 2.
2.3. Data Management
Statistical analysis was done using SPSS statistical package version 19 and results were got as mean ± standard deviation (mean ± SD). For statistical analysis, independent sample t test was used and the relation between variables was examined with Pearson correlation. P < 0.05 value was accepted meaningful statistically.
3. Results
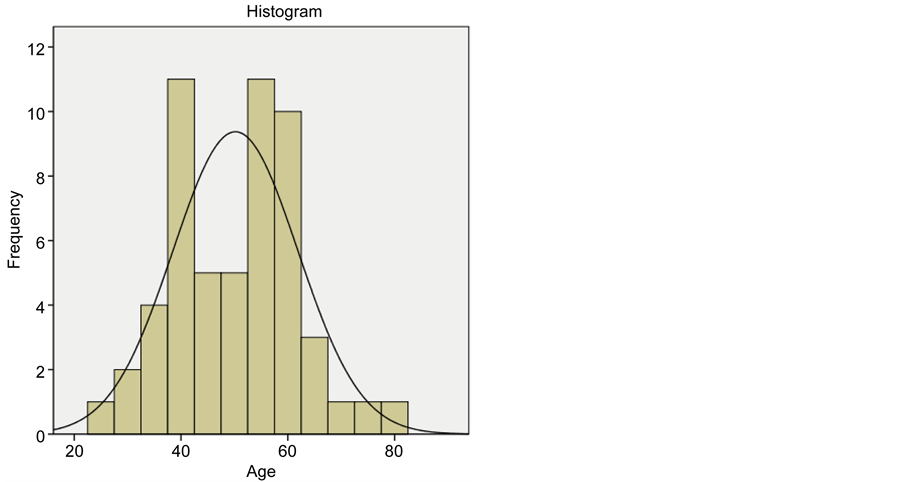
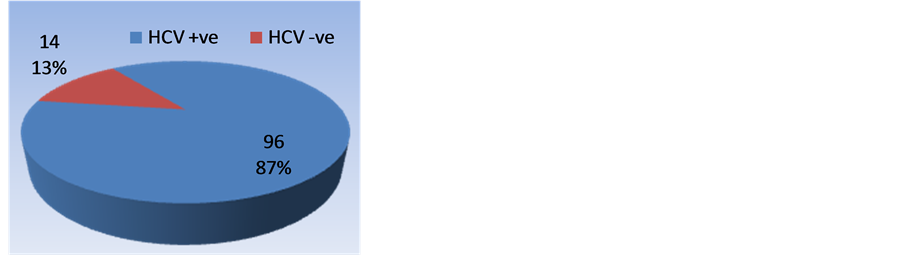
There were 44 (40%) male and 66 (60%) female patients. Mean age of the patients was 50.18 (±11.70) years. Most of the cases belonged to middle age group. Age and gender distribution of the 110 patients included in the study is shown in Figure 1 & Figure 2, while Figure 3 shows prevalence of HCV among study patients. Table 3 summarizes the laboratory parameter of patients included in study along with their child and MELD scores which were comparable in the two groups.
In our group of study, total cholesterol was decreased in 76 (69.09%) patients while
Table 2. ATP III classification of triglycerides and total, LDL & HDL cholestrols (mg/dl) [14] [15] [16] .
Figure 1. Histogram showing age frequency of patients included in study.
Figure 2. Gender distribution of patients included in study.
Figure 3. Prevalence of hepatitis C among study group.
normal range was present in 34 (30.91%) patients. None of the patient had hypercholesterolemia.
Serum triglyceride levels were low in 14 (12.72%) patients, normal in 82 (74.54%), borderline high in 7 (6.36%) and hypertriglyceridemia was seen in 7 (6.36%). HDL-c
Table 3. Laboratory parameters of patients enrolled in study.
NA: Not applicable; *Significant P-value.
was below normal in 26 (23.63%) cases, normal in 78 (70.91%), and high in 6 (5.45%).
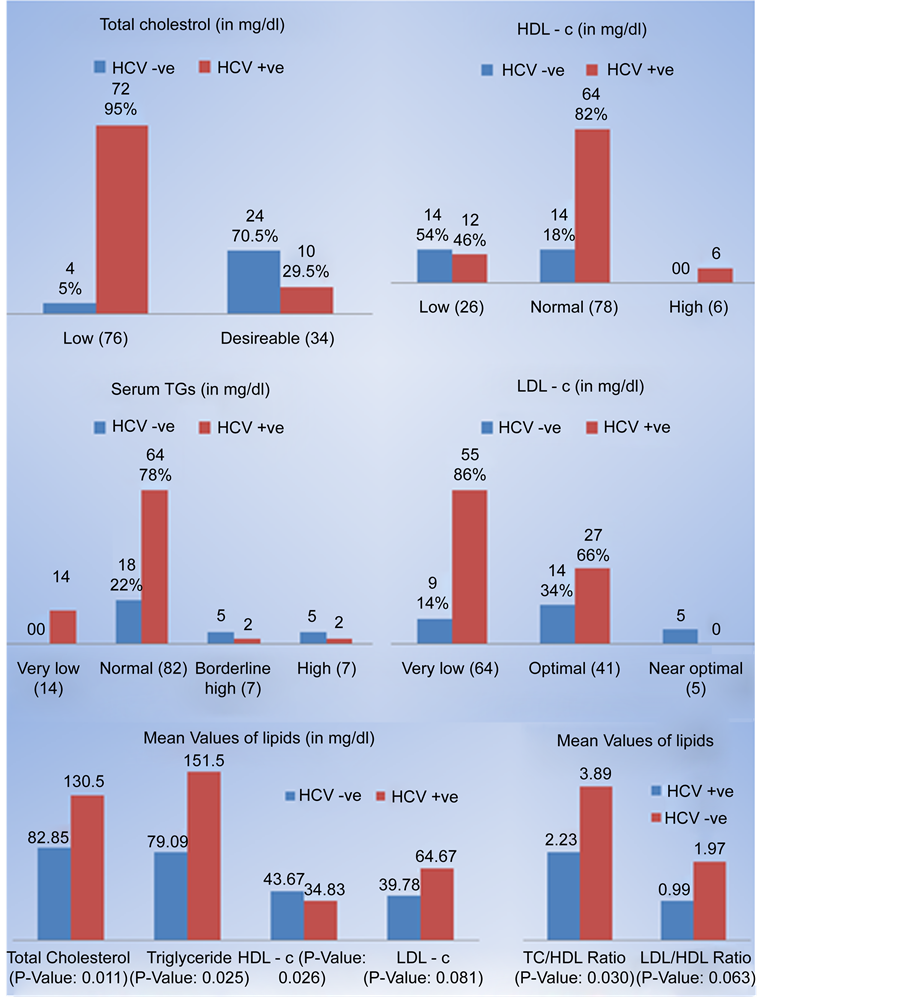
LDL was near optimal/above optimal in only 5 (4.5%) patients. Mean TC/HDL ratio was 2.53 (±1.02). Mean LDL/HDL ratio was 1.23 (±0.73). When compared, there was significant difference among patients who were HCV positive vs HCV negative patients in terms of total cholesterol [82.85 mg/dl vs 130.5 mg/dl (p-value 0.011)], triglycerides [79.09 mg/dl vs 151.5 mg/dl (p-value 0.025)], HDL cholesterol [43.67 mg/dl vs 34.83 mg/dl (p-value 0.026)], TC/HDL ratio [2.23 vs 3.89 (p-value 0.030)], however difference between LDL cholesterol and LDL/HDL ratio between the two groups was not significant as shown in Figure 4.
4. Discussion
Dyslipidemia is a frequent finding in chronic liver disease and HCV. Internationally this subject has been dealt in detail. But to the best of our knowledge no study was done for dyslipidemia in chronic liver disease secondary to HCV in Pakistan. So it was aimed to contribute to the literature through this study.
When it comes to its relationship with lipid metabolism, HCV is a remarkable virus. Its interaction with lipoproteins and its ability to induce massive steatosis are quite unique and idiosyncratic. In addition, HCV infection is generally associated with glucose tolerance disorder or diabetes and both these two situations are closely related with steatosis. Due to these two situations, HCV infection can be related with lipid metabolism indirectly.
Although changed serum lipid is commonly found in patients with chronic liver disease of any etiology, the relationship between HCV and lipid metabolism seems to be more specific.
Fernandez-Rodriguez et al. [17] documented in their study that Hepatitis C genotype 3 chronic liver diseases is associated with serum lipid changes and these changes are
Figure 4. Comparison of lipids among patients with hepatitis C Vs non hepatitis C CLD.
reversible with sustained viral response. This interference with lipid pathway is related to viral load.
Patients in the HCV Group had significantly lower total cholesterol and LDL-c levels than the HCV negative control group, (P: 0.011 and 0.081 respectively). These results agree with Fabris et al. [5] , Serfaty et al. [6] , Marzouk et al. [11] , Floris-Moore et al. [18] , Corey et al. [19] , Ehab H Nashaat [20] , Mustafa Güçlü [21] , and Mahmoud A. Khattab, 2012 [22] , who observed that frequency of hypocholesterolemia in noncirrhotic HCV-infected patients was five times higher than in their reference population.
Also patients in the HCV group had significantly lower triglycerides levels when compared to the uninfected control group (p = 0.025). These results agree with Perlemuter et al. [10] , Marzouk et al. [11] , Ehab H Nashaat [20] , and Mahmoud A. Khattab [22] , which refers this drop to the metabolic processes associated with viral replication. But these results disagree with Corey et al. [19] and Mustafa Güçlü [21] , who did not found significant difference as regard triglycerides between patients and controls.
HDL levels were also statistically significant between the HCV group and uninfected controls (p = 0.026). These results agree with Mahmoud A. Khattab et al. [22] , but disagree with Corey et al. [19] , Ehab H. Nashaat [20] , and Mustafa Güçlü et al. [21] .
Smaller sample size and unequal number of patients in the two groups due to consecutive sampling seems to be potential limitation of our study which actually reflects the commonest cause of CLD in our population. A larger study with good number of Non HCV CLD patients may overcome this limitation.
5. Conclusion
When it comes to its relationship with lipid metabolism, HCV is a remarkable virus. Its interaction with lipoproteins and its ability to induce massive steatosis are quite unique and idiosyncratic. Despite of causing hepatic steatosis, chronic HCV infection is associated with a paradoxically favorable lipid profile, although its reason cannot be enlightened precisely. There is a need for very well settled molecular and genetic studies to well understand HCV infection and lipid metabolism.
Acknowledgements
Dr. Tariq Aziz
Dr. Tahir Ansari
Prof. Jamal Ara
Cite this paper
Arisar, F.A.Q., Khan, S.B., Umar, A., Shaikh, N.S. and Choudhry, F. (2016) Changes in Serum Lipid Profile among Patients Suffering from Chronic Liver Disease Secondary to Hepatitis C. Open Journal of Gastroenterology, 6, 333-342. http://dx.doi.org/10.4236/ojgas.2016.611036
References
- 1. Mohd Hanafiah, K., Groeger, J., Flaxman, A.D. and Wiersma, S.T. (2013) Global Epidemiology of Hepatitis C Virus Infection: New Estimates of Age-Specific Antibody to HCV Seroprevalence. Hepatology, 57, 1333-1342.
https://doi.org/10.1002/hep.26141 - 2. Lauer, G.M. and Walker, B.D. (2001) Hepatitis C Virus Infection. New England Journal of Medicine, 345, 41-52.
https://doi.org/10.1056/NEJM200107053450107 - 3. Zein, N.N., Rakela, J., Krawitt, E.L., Reddy, K.R., Tominaga, T. and Persing, D.H. (1996) Hepatitis C Virus Genotypes in the United States: Epidemiology, Pathogenicity, and Response to Interferon Therapy. Annals of Internal Medicine, 125, 634-639.
https://doi.org/10.7326/0003-4819-125-8-199610150-00002 - 4. Idrees, M. and Riazuddin, S. (2008) Frequency Distribution of Hepatitis C Virus Genotypes in Different Geographical Regions of Pakistan and Their Possible Routes of Transmission. BMC Infectious Diseases, 8, 1.
https://doi.org/10.1186/1471-2334-8-69 - 5. Fabris, C., Federico, E., Soardo, G., Falleti, E. and Pirisi, M. (1997) Blood Lipids of Patients with Chronic Hepatitis: Differences Related to Viral Etiology. Clinica Chimica Acta, 261, 159-165.
https://doi.org/10.1016/S0009-8981(97)06532-7 - 6. Serfaty, L., Andreani, T., Giral, P., Carbonell, N., Chazouillères, O. and Poupon, R. (2001) Hepatitis C Virus Induced Hypobetalipoproteinemia: A Possible Mechanism for Steatosis in Chronic Hepatitis C. Journal of Hepatology, 34, 428-434.
https://doi.org/10.1016/S0168-8278(00)00036-2 - 7. Diaz, O., Delers, F., Maynard, M., Demignot, S., Zoulim, F., Chambaz, J., et al. (2006) Preferential Association of Hepatitis C Virus with Apolipoprotein B48-Containing Lipoproteins. Journal of General Virology, 87, 2983-2991.
https://doi.org/10.1099/vir.0.82033-0 - 8. Andre, P., Komurian-Pradel, F., Deforges, S., Perret, M., Berland, J., Sodoyer, M., et al. (2002) Characterization of Low- and Very-Low-Density Hepatitis C Virus RNA-Containing Particles. Journal of Virology, 76, 6919-6928.
https://doi.org/10.1128/JVI.76.14.6919-6928.2002 - 9. Dubuisson, J., Penin, F. and Moradpour, D. (2002) Interaction of Hepatitis C Virus Proteins with Host Cell Membranes and Lipids. Trends in Cell Biology, 12, 517-523.
https://doi.org/10.1128/JVI.76.14.6919-6928.2002 - 10. Perlemuter, G., Sabile, A., Letteron, P., Vona, G., Topilco, A., Chrétien, Y., et al. (2002) Hepatitis C Virus Core Protein Inhibits Microsomal Triglyceride Transfer Protein Activity and Very Low Density Lipoprotein Secretion: A Model of Viral-related Steatosis. The FASEB Journal, 16, 185-194.
https://doi.org/10.1096/fj.01-0396com - 11. Marzouk, D., Sass, J., Bakr, I., El Hosseiny, M., Abdel-Hamid, M., Rekacewicz, C., et al. (2007) Metabolic and Cardiovascular Risk Profiles and Hepatitis C Virus Infection in Rural Egypt. Gut, 56, 1105-1110.
https://doi.org/10.1136/gut.2006.091983 - 12. Child, C.G. and Turcotte, J. (1964) Surgery and Portal Hypertension. Major Problems in Clinical Surgery, 1, 1-85.
https://doi.org/10.1016/S0011-3840(64)80003-4 - 13. Pugh, R., Murray-Lyon, I., Dawson, J., Pietroni, M. and Williams, R. (1973) Transection of the Oesophagus for Bleeding Oesophageal Varices. British Journal of Surgery, 60, 646-649.
https://doi.org/10.1002/bjs.1800600817 - 14. Grundy, S.M., Cleeman, J.I., Merz, C.N.B., Brewer, H.B., Clark, L.T., Hunninghake, D.B., et al. (2004) Implications of Recent Clinical Trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. Journal of the American College of Cardiology, 44, 720-732.
https://doi.org/10.1016/j.jacc.2004.07.001 - 15. National Cholesterol Education Program (2002) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation, 106, 3143-3421.
- 16. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (2001) Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA, 285, 2486-2497.
https://doi.org/10.1001/jama.285.19.2486 - 17. Fernández-Rodríguez, C., López-Serrano, P., Alonso, S., Gutierrez, M., Lledo, J., Pérez-Calle, J., et al. (2006) Long-Term Reversal of Hypocholesterolaemia in Patients with Chronic Hepatitis C Is Related to Sustained Viral Response and Viral Genotype. Alimentary Pharmacology & Therapeutics, 24, 507-512.
https://doi.org/10.1111/j.1365-2036.2006.03000.x - 18. Floris-Moore, M., Howard, A.A., Lo, Y., Schoenbaum, E.E., Arnsten, J.H. and Klein, R.S. (2007) Hepatitis C Infection Is Associated with Lower Lipids and High-Sensitivity C-Reactive Protein in HIV-Infected Men. AIDS Patient Care and STDs, 21, 479-491.
https://doi.org/10.1089/apc.2006.0150 - 19. Corey, K.E., Kane, E., Munroe, C., Barlow, L.L., Zheng, H. and Chung, R.T. (2009) Hepatitis C Virus Infection and Its Clearance Alter Circulating Lipids: Implications for Long-Term Follow-Up. Hepatology, 50, 1030-1037.
https://doi.org/10.1002/hep.23219 - 20. Nashaat, E.H. (2010) Comparative Study of Serum Lipid Profile between Chronic Hepatitis C Egyptian Patients and Normal Controls and the Effect of Viral Eradication on Lipids Profile. Report and Opinion, 2, 14-20.
- 21. Güçlü, M. (2011) Evaluation of Serum Lipid Profile in Turkish Patients with Chronic Hepatitis C. European Journal of General Medicine, 8, 7-12.
- 22. Khattab, M.A., Eslam, M., Aly, M.M., Shatat, M., Mousa, Y.I., Abd-Aalhalim, H., et al. (2012) Serum Lipids and Chronic Hepatitis C Genotype 4: Interaction and Significance. Annals of Hepatology, 11, 37-46.