Open Journal of Organic Polymer Materials
Vol. 2 No. 4 (2012) , Article ID: 23983 , 6 pages DOI:10.4236/ojopm.2012.24010
Synthesis, Characterization and Photoinduced Cis-Trans Isomerisation Studies on Lignin Modified with 4-[(E)-2-(3-Hydroxynaphthalen-2-yl)diazen- 1-yl]benzoic Acid
1Research and Post Graduate Department of Chemistry, St. Thomas College, Mahatma Gandhi University, Kerala, India
2Research and Post Graduate Department of Chemistry, St. George College, Mahatma Gandhi University, Kerala, India
Email: *skresearchgroup@rediffmail.com
Received August 24, 2012; revised September 25, 2012; accepted October 5, 2012
Keywords: Lignin; Photoresponsive; Environment Friendly; Light Fastening; Cis-Trans Isomerisation
ABSTRACT
In the present study, a photoresponsive chromophoric system such as 4-[(E)-2-(3-hydro xynaphthalen-2-yl)diazen-1-yl] benzoic acid was incorporated on to lignin core by functional transformation reactions and the photoresponsive behavior of the green, environment friendly product was investigated. The end hydroxyl group of lignin was modified with the chromophoric systems by DCC coupling. The chromophoric systems as well as the chromophore-bound biopolymer core systems were purified by column chromatography. The products were characterized by UV-visible, fluorescence, FT-IR and NMR spectroscopic methods. The results of the studies show that incorporation of the chromophoric system on to the lignin core enhanced the light absorption, emission and light stabilization properties of the chromophoric system. The light fastening properties of chromophoric system and the modified product were compared. It shows that stability of the chromophoric system greatly enhanced on attaching to the polymeric system. The trans-cis photoisomerisation and the reverse cis-trans thermal conversions were also assisted by the lignin core. The remarkable stability on irradiation shows that this is a novel photoresponsive system with excellent light fastening properties which would find application in coating materials, dyes, paints, inks, therapeutic agents and many more.
1. Introduction
The incorporation of a photochromic moiety in polymers is very attractive because of the possibility of creating new light sensitive materials, storage and optical devices, diagnostic and therapeutic agent’s etc. [1-4]. A main interest of this azo containing polymer system is their photoisomerization and light fastening properties. Modification of biopolymers leads to environment friendly photo responsive systems. Common modes of modifications in biopolymers are esterification, etherification, phosphorylation, pregelatiniztion etc. In the present work, an attempt has been done to explore present and future industrial prospects of modified biopolymers using photoresponsive systems by the method of esterification. In the present study lignin is modified due to its usefulness in different industrial products in modified form [5,6]. Lignin is an amorphous, aromatic biopolymer second in natural abundance only to cellulose and is obtained from all types of natural wood based resources. Lignin makes up about one quarter to one third of the dry mass of wood. It has several unusual properties for being a biopolymer such as having a network structure and lacking a defined primary structure. Lignin fills the spaces in the cell wall between cellulose, hemi cellulose and pectin components. It also forms covalent bonds to polysaccharides and thereby cross links different plant polysaccharides. It confers mechanical strength to the cell wall and therefore the entire plant. It is particularly abundant in compression wood [7-12]. Lignin plays a crucial part in conducting water in plant stem. The polysaccharide components of plant cell walls are highly hydrophilic and thus permeable to water. Lignin is a large, crosslinked macromolecule with molecular mass in excess of 10,000. It is relatively hydrophobic and aromatic in nature [13,14].
2. Experimental
2.1. Materials and Methods: General
Lignin was purchased from Merck (Germany). Dimethylaminopyridine (DMAP) and dicyclohexylcarbodiimide (DCC) are commercial samples and used as purchased from E. Merck India Ltd. The solvents used for the study such as dimethylformamide (DMF) were purified by literature procedure. NMR spectra were recorded on a Bruker 500 MHz NMR instrument. IR spectra were recorded on a Shimadzu FT-IR instrument operating in the range 4000 - 400 cm–1. UV-visible spectra were recorded on a Shimadzu UV-visible-NIR spectrophotometer operating in the range 200 - 1100 nm.
2.2. Synthesis of 4-[(E)-2-(3-Hydroxynaphthalen- 2-yl)diazen-1-yl]benzoic Acid
p-Amino benzoic acid (1.37 g) was dissolved in dil. HCl (2 N, 15 ml) and cooled in ice. It was diazotized with an ice cold saturated solution of sodium nitrite and coupled with ice cold solution of β-naphthol (1.46 g) in 20% sodium hydroxide (20 ml). The product was filtered, washed with water and dried in vacuum and purified by column chromatography using 10:4 hexane-ethyl acetate solvent system. The yield is noted as 81%.
2.3. Synthesis of Lignin Functionalized with 4- [(E)-2-(3-Hydroxynaphthalen-2-yl)diazen-1- yl]benzoic Acid
Lignin (1 g), DMAP(200 mg), DCC (1 g) and the chromophoric system (2.8 g) in molar ratio were separately dissolved in DMF and the mixture was stirred at room temperature for 2 hours and at 80˚C for 6 hours. The by-product dicyclohexyl urea (DCU) was removed by warming-cooling-filtration process. The solvents were removed in a vacuum rotory evaporator. It was dried and purified by column chromatography using hexane-ethylacetate solvent system (4:1) and dried in vacuum.
3. Results and Discussion
Lignins are complex, amorphous, phenolic polymers, whose aromatic and aliphatic hydroxyl groups can be easily modified by estrification reaction. Spectroscopic analysis such as UV-visible, FT-IR, and 1HNMR has been done in order to visualize the chemical changes in the structure of lignin. The green, nature friendly lignin functionalized with 4-[(E)-2-(3-hydroxynaphthalen-2-yl) diazen-1-yl]benzoic acid system is a novel photoresponsive system and reported in the present paper for the first time.
3.1. Synthesis and Characterisation of 4-[(E)-2-(3-Hydroxynaphthalen-2-yl)diazen-1-yl] benzoic acid
p-Aminobenzoic acid was dissolved in dil. HCl and cooled in ice. It was diazotized with an ice cold saturated solution of sodium nitrite and coupled with ice cold solution of β-naphthol in sodium hydroxide. The product was filtered, washed with water and dried in vacuum and purified by column chromatography (Scheme 1).
IR spectrum of 4-[(E)-2-(3-hydroxynaphthalen-2-yl) diazen-1-yl]benzoic acid was recorded in the solid state as KBr discs in the operating frequency range 4000 - 400 cm–1. The IR spectrum is given in Figure 1. IR (KBr): 3294 cm–1 (broad):νO-H(str), 3050 cm–1:νC-H of CH2 , 1697 cm–1:νC=O(str), 1600 cm–1:νC=C(str), 1508 cm–1:νN=N(str), 1350 cm–1:νC-N(str), 1165 cm–1:νC-O(str).
The proton NMR spectrum of 4-[(E)-2-(3-hydroxynaphthalen-2-yl)diazen-1-yl]benzoic acid was recorded in chloroform using a 500 MHz 1H NMR spectrophotometer. The spectrum is given in Figure 2. 1H NMR: 9.70 ppm (1H, s: -COOH), 7.9 ppm (2H, d: Ha), 7.7 ppm (4H, m: C3-H, C4-H, C5-H and C6-H) 7.6 ppm (2H, d: Hb), 7.3 ppm (1H, s: C1-H), 6.85 ppm (1H, s: C2-H), 5.9 ppm (1H, s: OH).
3.2. Synthesis and Characterisation of Lignin Functionalised with 4-[(E)-2-(3-Hydroxynaphthalen-2-yl)diazen-1-yl]benzoic Acid
The hydroxyl functionalities of lignin were esterified with the free carboxyl group of 4-[(E)-2-(3-hydroxynaphthalen-2-yl)diazen-1-yl]benzoic acidthrough DCC coupling using DMAP as the catalyst (Scheme 2).
IR spectrum was recorded in the solid state as KBr discs in the operating frequency range 4000 - 400 cm–1 (Figure 3). IR(KBr):3330cm–1 (broad):νO-H(str), 2913 cm–1:νC-H(str), 1745 cm–1:νC=O(str), 1612 cm–1:νC=C(str), 1541 cm–1:νN=N(str), 1318 cm–1:νC-N(str), 1033 cm–1:νC-O-C (str). The peak at 1745 cm–1 is due to the presence of ester linkage.
The proton NMR spectrum of the product was recorded in chloroform using a 500 MHz 1H NMR spectrophotometer. 1H NMR: 8.25 ppm (2H, s: aromatic protona), 7.65 (4H,m:C3-H,C4-H,C5-H and C6-H), 7.50 ppm (1H,s: C2-H), 7.01 ppm (1H,s:C1-H), 6.85 ppm (2H,d: Hb), 4.15 ppm (s: unreacted OH groups in lignin), 3.48 ppm (1H, s: OH proton in the chromophore), 1 - 1.4 ppm (aliphatic protons of lignin core) (Figure 4). The carboxylic proton signal of the chromophore system was disappeared when it coupled with the core. This is due to the esterification of COOH group.
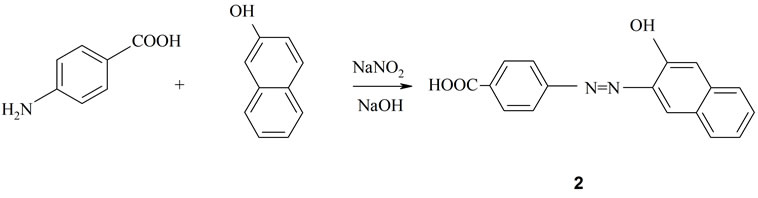
Scheme 1. Synthesis of 4-[(E)-2-(3-hydroxynaphthalen-2-yl) diazen-1-yl]benzoic acid.
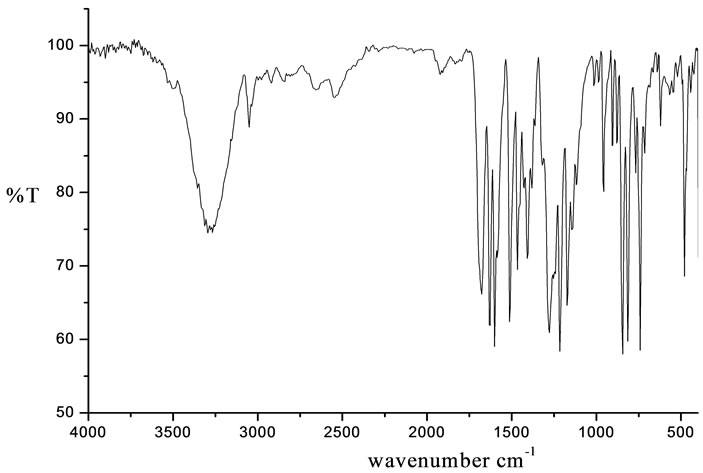
Figure 1. IR spectrum of 4-[(E)-2-(3-hydroxynaphthalen-2- yl)diazen-1-yl]benzoic acid.
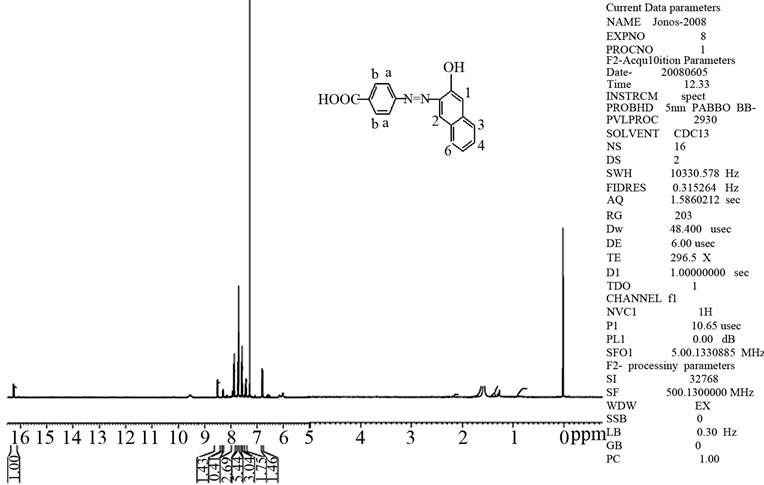
Figure 2. 1H NMR spectrum of 4-[(E)-2-(3- hydroxynaphthalen-2-yl)diazen-1-yl]benzoic acid.
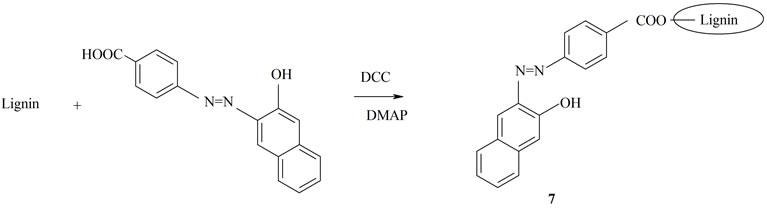
Scheme 2. Synthesis of lignin functionalized with 4-[(E)-2- (3-hydroxynaphthalen-2-yl)diazen-1-yl]benzoic acid.

Figure 3. IR spectrum of lignin modified with 4-[(E)-2-(3- hydroxynaphthalen-2-yl)diazen-1-yl]benzoic acid.
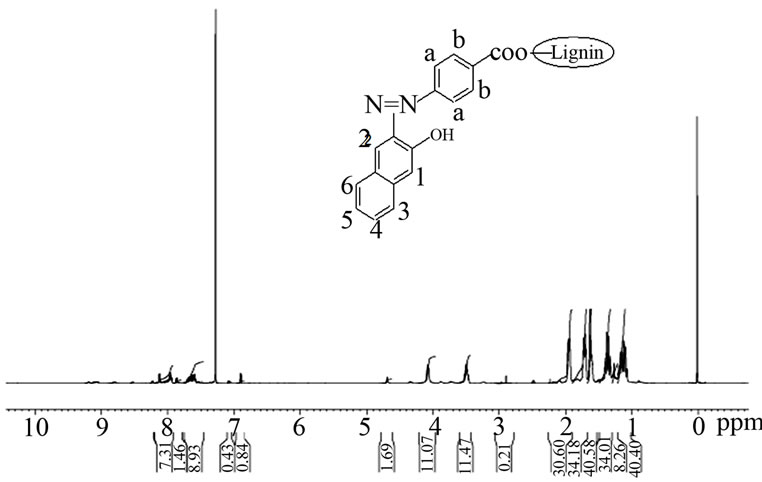
Figure 4. 1H NMR spectrum of lignin modified with 4-[(E)- 2-(3-hydroxynaphthalen-2-yl)diazen-1-yl]benzoic acid.
3.3. Light Absorption Properties of Lignin Modified with 4-[(E)-2-(3-Hydroxynaphthalen-2-yl)diazen-1-yl]benzoic Acid
UV-visible absorption spectrum of lignin modified with 4-[(E)-2-(3-hydroxynaphthalen-2-yl)diazen-1-yl]benzoic acid was recorded in chloroform. The λmax of the chromophoric system was observed at 483 nm whereas the λmax of modified system was found at 494 nm. The spectrum shows a characteristic red shift of 11 nm when attached to lignin core. The intensity of absorption was 1.9 in the case of chromophoric system and 2.4 for functionally modified system. The observed red shift is due to the notably decreased HOMO-LUMO energy gap of the conjugated π system of the chromophoric group on attaching to the core system. The peak at 303 nm is due to the π-π* transition of azo group present in the chromophoric system. The peak at 494 nm is due to the n-π* transition of the group present in the polymer supported chromophoric system (Figure 5).
The fluorescence emission of lignin coupled with 4-[(E)-2-(3-hydroxynaphthalen-2-yl)diazen-1-yl]benzoic acid in chloroform was recorded at room temperature. The excitation wavelength for emission was 400 nm. The emission maximum for the product was observed at 575 nm. The efficiency of fluorescence emission was enhanced on attaching to the polymer chain. When attached to an aromatic biopolymer, electronic excitation of the compound leads to increase in the dipole moment and observable red shift. The emission spectra show red shift of 23 nm and an increase in intensity. The fluorescence spectra of the chromophoric system and the modified lignin are shown in Figure 6.
3.3.1. Light Fastening Studies
The light fastening properties of the chromophoric system and the modified biopolymeric system were examined using chloroform as the solvent. The experiments

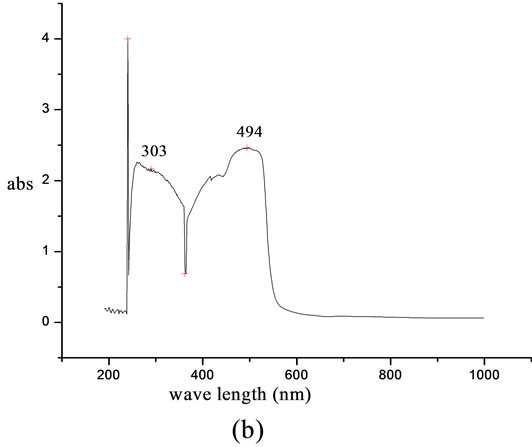
Figure 5. UV-visible spectra of (a) 4-[(E)-2-(3-hydroxynaphthalen-2-yl) diazen-1-yl] benzoic acid and (b) lignin modified with 4-[(E)-2-(3-hydroxynaphthalen-2-yl) diazen-1-yl] benzoic acid.
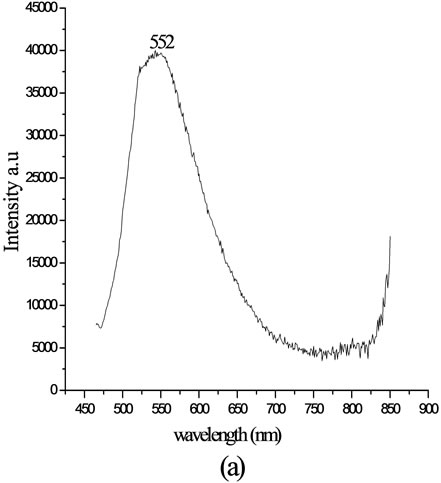
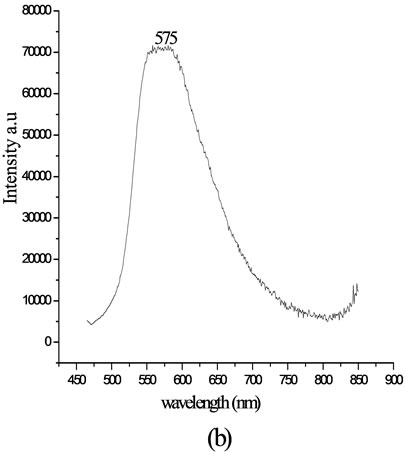
Figure 6. Fluorescence spectra of (a) pure 4-[(E)-2-(3-hydroxynaphthalen-2-yl) diazen-1-yl] benzoic acid and (b) lignin modified with 4-[(E)-2-(3-hydroxynaphthalen-2-yl) diazen-1-yl]benzoic acid
were carried out in chloroform using equi molar concentrations. In this experiment the changes in the intensity of absorption as a function of time of irradiation were noted. The intensity of absorption at zero time was noted as 1.9. Absorbance of the n-π* band at about 483 nm progressively decreased with irradiation time. After 5 hours of irradiation, the intensity of absorption notably decreased and the intensity observed after 5 hours was 1.1. On comparing the pure chromophoric system and the unmodified lignin the modified system shows remarkable stability on prolonged irradiation. The intensity of the modified system remained intact at 2.4. This light fastening study reveals that stability of chromophoric system is greatly enhanced on attaching the chromophoric system to the core material. The spectra are shown in Figure 7.
3.3.2. Trans-Cis-Trans Photo and Thermal Isomerization Studies
The photo isomerisation of chromophore functionalized biopolymer was monitored using UV-visible spectroscopy. UV-visible spectra were recorded as a function of time. In the case of modified lignin, a peak at 303 nm was obtained in the UV region and another in the visible region at 494 nm. The intensity of band in the visible region was enhanced as the irradiation progressed. Where as in the case of UV region the  band at 303 nm is progressively decreased with irradiation time and reached an equilibrium state in about 4 minutes under the irradiation conditions employed in this experiment. This shows the trans-cis isomerisation and after 4 minutes, a photo stationary state was reached. The more stable trans isomer is characterized by a strong
band at 303 nm is progressively decreased with irradiation time and reached an equilibrium state in about 4 minutes under the irradiation conditions employed in this experiment. This shows the trans-cis isomerisation and after 4 minutes, a photo stationary state was reached. The more stable trans isomer is characterized by a strong  band at 303 nm. By exposing trans compound to UV light the
band at 303 nm. By exposing trans compound to UV light the 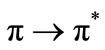 transition is activated. Remarkable change observed when we kept the sample solution at room temperature in darkness. The trans isomer is regenerated fully after 24 hr. This shows the reversible cis-trans isomerization. The chromophoric system also exhibits an increase in intensity with irradiation time in the visible region and decrease in the UV region. On keeping the samples in darkness for 24 hr, the original trans-cis equilibrium state was reached and this was evident from the attainment of the initial λmax and absorption intensity values of the samples as before irradiation. The results show that the polymer bound chromophoric system performs the reversible trans-cis-trans isomerization more efficiently than the low molecular chromophoric system (Figure 8).
transition is activated. Remarkable change observed when we kept the sample solution at room temperature in darkness. The trans isomer is regenerated fully after 24 hr. This shows the reversible cis-trans isomerization. The chromophoric system also exhibits an increase in intensity with irradiation time in the visible region and decrease in the UV region. On keeping the samples in darkness for 24 hr, the original trans-cis equilibrium state was reached and this was evident from the attainment of the initial λmax and absorption intensity values of the samples as before irradiation. The results show that the polymer bound chromophoric system performs the reversible trans-cis-trans isomerization more efficiently than the low molecular chromophoric system (Figure 8).
4. Conclusion
The paper reports the development of a novel photo-responsive, nature friendly, biomaterial by modifying biopolymeric core of lignin by incorporating photo isom-
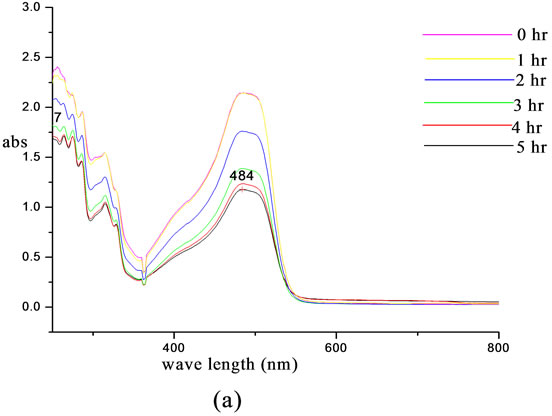

Figure 7. Light fastening studies of (a) 4-[(E)-2-(3-hydroxynaphthalen-2-yl)diazen-1-yl]benzoic acid and (b) lignin modified with 4-[(E)-2-(3-hydroxynaphthalen-2-yl)diazen-1-yl] benzoic acid.
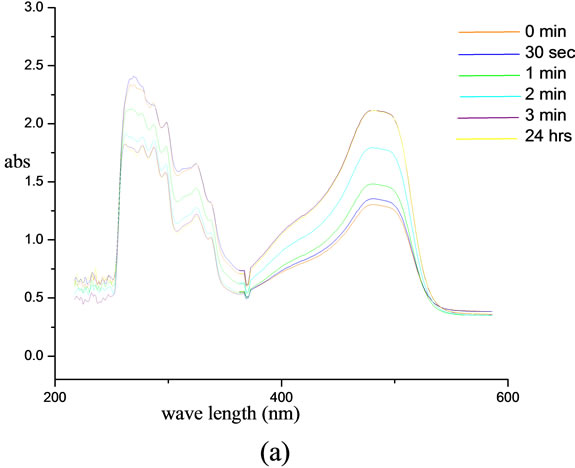
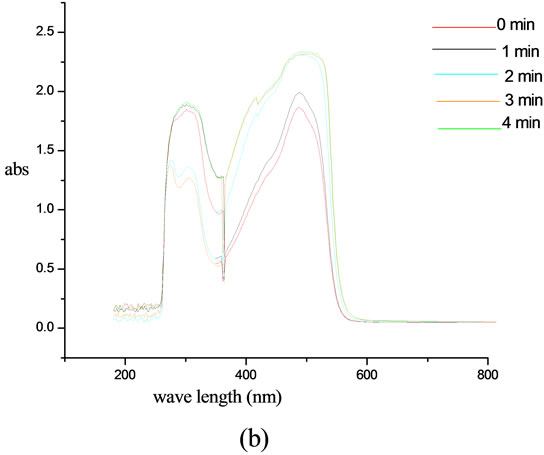
Figure 8. UV-irradiation studies of (a) 4-[(E)-2-(3-hydroxynaphthalen-2-yl) diazen-1-yl]benzoic acid and (b) lignin modified with 4-[(E)-2-(3-hydroxynaphthalen-2-yl) diazen-1-yl] benzoic acid.
erizable units. The designs of such intelligent bio materials are green and environment friendly. In this work a polymer-based photo-responsive system was developed by the functional modification of lignin biopolymer. Lignin was functionally modified with a chromophoric system namely 4-[(E)-2-(3-hydroxynaphthalen-2-yl) diazen- 1-yl] benzoic acid by DCC coupling between the free carboxyl function of the chromophoric system and the end hydroxyl functionalities of lignin and their photochemical and photophysical properties have been investigated. The products were characterised by spectroscopic methods. The photochemical properties of the coupled lignin were compared with the pure chromophoric system. The modified system shows a considerable red shift and remarkable increase in intensity of absorption and emission. The strong red shift observed on binding the chromophoric system to lignin and the remarkable stability on irradiation provides a novel photoresponsive system with excellent light fastening properties. More over it shows excellent emission behaviour which was demonstrated by recording the fluorescence spectra. Photoisomerization of azo compounds and its derivatives have been extensively studied and this system can be used as a photo switch in numerous molecular systems and functional materials. The cis-trans isomerism in azo modified polymers, makes the system good candidates as constituents of molecular switches, image storage devices and materials with photomodulable chemical and physical properties. Since lignin constitutes the major renewable organic substance available on the earth, research in this field is a good milestone for determining the basis of novel polymeric products and future technologies that combine environmental demands, economic realities and a high efficiency of biomass conversion.
5. Acknowledgements
The authors thank the Department of Science and Technology (Ministry of Science and Technology), Govt. of India, New Delhi for financial support by awarding major research project No. SR/S1/OC-24/2006, dtd. 26.10. 2006.
REFERENCES
- J. F. Matte and J. Doucet, “Recent Developments in Lignin Utilization as Wood Adhesive: A Review,” Cellulose Chemistry and Technology, Vol. 22, No. 1, 1988, pp. 71- 85.
- D. W. Sundstrom and H. E. Klei, “Uses of By-Product Lignins from Alcohol Fuel Processes,” Biotechnology and Bioengineering Symposium, Vol. 12, 1982, pp. 45-56.
- J. Ralph, R. D. Hatfield, S. Quideau, R. F. Helm, J. H. Grabber and H.-J. G. Jung, “Pathway of P-Coumaric Acid Incorporation into Maize Lignin as Revealed by NMR,” Journal of the American Chemical Society, Vol. 116, No. 21, 1994, pp. 9448-9456. doi:10.1021/ja00100a006
- J. Ralph, R. F. Helm, S. Quideau and R. D. Hatfield, “Lignin-Feruloyl Ester Cross-Links in Grasses. Part 1. Incorporation of Feruloyl Esters into Coniferyl Alcohol Dehydrogenation Polymers,” Journal of the Chemical Society, Perkin Transactions, Vol. 1, 1995, pp. 2961-2969.
- J. Ralph and F. Lu, “The DFRC Method for Lignin Analysis. Part 6. A Modified Method to Determine Acetate Regiochemistry on Native and Isolated Lignins,” Journal of Agricultural and Food Chemistry, Vol. 46, No. 11, 1998, pp. 4616-4619. doi:10.1021/jf980680d
- J. H. Grabber, J. Ralph and R. D. Hatfield, “Ferulate Cross-Links Limit the Enzymatic Degradation of Synthetically Lignified Primary Walls of Maize Digestibility,” American Society of Agronomy Inc., Maidson, 1993.
- S. Sarkanen, P. C. Teller, C. R. Stevens, J. L. Mccarthy, “Lignin. 20. Associative Interactions between Kraft Lignin Components,” Macromolecules, Vol. 17, No. 12, 1984, pp. 2588-2597. doi:10.1021/ma00142a022
- D. C. C. Smith, “Ester Groups in Lignin,” Nature, Vol. 176, No. 4475, 1955, pp. 267-268. doi:10.1038/176267a0
- J. Ralph, K. Lundquist, G. Brunow, F. Lu, H. Kim, P. F. Schatz, J. M. Marita, R. D. Hatfield, S. A. Ralph, J. H. Christensen and W. Boerjan, “Lignins: Natural Polymers from Oxidative Coupling of 4-hydroxy Phenyl Propanoids,” Phytochemistry Reviews, Vol. 3, No. 1, 2004, pp. 29-60. doi:10.1023/B:PHYT.0000047809.65444.a4
- W. Boerjan, J. Ralph and M. Baucher, “Lignin Biosynthesis,” Annual Review of Plant Biology, Vol. 54, No. 1, 2003, pp. 519-546. doi:10.1146/annurev.arplant.54.031902.134938
- J. Ralph and F. Lu, “The DFRC Method for Lignin Analysis. Part 6. A Modified Method to Determine Acetate Regiochemistry on Native and Isolated Lignins,” Journal of Agricultural and Food Chemistry, Vol. 46, No. 11, 1998, pp. 4616-4619. doi:10.1021/jf980680d
- J. H. Grabber, J. Ralph and R. D. Hatfield, “Ferulate Cross-Links Limit the Enzymatic Degradation of Synthetically Lignified Primary Walls of Maize,” Journal of Agricultural and Food Chemistry, Vol. 46, No. 7, 1998, pp. 2609-2614. doi:10.1021/jf9800099
- R. D. Hatfield, J. Grabber and J. Ralph, “A Potential Role of Sinapyl P-Coumarate in Grass Lignin Formation,” Annual Meeting of the American Society of Plant hysiologists, Vol. 114, 1997, p. 346.
- N. Fukagawa, G. Meshitsuka and A. Ishizu, “A Two Dimensional NMR Study of Birch Milled Wood Lignin,” Journal of Wood Chemistry and Technology, Vol. 11, No. 3, 1991, pp. 373-396. doi:10.1080/02773819108050280
NOTES
*Corresponding author.

