Open Journal of Composite Materials
Vol.06 No.04(2016), Article ID:70677,9 pages
10.4236/ojcm.2016.64011
Effect of Degree of Cure on Sandwich Structural Capacitor Using Ion-Conductive Polymer with Carbon Fabric Skins
Akira Todoroki
Department of Mechanical Sciences of Engineering, Tokyo Institute of Technology, Tokyo, Japan

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: August 17, 2016; Accepted: September 16, 2016; Published: September 19, 2016
ABSTRACT
Structural capacitors are composite structures that function as energy storage capacitors. An electric double-layer capacitor with a composite structure using a solid polymer electrolyte matrix with a glass fiber fabric separator has recently been developed. In the present study, new foam core sandwich structure is adopted and the effect of the degree of cure is experimentally investigated. Carbon fiber fabric cloth is used as electrodes, and the polystyrene foam core is used as separator. Material system of Poly Ethylene Glycol DiGlycidyl Ether (PEGDGE) with Lithium bisTriFluoromethane Sulfonyl Imide (LiTFSI) and hardener of TriEthylene TetrAmine (TETA) is adopted as ion-conductive polymer matrix. The effect of the cure degree is experimentally investigated by using 100% cure degree, 70% cure degree and 0% cure degree specimens. As a result, the polystyrene foam-core sandwich system is proved to be effective, but the capacitance is not enough because of the lack of surface area of the carbon fiber electrodes. As the remained TETA impedes the movement of Li+ cation in the solid polymer by means of the segment-motion-assisted diffusion process, the low degree of cure causes small capacitance with this material system.
Keywords:
Composites, Woven Carbon Fabric, Capacitor, Supercapacitor, Sandwich, Foam Core

1. Introduction
Structural capacitors are composite structures that function as energy storage capacitors. Luo and Chung have fabricated structural capacitors by using a paper separator [1] that exhibited as capacitance of 12 nF/cm2. Lin and Sodano made a cylindrical capacitor by using piezoelectric material BaTiO3 that can be used as reinforcements of composites [2] . Carlson et al. used a paper and polymer sheet as dielectric separator of a capacitor, and obtained a capacitance of 25 nF/cm2 [3] [4] . O’Brien et al. also developed a parallel plate type structural capacitor [5] . The parallel plate type capacitor has advantage of the higher dielectric breakdown strength. The capacitor has, however, the drawback of the lower capacitance.
Shirshova et al. have developed an electric double layer capacitor (supercapacitor) of composite structures using electrolyte solid polymer matrix and glass fiber fabric separator [6] [7] . They obtained the capacitance of 8.9 mF/cm2. Qian et al. increased the surface area of the electrodes of the supercapacitor by using carbon aerogel [8] , and they obtained the best capacitance of 640 mF/cm2. The supercapacitor enabled the higher capacitance of order of 1 F/cm2, although the dielectric breakdown strength became smaller than that of a film type capacitor. The dielectric breakdown strength of a supercapacitor is approximately order of 1 V, although the dielectric breakdown strength of the film capacitor is approximately order of 10 kV. As the extremely high voltage is not safe for regeneration-energy-storage capacitors in automobiles in service, the high capacitance of the supercapacitor is appropriate for the actual automobiles that require higher energy density compared with the film type capacitor. Therefore the structural supercapacitor is preferable for regenerated-energy storage of an automobile. The supercapacitor of a composite structure using electrolyte solid polymer matrix and glass fiber fabric separator, however, has a problem of electrical short between the carbon fiber plies because of the movement of glass fiber cloth during curing process [9] .
In the present study, a new method to use foam core as a separator for a sandwich structural capacitor is proposed by using electrolyte polymer matrix to prevent the electrical short between the electrodes through the fabrication process. As a separator ply, a sandwich foam core is selected and experimentally confirmed to be effective. Moreover, the effect of the degree of cure of the electrolyte polymer matrix on the capacitance is also experimentally investigated.
2. Concept of Foam-Core Sandwich Capacitor
The concept of the foam-core-capacitor sandwich proposed in the present study is schematically shown in Figure 1. The skin of the sandwich structure is woven fabric
Figure 1. Schematic representation of a foam-core structural supercapacitor.
carbon fiber reinforced polymer (CFRP) cloth. The skin woven fabric CFRP sheets play triple roles of structures, lead wires and electrodes of a capacitor. For the matrix of the CFRP, ion conductive solid polymer electrolyte is adopted.
3. Fabrication of a Foam-Core Sandwich Capacitor
Polystyrene foam core (moisture absorption type, Sekisui Plastic Co. Ltd, Osaka, Japan) is selected in the present study because of its higher liquid retentivity as shown in the reference [10] . PolyEthylene Glycol DiGlycidyl Ether (PEGDGE) with Lithium bisTriFluoromethaneSulfonylImide (LiTFSI) is selected as ion―conductive polymer and TriEthyleneTetrAmine (TETA) is adopted as harder as shown in the reference [7] [8] . The difference from the published papers is the selection of the foam core as a separator in the present study. To make sure that the polystyrene foam core can retain the ion-conductive polymer experimentally, red color ink is added into ion-conductive polymer PEGDGE with LiTFSI, and the red-colored PEGDGE with LiTFSI is immersed into the polystyrene foam core. Figure 2 shows the pre-investigation of this foam core. The red-colored PEGDGE with LiTFSI is immersed from the top surface of the polystyrene form core. The backside shows the polymer is partially immersed but not fully immersed. The ion-conductive polymer, therefore, is immersed from the both sides of the foam core. Figure 3 shows the cross sectional view of the immersed foam core from the both sides. Figure 3 shows the entire cross section is red. This means ion-conduc- tive polymer is perfectly immersed into the polystyrene foam core.


Figure 2. PEGDGE + LiTTFSI immersion test using red ink. (a) Top side of specimen; (b) Backside of specimen.
Figure 3. Cross sectional view of a polystyrene foam with red-colored PEGDGE with LiTTFS.
Woven carbon fiber cloth (SA-3103, thickness 0.013 mm, Sakaiovex Co. Ltd., Fukui Japan) was used as skin surface structure and lead wires. Polystyrene foam core (Sekisui plastics Co. Ltd., 0.8 mm thickness) was selected as a sandwich-foam core and a separator of a capacitor.
To make the ion-conductive polymer, a globe box is used to keep dry air condition. First, 10 g of PEGDGE was prepared and 0.1 mol LiTFSI is added into the PEGDGE. The PEGDGE with LiTFSi is mixed well using an agitator NBK-1 made by Nihonseiki Kaisha Ltd for five minutes at 200 rpm. After this, TriEthylene TetrAmine (TETA) was added at the mol ratio of PEGDGE:TETA = 4:1. The PEGDE with TETA was mixed for ten minutes at 200 rpm. Using this mixing process, the ion-conductive polymer was prepared as described in the reference [8] .
The prepared ion-conducting polymer was immersed into the woven CF cloth and the foam core. The prepared woven CF cloth is 100 mm long and 50 mm wide. The 50 mm length of the woven CF cloth was used as electrodes for the structural capacitor. This means that the area of the capacitor is 50 × 50 (mm2). The specimen was sandwiched by aluminum plates as shown in Figure 4. The weight of the aluminum plates (3.3 kg) was loaded during the curing process. Figure 5 shows the specimen configuration.
Figure 4. Manufacturing of CFRP structural capacitor using ion conductive polymer.
Figure 5. Specimen configuration.
4. Experimental Setup
In the present study, three types of the degree of cure are investigated: 100%, 70% and 0%. To measure the degree of cure, a Difference Scanning Calorimetry (DSC-60 Plus, Shimadzu Co., Kyoto, Japan) was used. To make a specimen of 100% degree of cure, the specimen was elevated at 1˚C/min up to 140˚C. To make a specimen of 70% of degree of cure, the specimen was elevated at 1˚C/min up to 75˚C. To make a specimen of 0% degree of cure, no curing process was performed. As it is difficult to control furnace temperature at lower temperature, specimens of the lower degree of cure was not prepared in the present study.
To measure the capacitance of the foam-core-sandwich supercapacitor specimen, the cyclic voltammetry method was employed. The cyclic voltammetry method uses constant rate voltage increase and decrease. The current is measured during sweeping the voltage. The capacitance C(t) at time t can be calculated as follows.
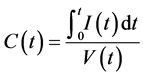 (1)
(1)
where V(t) is the voltage at time t, and I(t) is electric current at time t.
For the measurements of the capacitances, a versatile potentiostat/galvanostat machine Versastat 4 (Princeton Applied Research, Oak Ridge USA) was used. In the present study, the maximum voltage charged to the capacitor was set to 0.5 V to prevent dielectric breakdown of the electric double layer capacitor in the solid ion-con- ductive polymer. Since the low voltage causes the lower charged electric energy, the maximum limit voltage should be larger for actual usage. The maximum voltage can be improved using serial connection of the supercapacitor.
5. Results and Discussion
Figure 6 shows the result of the DSC at 1˚C/min up to 150˚C test. The abscissa shows the temperature and the ordinate shows the measured reaction heat. The figure shows that the curing ended at the temperature of 131.8˚C. This experimental result reveals
Figure 6. Measured DSC result at 1˚C/min up to 150˚C.
that the 140˚C at the rate of 1˚C/min is enough for 100% curing. After this test, the specimen temperature was elevated again with the DSC. The results showed that there is no curing reaction.
In order to obtain the specimen of 70% degree of cure, a specimen was heated at 1˚C/min up to 70˚C and cooled down to the room temperature. During the process, the DSC is used to measure the heat reaction. The result is shown in Figure 7.
In Figure 7, the abscissa shows the time and the ordinate shows the measured reaction heat. The total reaction heat was measured from the elevation of the temperature to the cooling down to the room temperature. The degree of cure can be calculated as follow.
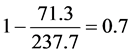 (2)
(2)
This shows the degree of cure is 70%.
Figure 8 shows the measured results of the specimen of 100% cure using the voltammetry method. The abscissa is the charged voltage and the ordinate is the calculated capacitance obtained from the meas current. Let us consider the capacitance:
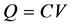 (3)
(3)
Figure 7. Measured DSC result 70 % degree of curing test.
Figure 8. Measured results of the voltammetry method of 100 % cure specimen.
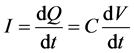 (4)
(4)
where Q is electrical charge, V is voltage, C is capacitance, and I is electric current. Equation (4) shows that capacitance can be easily calculated when the voltage change ratio (dV/dt) is constant. The voltage change rate is 0.1 mV/s in the present study. Three cycles are performed to obtain the averaged capacitance. Figure 8 shows the electric current and voltage curve is almost rectangular shape.
In the present study, the capacitance was calculated using an increasing time period of voltage. The measured average capacitance is 3.66 mF. As the area used is 50 mm × 50 mm = 25 cm2, the capacitance per unit square centimeter is 0.146 mF/cm2. Although the capacitance is larger than that of film type capacitor, the capacitance is smaller than that of the research results obtained by Shirshova et al. [6] - [8] . This is because of the smaller surface area of carbon fibers at the electrodes. Shirshova et al. used special surface treatment of the carbon fibers to increase the surface area of the electrode carbon fibers. In the present study, the surface treatment was not performed.
Figure 9 shows the measured results of the specimen of 70% cure using the voltammetry method. The capacitance was calculated using increasing time period of voltage. The measured average capacitance is 2.57 mF. The capacitance is 30% decrease compared with that of 100% degree of cure. The specimen of 0% degree of cure shows no capacitance as shown in Figure 10. This means the degree of cure greatly affect the ion- conductivity in this polymer.
Figure 9. Measured results of the voltammetry method of 70% cure specimen.
Figure 10. Measured results of the voltammetry method of 0 % cure specimen.
As shown in the reference [11] , Li+ cations are transported by the segmental-motion- assisted diffusion process in solid polymer. When the degree of cure is low, this means the number of cross-links is small in the solid polymer. The long segments usually bring lower glass transition temperature [12] . This means the segment motion is easy when the degree of cure is low. Although the segment motion is easy when the degree of cure is low, the measured results show that the capacitance decreases along with the decrease of the degree of cure. This is because of the existence of the remained hardener TETA. TETA is amino group. The amino group has strong basicity [13] , and this basicity impedes the diffusion movement of Li+ cations. The selection of hardener is the important issue for the electrolyte solid polymer structural capacitor.
As the foam core plays a role of separator as shown in Figure 8, the polystyrene foam core is useful for the separator instead of the woven glass cloth that causes electrical short [9] .
6. Conclusion
Experimental study shows that the polystyrene foam core is applicable as a separator for the structural capacitor. The foam core separator does not cause the electrical short during fabrication process. The degree of cure for PEGDGE with LiTFSI using TETA as hardener affects the capacitance significantly. Insufficient degree of cure causes remained harder TETA. The remained TETA impedes the movement of Li+ cation in the solid polymer by means of the segment-motion-assisted diffusion process. To increase the capacitance, increase of the surface area of the electrodes is indispensable.
Acknowledgements
This research is supported by the JSPS Grants-in-Aid for scientific research of scientific research (part C) general FY2015 #15K05673. This research was performed with the help of Mr. Kento Kawamura who was a student of Tokyo Institute of Technology. I would like to express sincerely thanks for his work.
Cite this paper
Todoroki, T. (2016) Effect of Degree of Cure on Sandwich Structural Capacitor Using Ion-Con- ductive Polymer with Carbon Fabric Skins. Open Journal of Composite Materials, 6, 112-120. http://dx.doi.org/10.4236/ojcm.2016.64011
References
- 1. Luo, X. and Chung, D.D.L. (2001) Carbon-Fiber/Polymer Matrix Composites as Capacitors. Composites Science and Technology, 61, 885-888.
http://dx.doi.org/10.1016/S0266-3538(00)00166-4 - 2. Lin, Y. and Sodano, H.A. (2009) Characterization of Multifunctional Structural Capacitors for Embedded Energy Storage. Journal of Applied Physics, 106, 114108.
http://dx.doi.org/10.1063/1.3267482 - 3. Carlson, T., Ordéus, D., Wysocki, M. and Asp, L.E. (2010) Structural Capacitor Materials Made from Carbon Fibre Epoxy Composites. Composites Science and Technology, 70, 1135-1140.
http://dx.doi.org/10.1016/j.compscitech.2010.02.028 - 4. Carlson, T., Ordéus, D., Wysocki, M. and Asp, L.E., (2011) CFRP Structural Capacitor Materials for Automotive Application. Plastics, Rubber and Composites, 40, 311-316.
http://dx.doi.org/10.1179/174328911X12948334590286 - 5. O’Brien, D.J., Baechie, D.M. and Wetzel, E.D. (2011) Design and Performance of Multifunctional Structural Composite Capacitors. J. of Composite Materials, 45, 2797-2809.
http://dx.doi.org/10.1177/0021998311412207 - 6. Shirshova, N., Qian H., Shaffer M.S.P., Steinke J.H.G., Greenhalgh, E.S., Curtis, P.T., Kucernak, A. and Bismarck, A. (2013) Structural Composite Supercapacitor. Composites: Part A, 46, 96-107.
http://dx.doi.org/10.1016/j.compositesa.2012.10.007 - 7. Shirshova, N., Bismarck, A., Carreyette, S., Fontana, Q.P.V., Greenhalgh, E.S., Jacobsson, P., Johansson, P., Marczewski, M.J., Kalinka, G., Kucernak, A.R.J., Sheers, J., Shaffer, M.S.P., Steinke, E.S. and Wienrich, M. (2013) Structural Supercapacitor Electrolytes Based on Bicontinuousionic Liquid-Epoxy Resin System. Journal of Materials Chemistry A, 1, 15300-15309.
http://dx.doi.org/10.1039/c3ta13163g - 8. Qian, H., Kucernak, A.R.J., Greenhalgh, E.S., Bismarck, A. and Shaffer, M.S.P. (2013) Multifunctional Structural Supercapacitor Composites Based on Carbon Aerogel Modified High Performance Carbon Fiber Fabric. ACS Applied Materials and Interfaces, 5, 6113-6122.
- 9. Todoroki, A., Shiomi, H., Mizutani, Y. and Suzuki, Y. (2014) Electrical Shorting between the Carbon-Fiber Cloth Electrodes of Structural Capacitors with a Glass-Fiber Cloth Separator. Open Journal of Composite Materials, 4, 140-147.
http://dx.doi.org/10.4236/ojcm.2014.43016 - 10. Todoroki, A., Sawada, T., Mizutani, Y. and Suzuki, Y. (2015) Supercapacitor Consisting of a Form Core Sandwich with Woven Carbon Fiber Skin. Open Journal of Composite Materials, 5, 101-109.
http://dx.doi.org/10.4236/ojcm.2015.54013 - 11. Yang, M. and Hou, J. (2012) Membrances in Lithium Ion Batteries. Membrances, 2, 367- 383.
http://dx.doi.org/10.3390/membranes2030367 - 12. Wise, C.W., Cook, W.D. and Goodwin, A.A. (1997) Chemico-Diffusion Kinetics of Model Epoxy-Amine Resins. Polymer, 38, 3251-3261.
http://dx.doi.org/10.1016/S0032-3861(96)00882-8 - 13. McMurry, J. and Simanek, E. (2006) Fundamentals of Organic Chemistry. 6th Edition, Brooks/Cole Pub Co., Belmont.










