Open Journal of Medicinal Chemistry
Vol.4 No.2(2014), Article
ID:47132,9
pages
DOI:10.4236/ojmc.2014.42005
Experience of Using the Recombinant Human Superoxide Dismutase Drug in Neurological and Ophthalmological Practice
Elena E. Dubinina1, Irina V. Churilova2, Liudmila V. Lipatova1*, Liudmila V. Zhuravleva2
1St. Petersburg V. M. Bekhterev Psychoneurological Research Institute, St. Petersburg, Russian Federation
2State Research Institute of Highly Pure Biopreparations, St. Petersburg, Russian Federation
Email: *l_lipatova@mail.ru
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 22 April 2014; revised 20 May 2014; accepted 20 June 2014
ABSTRACT
Recsod® has been used for treating epilepsy and ophthalmological disease. Oxidative stress has been demonstrated to be a pathogenic chain of these diseases. Parameters of proand antioxidant systems were studied in all the patients treated. Recsod® drug was shown to produce positive effect in all the patients. Improvement of patients’ clinical condition correlated with an increase in antioxidant activities. Antioxidants, in particular, the recombinant human SOD drug, proved to be effective in treatment of some neurological and ophthalmological diseases.
Keywords:Superoxide Dismutase, Oxidative Stress, Reactive Oxygen Species, Epilepsy, Age-Related Macular Degeneration

1. Introduction
Metabolic processes in tissues are accompanied by generation of highly reactive oxygen species (ROS). The intensive ROS generation at different pathologies causes oxidative destruction of proteins, lipids, nucleic acids, and carbohydrates. In the body, toxic effects of ROS are prevented by antioxidant protection (AOP), which involves enzymes and nonenzymatic components. Effects of enzymatic antioxidants are closely connected with each other and accurately balanced among themselves [1] [2] . Each tissue possesses a certain buffer capacity for AOP, represented by AOP of intracellular fluid and of a cell itself. Some tissues due to special features of their functional and metabolic activities possess hypersensitivity to oxidative stress (OS). Among these first are brain tissue and retina [3] [4] .
High sensitivity of brain tissue and retina to OS is caused by high degree of dependence of metabolic processes from extent of saturation by oxygen. The probability of oxidative damage of the retina depends on high partial pressure of oxygen, on UVand visible light effects and on the high content of polyunsaturated fatty acids in the external segments of photoreceptors [5] . The certain ROS levels in the retinal pigmentary epithelium (RPE) and in retina photoreceptors are due to phagocytosis reaction of external segments of rod and conus and also due to photochemical damage (autooxidation of lipofuscin) [6] . In addition, ROS can be generated by activated neutrophils and macrophages in case of the unfavorible environment, especially tobacco smoke, and can also be due to pro-oxidant effects of synthetic vitamins [7] .
The brain tissue oxidative damage probability also mainly depends on high oxygen partial pressure (the brain uses for its own purpose 1/5 of incoming oxygen and possesses the intensive aerobic oxidation rate) and on the presence of polyunsaturated fatty acids in nervous tissue membranes which are exposed to oxidative destruction during stress. An increase of ROS levels in the brain tissue may be associated with high rate of biogenic amine metabolism and with the presence of variable valence metal ions bound with low molecular substances. High sensitivity of brain tissue to free radical effects is caused by low activities of separate antioxidant enzymes, in particular catalase, which is localized in microperoxisomes of neurons [8] -[12] .
The activities of superoxide dismutase (SOD) and of glutathione peroxidase are moderate, but these enzymes, similar to nonenzymatic antioxidants, are unequally distributed among different parts of the brain. Nonenzymatic antioxidant protection is presented by vitamin E, carotinoid, ubiquinones Q9 and Q10 and the derived ubiquinoles as lipid soluble antioxidants. Ascorbic acid and reduced thiols function as the main antioxidants of neuron cytosol [3] [13] .
The retina contains enough levels of vitamin E, carotinoids, SOD, catalases, glutathione peroxidase to prevent development of oxidative stress. However, under certain conditions, especially late in life, the retina antioxidant (AO) concentrations may be insufficient and this may result in growth and accumulation of oxidative damages.
There is evidence that oxidative stress has been implicated in the retina and brain tissue pathologies. Hence, use of antioxidant drugs in therapy of some diseases of the retina and brain is pathogenic reasonable and necessary.
In particularly, it is generally accepted, that along with heredity, age and other risk factors, OS is one of the leading factors of age-related macular degeneration (AMD) [6] [14] [15] and epilepsy development [16] -[19] .
Among the antioxidants useful for prevention and treatment of AMD and epilepsy, those are primarily used, the components of which inhibit final processes of free radical oxidation (FRO) and can’t generally reduce OS severity. The use of antioxidants capable to prevent generation of primary ROS (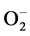 , H2O2), in particular, the drugs based on SOD, a key enzyme of AO protection, is more advisable. Among these is Recsod® drug, which contains recombinant human SOD as the main active ingredient.
, H2O2), in particular, the drugs based on SOD, a key enzyme of AO protection, is more advisable. Among these is Recsod® drug, which contains recombinant human SOD as the main active ingredient.
The goal is to assess Recsod® drug efficacy in complex therapy of patients with AMD and epilepsy based on examination of the proand antioxidant status and on functional ophthalmological and neurological studies.
2. Materials and Methods
40 patients with “dry” form of AMD aged from 50 to 90 years (men—14, women—26) and 58 patients with epilepsy (PE), (20 women, 38 men; middle age 36.8 years) were examined. Control group included 40 healthy volunteers aged from 40 - 70 years. Changes of retina’s macular zone which mostly contained “hard”, small, clearly defined druses were observed in 22 ophthalmologic patients (55%) and different degree of RPE destruction, until it’s atrophy—in 18 (45%).
Patients were divided into two groups: 1 group (n = 15)—without antioxidant therapy; 2 group (n = 25) took Adruzen Zinko (nutraceuticals, SIF, Italy): 1 capsule twice a day for 6 months. Patients of both groups were given Recsod® drug 3.2 × 106 U, lyophilized powder for preparation of solution for injections (State Research Institute of Highly Pure Biopreparations, Russian Federation). Recsod® is a trade mark common for novel enzymatic drugs, which possess antioxidant, anticytolytic, and anti-inflammatory activities. Recombinant human SOD produced from yeast Saccharomyces cerevisiae, strain Y2134, is the main active ingredient of Recsod®.
All ophthalmologic patients were examined for diagnosing “dry” form of AMD. Epileptic patients were subjected to complex clinical, neurologic, electroencephalographic (EEG), magnetic resonance imaging of the brain, psychological test, National Health Seizure Severity Scale—“NHS-3” (O’Donoghue, M.F., et al., 1996) and Clinical Global Impression scale—“CGI” (Guy, W., 1976).
These patients received basic antiepileptic drugs before course of Recsod®. The drug was intravenous injected by drop infusion at 200 ml/hour for 7 - 10 days. A single dose of Recsod® was 3.2 × 106 U in 200 ml of 0.9% NaCl for neurological patients and 6.4 × 106 U in 100 ml of 0.9% NaCl for AMD patients.
The presense of oxidative stress was assessed by the activity of proand antioxidant systems in both groups.
Chemiluminescence (CL, relative value unit) of a whole blood was determined with luminometer LKB-1251 (Sweden) in the presence of luminol (L), phorbol-miristatacetate (PMA) was used as the activator [20] . Reaction mix contained 100 µl of a whole blood on heparin, 2 × 10−5 МL, 1.6 µM PMA, HENKS buffer with 0.1% glucose. Lightsum of CL in mV was registered within 30 min. Reaction temperature was 37˚C.
Final products the lipid peroxidation (LPO) (TBA-active products) was analysed by spectrophotometric method with the use of thiobarbituric acid (TBA-AP) [21] . Oxidized and reduced glutathione in erythrocytes were determined by color reaction with chlorpromazine in the presence of palladium ions [22] . Thiol status was judged from the SH groups (µM/ml) in blood plasma [23] .
To evaluate the superoxide dismutase activity in a whole blood the spectrophotometric method based on inhibition of oxidation reaction was used [24] . The quantity of SOD which inhibited quercetin oxidation for 50% was taken as a unit of activity.
The activity of erythrocytes catalase (U/mg hemoglobin) was estimated by a decrease in concentration of hydrogen peroxide in 1 min [25] .
Oxidative modification of plasma proteins was estimated by the level of carbonyl groups generated based on their interaction with 2,4-DNFG [26] .
The experiments on distribution of I125-labelled SOD in eye tissues were carried out in rabbits weighing 2.5 kg. Five rabbits received the drug into an ear vein at a dose of 0.5 ml. Just after administration, rabbits were killed with air embolism and eyes enukleation was made. From denucleated eyes sections were prepared, in which concentration of I125 was determined by a radio isotope method by means of the counter Delta 300. Initial activity was 7.8 × 106 imp/min·ml.
Statistical analysis of the results was made with Student (t) and Mann-Whitney (U) criteria.
3. Results
Several parameters of free radical oxidation and of AO system were evaluated in all the patients with dry AMD and in healthy volunteers (Table 1). Based on the data of evaluation a statistically significant increase in free radical oxidation (PMA-CL-L and carbonyl protein groups) intensity as well as a decrease in antioxidant system components (SOD and the reduced glutathione) were observed. In these patient groups, no significant changes in TBA-AP and the oxidized glutathione were revealed.
Treatment with Adruzen-Zinko drug given peros demonstrated none drug effect on the course of disease or the indices examined. Possibly, some other indices, such as plasma levels of tocopherol or carotenoids, not studied in this work, were affected.
The second drug studied in patients with dry AMD was Recsod®. We studied experimentally the drug distribution in rat eye tissue after i.v. administration of I125-labeled Recsod® (Table 2).
This study showed that at slow intravenous administration exogenous superoxide dismutase reaches the retina.
Table 1. Indices of proand antioxidative status in AMD patients.
Evaluating the Recsod® drug administration in AMD patients, we observed the following changes in the indices examined: the enzymatic activity of antioxidant system (SOD and catalase) and concentrations of the oxidized and reduced glutathione increased, while the ratio between these indices did not change. In parallel, a fall in the intensity of oxidative destruction of plasma proteins, final PLO-MDA product, and PMA-CL-L value was observed. However, only SOD and PMA-CL-L indices were statistically significant (Table 3).
In both patient groups, the proand antioxidant status indices normalized after Recsod® treatment. However, a significant improvement of patient clinical condition followed by stabilization of visual functions, and of life quality was found only in the group, which received Adruzen-Zinko drug in combination with Recsod®. In these patients, the visual acuity rose by a mean of 0.4 units, scotoma in central visual field decreased, brightness sensitivity inparaphovea increased, and AOS improved. In this group, visual functions were stable for 6 months.
The second series of experiments were aimed at study of some AOS indices in dynamics after Recsod® administration on the background of basic therapy in PE. Statistically significant decreases in some AO components (SOD activity and SH-group concentration) in PE blood as compared with that in control were found. The SOD activity was almost twice lower compared to that in healthy persons. A reduction in both SOD activity and thiol group levels in blood of patients with epilepsy suggested that at OS there was a depletion of antioxidant cell protection, including neurons.
This was a reason for Recsod® drug introduction to therapy of patients with epilepsy. The data obtained are presented in Diagram 1 and Diagram 2.
Administration of Recsod® drug in patients with epilepsy resulted in an increase of AOS activity. This is confirmed by a statistically significant increase in SOD activity as well as by a tendency for SH-group concentrations to increase without statistical reliability. Positive correlation among the blood AOS biochemical indices, improvement of clinical encephalography data and patient general well-being were observed in PE after therapy with Recsod®. These parameters were NHS3 and CGI scores and were as follows: the severity of epileptic attacks fell from 13.75 ± 4.41 to 7.54 ± 3.05, p ≤ 0.5, suggesting that the severe and complicated attack rates decreased.
In patients with epilepsy subjected to Recsod® treatment, the composite CGI score migrated from moderate and marked disorder range to light and moderate one (6.01 ± 1.74 to 2.37 ± 0.5).
Table 2. I125-SOD distribution in rabbit eye tissue sections after intravenous injection (imp/min/g).
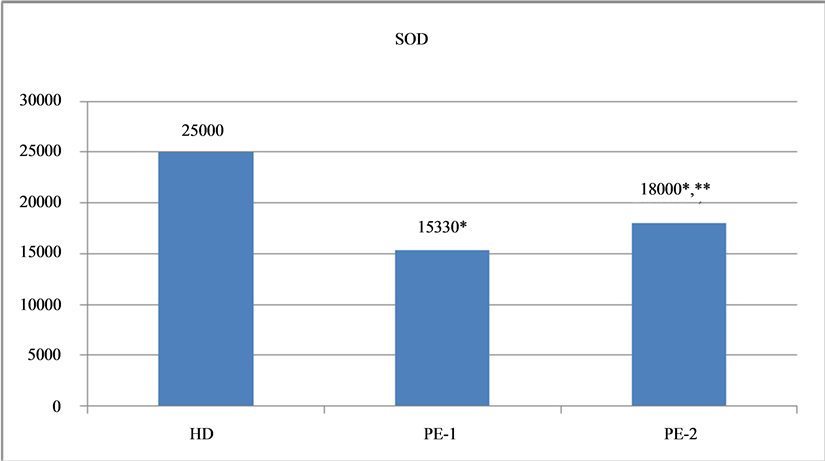
Diagram 1. SOD activity in the blood of healthy donors and of patients with epilepsy before and after Recsod® treatment. SOD activity in the blood of healthy donors (HD) and of PE-1—before treatment, of PE-2— after treatment with Recsod,*p < 0.05 between HD-PE1, HD-PE2, **p < 0.05 PE1-PE2.
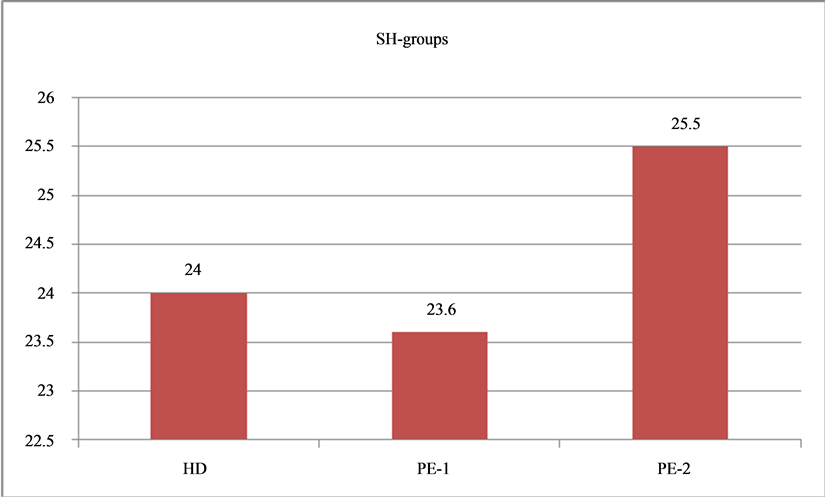
Diagram 2. Concentrations of SH-groups in the blood at healthy donors and of patients with epilepsy before and after Recsod® treatment. SH-groups plasma levels in HD-healthy donors, in PE-1—before treatment, in PE-2—after treatment with Recsod®.
4. Discussion
Free radical oxidation and its intensification are thought to contribute to the development of a wide range of diseases including ophthalmological and psychoneurological ones. Intensive generation of ROS may be caused by tissue energy metabolism disturbance in development of macular degeneration, primary open-angle glaucoma associated with tissue hypoxia during aging or with energy consumption jump and its quick depletion at epileptic seizure. In fact, we can observe chronic stress development accompanied by a decrease in antioxidant defense, which is a body adaptation system under stress. Depletion of enzymatic antioxidant defense, in particularly SOD, and activation of pro-oxidative systems were the indicators we revealed in patients examined.
Neither prophylaxic actions to prevent blindness in atrophic AMD nor effective treatment of the disease existed up to now. Evaluation of effects of widely used antioxidants lutein, zeaxatin, carotin, vitamins E and C on retina antioxidant state is quite a challenging task. This is caused, first of all, by the lack of simple, reliable, non-aggressive measurements of oxidative balance of retina. For this reason, indirect tests for estimation of the retina antioxidant system are relevant and important for clinical practice.
The results of this study pointed to a decrease in blood SOD levels of AMD patients compared to that in control group, which coincided with literature data [27] . At the same time, data of other studies showed that SOD and glutathione peroxidase activities did not differ from that in control group [28] . Such a difference suggests that personal characteristics of an AMD patient are significant for studying oxidative stress and aging. These depend a lot on patient’s physiological condition, genetic predisposition, diet, life style and physical activity. The mechanisms underlying the involution-dependent effects are multifactorial and changeable.
We showed that the reduced glutathione concentrations were significantly lower in AMD patients compared to age norm rates. These results coincided with literature data [29] . The blood RMA-CL-L is a reporting marker of pro-oxidative status. This marker is indicative of the phagocytizing cells (polymorphonuclear leukocytes) activiation and is responsible for their maximal biocidal ability, as RMA activates phagocyte NADPH-oxigenase through action of proteinkinase C. This is a kind of loading tests which may reveal the capabilities of phagocytosis system [30] .
Retina is very sensitive to damaging action of activated inflammatory cells. Such cells may cause additional damage of RPE due to generation of toxic factors (AFO, lysosomal enzymes and proteins). Phagocytosis activation is associated with oxidative destruction of biomolecules under oxidative stress. This is especially evident in AMD patients.
The results of this study showed that the blood RMA index in the majority of AMD patients was higher than norm rate that may be due to concomitant atherosclerosis and artherial hypertention. In particularly, atherosclerosis may cause formation of lipid-comprising immune complexes capable of activating the complement system and of supporting afflux of new activated cells [30] .
The blood RMA levels in the group studied did not change for a long period of patient observation. This fact confirms the existance of constant pool of activated cells indicating chronic oxidative stress. A course of nonenzymic antioxidant drug Adruzen-Zinko given peros in AMD patients did not produce a significant effect on visual fuction and on biochemical indices studied.
Recsod® was the second antioxidant drug used for treatment of AMD patients. Recsod® has proven itself not only as ophthalmological drug for treating open angle glaucoma, herpes and viral eye diseases, anterior uveitis, injuries and burns of the eyes, but showed high efficacy in a variety of diseases involving the so-called systemic inflammatory response [31] [32] .
After Recsod® treatment all patients noted improvement of general well-being. However, the results of this study showed that only combination of enzymatic and non-enzymatic antioxidants may result in simultaneous improvement of visual functions and of proand antioxidant markers in patients with AMD. The studies conducted revealed the pathogenic role of SOD in development of AMD and the need of including Recsod® drug in complex treatment of AMD patients to prevent the disease progression.
Previously we revealed low blood SOD and catalase levels in immature infant which suggested the immaturity of enzymatic antioxidant. The cause of retinopathies in immature infant leading to total blindness is the uterine fetus hypoxia. This group of immature infant represents the retinopathies risk group. That particular group of infant should timely receive antioxidant drugs, in particular Recsod®, as well as careful oxygen therapy [33] .
The same changes in the blood SOD activities were observed in EP and this coincided with literature data [35] . There is evidence that 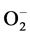 may play an important role in seizure-induced brain damage (16 - 19) and that SOD1 activity was significantly lower in PE with myoclonic epilepsy than that in healthy controls [34] . The study of Dan Chen et al. (2012) demonstrated that the SOD1 activity was reduced in the cerebral spinal fluid (CSF) of EP compared to that in healthy controls, and the reduced CSF SOD1 is more obvious in the subgroup of drug-resistant epilepsy. The authors suggest that a decrease of CSF-SOD1 level may be a marker for intractable epilepsy [35] .
may play an important role in seizure-induced brain damage (16 - 19) and that SOD1 activity was significantly lower in PE with myoclonic epilepsy than that in healthy controls [34] . The study of Dan Chen et al. (2012) demonstrated that the SOD1 activity was reduced in the cerebral spinal fluid (CSF) of EP compared to that in healthy controls, and the reduced CSF SOD1 is more obvious in the subgroup of drug-resistant epilepsy. The authors suggest that a decrease of CSF-SOD1 level may be a marker for intractable epilepsy [35] .
A decrease in SOD levels found in EP indicated that there is reduced cell oxidant protection, including neurons. Use of Recsod® in patients with epilepsy resulted not only in improvement of patients’ well-being, but also in increased AOS activities, suggesting that OS plays a pathogenic role in epilepsy.
Thus, in case of diseases accompanied by OS, the activities of AOS components, especially of antioxidant enzymes, including SOD, should be examined to include antioxidants (Recsod®) in complex therapy. It is our opinion that the mechanism of Recsod® drug protection is related to its ability of effectively removing reactive oxygen species (superoxide radical) and reducing the intensity of free-radical oxidation in peripheral blood of AMD and epileptic patients.
5. Conclusions
1) An increase in the intencity of free-radical oxidation and a reduction in the antioxidant system components were found in AMD and epileptic patients, which is typical for oxidative stress.
2) Complex therapy of AMD patients with enzymatic and nonenzymatic antioxidants permits the reduction of oxidative stress severity and stabilization of visual functions, which results in a significant improvement of health-related quality of life.
3) Use of Recsod® drug as add-on therapy in PE resulted in patients’ clinical response and in reduction of the oxidative stress severity, suggesting that enzymatic protection is of pathogenic significance at epilepsy.
References
- Gutteridge, J.M.C. and Halliwell, B. (2000) Free Radicals and Antioxidants in the Year 2000. Annals of the New York Academy of Sciences, 899, 136-147. http://dx.doi.org/10.1111/j.1749-6632.2000.tb06182.x
- Menszikova, E.B., Lankin, V.Z., Zenkov, N.K., Bondar, I.A. and Krugovich, N.F. (2006) Oxidative Stress. Prooxidants and Antioxidants.
- Halliwell, B. (1992) Reactive Oxygen Species and the Central Nervous System. In: Packer, L., Philipko, L. and Christen, Y., Eds., Free Radical in the Brain. Aging, Neurological and Mental Disorders. Springer-Verlag, Berlin, 21-40.
- Dubinina, E.E. (2006) Oxygen Metabolism Products in Cell’s Functional Activity (Life and Death, Creation and Destruction).
- Goss-Sampson, M., Vivian, A.J. and Kelly, T.J. (1995) Freeradicals, Inflammationand Eye Diseases. In: Blake, D., Ed., Immunopharmacology of Free Radical Speacies, Academic Press Limited, London, 127-143.
- Mulroy, L., McGarvey, D.J., Truscott, T.G., Boulton, M. and Davies, S. (1998) Age-Related Macular Degeneration: Understanding the Roles of Lipofuscin, Macular Carotenoid Pigments and Reactive Oxygen Species. Investigative Ophthalmology & Visual Science, 39, S129.
- Chan, D. (1998) Cigarette Smoking and Age-Related Macular Degeneration. Investigative Ophthalmology & Visual Science (United States), 75, 476-484.
- Halliwell, B. (1992) Reactive Oxygen Species and the Central Nervous System. Journal of Neurochemistry, 59, 1609- 1623. http://dx.doi.org/10.1111/j.1471-4159.1992.tb10990.x
- Boldirev, A.A. (2003) The Role of Active Forms of Oxygen in the Life of the Neuron. The Success of Physiol. Sciences, 34, 21-34.
- Dubinina, E.E. and Pustigina, A.V. (2007) Free Radicals Process during Aging, Neurodegenerative Diseases and Other Pathological Conditions. Biomedicalchemistry, 53, 351-372.
- Youdim, M.B.H. (1988) Brainiron. Neurochemical and Behavioural Aspects. Taylor and Francis, New York, 148 p.
- Levadnaya, O.V., Dontchenko, G.V., Valutsina, V.M., et al. (1998) The Ratio between Values of Activity of the Antioxidant System in Various Tissues of Intact Animals. Ukrainian Biochemical Journal, 70, 53-58.
- Strain, J.J. and Mulholland, C.W. (1992) Vitamin C and Vitamin E—Synergistic Interactions in Vivo. In: Ement, I. and Chance, B., Eds., Free Radical and Aging, Birkhauser, Verlag, Basel, 419-422.
- Barry, S. Winkler, M.E., Boulton, J.D., Gottsch, P.S. and Snodderly, D.M. (1999) Oxidative Damageand Age-Related Macular Degeneration. Molecular Vision, 5, 32-45.
- Beatty, S., Koh, H., Phil, M., Henson, D. and Boulton, M. (2000) The Role of Oxidative Stress in the Pathogenesis of Age-Related Macular Degeneration. Survey of Ophthalmology, 45, 115-134. http://dx.doi.org/10.1016/S0039-6257(00)00140-5
- Aguiar, C.C., Almeida, A.B., Araújo, P.V., de Abreu, R.N., Chaves, E.M., do Vale, O.C., Macêdo, D.S., Woods, D.J., Fonteles, M.M. and Vasconcelos, S.M. (2012) Oxidative Stress and Epilepsy: Literature Review. Oxidative Medicine and Cellular Longevity, 2012, 7952-7959.
- Freitas, R. (2009) Investigation of Oxidative Stress Involvement in Hippocampus in Epilepsy Model Induced by Pilocarpine. Neuroscience Letters, 462, 225-229. http://dx.doi.org/10.1016/j.neulet.2009.07.037
- Rumiàa, J., Marmolb, F., Sanchezb, J., Giménez-Crouseillesc, J., Carrenod, M., Bargallód, N., Bogetd, T., Pintord, L., Setoaind, X., Donaired, A., Saeze, G.T., Ribaltaf, T., Ferrera, E. and Puig-Parelladaba, P. (2013) Oxidative Stress Markers in the Neocortex of Drug-Resistant Epilepsy Patient Submitted to Epilepsy Surgery. Epilepsy Research, 107, 75- 81. http://dx.doi.org/10.1016/j.eplepsyres.2013.08.020
- Chang, S.J. and Yu, B.C. (2010) Mitochondrial Matters of the Brain: Mitochondrial Dysfunction and Oxidative Status in Epilepsy. Journal of Bioenergetics and Biomembranes, 42, 457-459. http://dx.doi.org/10.1007/s10863-010-9317-4
- Fasmon, C.S., Cole, P.J., Williams, A.I. and Hastings, M. (1980) The Measurement of Opsonic and Phagocytic Function by Luminol-Depends Chemiluminescence. Immunology, 41, 67-74.
- Gavrilov, V.B., Gavrilov, A.R. and Mazhul, L.M. (1987) Analysis of Methods of Determining Products of Lipid Peroxidation in Serum Test with Thiobarbituric Acid. The Issues of Medical Chemistry, 33, 118-122.
- Kum-Tatt, L. and It-Koon, T. (1974) А New Colorimrtric Method for the Determination of Glutation in Erythrocytes. Clinica Chimica Acta, 53, 153-161.
- Ellman, G.L. (1959) Tissue Sulfhydryl Groups. Archives of Biochemistry and Biophysics, 82, 70-71.
- Kostyuk, V.A., Potapovitch, A.I. and Kovaleva, Z.V. (1990) Simple and Sensitive Method for Determining the Activity of Superoxide Dismutase, Based on the Oxidation Reaction of Quercetin. The Issues of Medical Chemistry, 36, 88-91.
- Korolyuk, M.A., Ivanova, L.I., Mayorova, I.G. and Tokareva, V.E. (1988) Method for Determining the Activity of Catalase. LBA. Thematter, 1, 16-19.
- Dubinina, E.E., Burmistrov, S.O., Khodov, D.A. and Porotov, I.E. (1995) Oxidative Modification of Proteins in Human Serum. Methods of Its Determination. The Issues of Medical Chemistry, 41, 24-26.
- Prashar, S., Pandav, S.S., Gupta, A. and Nath, R. (1993) Antioxidant Enzymes in RBCs Biological Index of Age Related Macular Degeneration. Acta Ophthalmologica (Copenh), 71, 214-218. http://dx.doi.org/10.1111/j.1755-3768.1993.tb04993.x
- Cohen, S.M., Olin, K.L., Feuer, W.J., Hjelmeland, L., Keen, C.L. and Morse, L.S. (1994) Low Glutation Reductase and Peroxidase Activity in Age-Related Macular Degeneration. British Journal of Ophthalmology, 78, 791-774. http://dx.doi.org/10.1136/bjo.78.10.791
- Delcourt, C., Cristol, J.P., Tessier, F., Léger, C.L., Descomps, B. and Papoz, L. (1999) Age-Related Macular Degeneration and Antioxidant Status in the POLA Study. Archives of Ophthalmology, 117, 1384-1390. http://dx.doi.org/10.1001/archopht.117.10.1384
- Mayansky, A.N. and Pikuza, O.N. (1993) Clinical Aspects of Phagocytosis. Magarif, Kazan, 76-78.
- Alekseev, V.N. and Usachev, V.V. (2003) Study of the Efficacy of the Drug Rexod in Patients with Primary Open-Angle Glaucoma. Saturday Papers Dedicated to the 90th Anniversary of Department, State Medical Academy of Them, Mechnikov, Glaucoma and Other Eye Diseases, SPb, 97-106.
- Stefanov, A.V., Derimedved, L.V., Churilova, I.V., Drogovoz, S.M., Kutsenko, T.A. and Shchekina, E.G. (2004) Clinical and Experimental Substantiation of Application of Superoxide Dismutase in Medicine. Publishing House of Pau, Golden, p. 288.
- Dubinina, E.E., Sofronova, L.N., Litvinenko, L.A. and Muschkatina, O.F. (1991) Diagnostic Value of Determines the State of the Antioxidant System in Retinopathy of Prematurity. Issues of Protection of Motherhood and Childhood, 36, 30-34.
- Ben-Menachem, E., Kyllerman, M. and Marklund, S. (2000) Superoxide Dismutase and Glutathione Peroxidase Function in Progressive Myoclonus Epilepsies. Epilepsy Research, 40, 33-39. http://dx.doi.org/10.1016/S0920-1211(00)00096-6
- Chen, D., Lu, Y., Yu, W., Luo, J., Xiao, Z., Xiao, F. and Wang, X. (2012) Clinical Value of Decreased Superoxide Dismutase 1 in Patients with Epilepsy. Seizure: European Journal of Epilepsy, 21, 508-511. http://dx.doi.org/10.1016/j.seizure.2012.05.003
NOTES

*Corresponding author.


