Open Journal of Radiology
Vol.05 No.02(2015), Article ID:56898,7 pages
10.4236/ojrad.2015.52012
Effectiveness and Safety of Splenic Microwave Ablation Combined with Hepatic Arterial Chemoembolization for Hepatocellular Carcinoma Associated with Hypersplenism: A Prospective Study in 5 Patients
Guobao Wang1, Fujun Zhang2, Sheng Peng2, Tao Zhang2, Mingjian Lu2, Yangkui Gu2, Jinhua Huang2, Fei Gao2*
1Department of Endoscopy and Laser, Sun Yat-Sen University Cancer Center and State Key Laboratory of Oncology in South China, Guangzhou, China
2Department of Interventional Radiology, Sun Yat-Sen University Cancer Center and State Key Laboratory of Oncology in South China, Guangzhou, China
Email: *gaof@sysucc.org.cn
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 1 April 2015; accepted 1 June 2015; published 4 June 2015
ABSTRACT
The aim was to prospectively evaluate the efficacy and safety of splenic microwave ablation (MWA) combined with transcatheter hepatic arterial chemoembolization (TACE) in treatment of hepatocellular carcinoma (HCC) associated with hypersplenism. Five patients suffering from primary HCC associated with hypersplenism caused by cirrhosis were received MWA combined with TACE. Follow-up examinations included calculation of peripheral blood cells (leukocytes, platelets and red blood cells) and treatment-associated complications. After treatment, leukocyte and platelet counts were significantly higher (P < 0.05) than prior-treatment. Right upper quadrant pain occurred in 3 patients, fever occurred in 4 patients, and mild nausea & vomiting occurred in 3 patients. Complications such as pleural effusion, ascites, bacterial peritonitis, and variceal bleeding did not occur after treatment. So, MWA combined with TACE is effective and safe for the patients with HCC associated with hypersplenism caused by cirrhosis.
Keywords:
Efficacy, Safety, MWA, TACE, HCC, Hypersplenism

1. Introduction
Transcatheter hepatic arterial chemoembolization (TACE) has become the first choice of treatment for unresectable hepatocellular carcinoma (HCC) [1] [2] . However, 70% - 90% of HCC patients are associated with liver cirrhosis, portal hypertension and hypersplenism. The treatment of HCC is usually affected by low peripheral blood cell counts and high incidence of hemorrhagic complications due to treatment and/or portal hypertension [3] [4] . Moreover, chemotherapeutics during TACE is another cause for low peripheral blood cell counts because of myelosuppression. Our preliminary clinical study has revealed that TACE combined with partial splenic embolization (PSE) appears to have a favorable effect on the patients of unresectable HCC with hypersplenism since PSE has been widely used in the treatment of leukocytopenia and thrombocytopenia caused by splenomegaly [5] -[7] . Nevertheless, it is difficult to accurately evaluate embolism volume of PSE in real time and sometimes it carries a risk of severe complications which are related to excessive embolism volume [5] [8] . So, thermal ablation methods such as microwave ablation (MWA), and radiofrequency ablation (RFA) were developed rapidly as minimally invasive techniques for hypersplenism [9] [10] . With thermal ablation, destruction volume of the spleen can be easily controlled [11] . This study was to prospectively evaluate the efficacy and safety of MWA for the treatment of unresectable HCC with hypersplenism.
2. Materials and Methods
2.1. Patients
From December 2010 to May 2012, 5 consecutive patients with HCC associated with hypersplenism caused by liver cirrhosis and portal hypertension were enrolled in this study. The diagnosis of HCC was established on the basis of clinical laboratory data, computed tomography (CT) and biopsy. The diagnosis of hypersplenism and splenomegaly was made in the light of clinical laboratory data and CT. The enrolling criteria for this study were patients with splenomegaly and thrombocytopenia (platelet count ≤ 60 × 109/L) and/or leukocytopenia (leukocyte count ≤ 3.0 × 109/L). All the 5 patients received MWA in combination with TACE. The patients’ sex, age, Child-Pugh grade, tumor diameter, mass pathology type and peripheral blood cell counts were summarized in Table 1.
2.2. Methods
2.2.1. Percutaneous MWA
Specifications of intravenous anesthesia were administered using propofol via the peripheral vein. The spleen was accessed percutaneously via the left subcostal approach under CT guidance. An applicator (water-cooled antenna: diameter 1.7 mm, model Ⅲwith high-power, made by Qi Ya Medical Treatment Facility Limited Company, Nanjing, China) was placed in the lower part of the spleen. The applicator was activated at 60 - 100 W for 5 - 10 minutes according to the results of a previous in vivo study [11] [12] . When withdrawing the applicator, another MWA emitting for 1 - 3 minutes was performed to prevent bleeding from the needle track. After the emission, the applicator was pulled back for about 2 - 3 cm more than 3 cm from the splenic surface. Two to three overlapping areas from the middle to inferior part of the spleen were ablated with one insertion of applicator. The emission facility was produced by Qi Ya Medical Treatment Facility Limited Company, Nanjing, China (Frequency: 2450 MHz; Output power: 0 - 120 W; Precision of temperature control: ±0.1˚C; Discharge waveform: continuous wave).
2.2.2. TACE
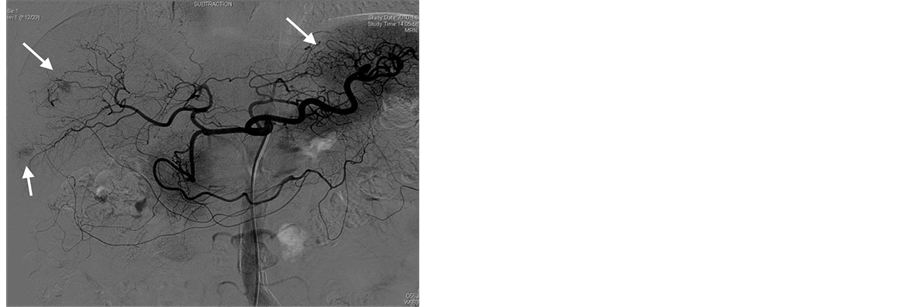
After splenic MWA, a 5.0 French catheter (Terumo, Tokyo, Japan) was inserted into the femoral artery with the Seldinger method after local anesthethia, celiac angiography and selective hepatic arterial angiography were routinely performed to observe the tumor blood-supply, distribution of hepatic arteries and collateral circulation routes (Figure 1(a)), the tip of the catheter was placed at the feeding artery of the tumor, and embolization was performed using an emulsion mixture of lipoidal ultra-fluid (Guerbet, France), perarubicin (50 mg/m2) and DDP (80 mg/m2). The maximum dose for embolization was based on the size of the tumor, blood supply and hepatic function of the patient. When the tumor was filled well with emulsifier, the embolization was terminated (Fig- ure 1(b)).
Table 1. Demographic, clinical, histological and laboratory characteristics of patients.
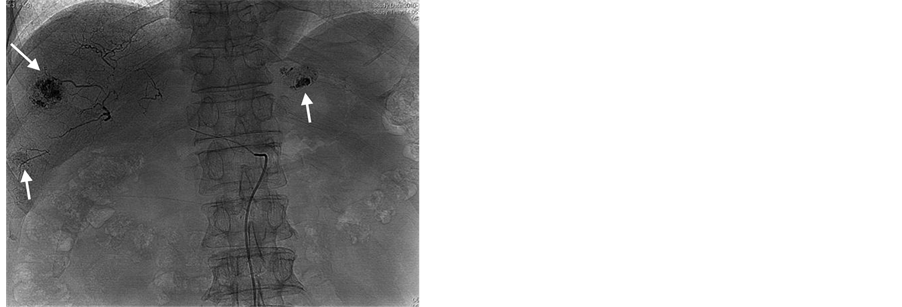
Figure 1. TACE treatment for a 63-year-old male case of HCC with splenomegaly and thrombocytopenia. (a) Celiac arteriography before TACE showing the tumor blood-supply image, as indicated by the arrowhead; (b) TACE is terminated when the three tumors were filled with emulsifier, as indicated by the arrowhead.
2.3. Follow-Up Protocol
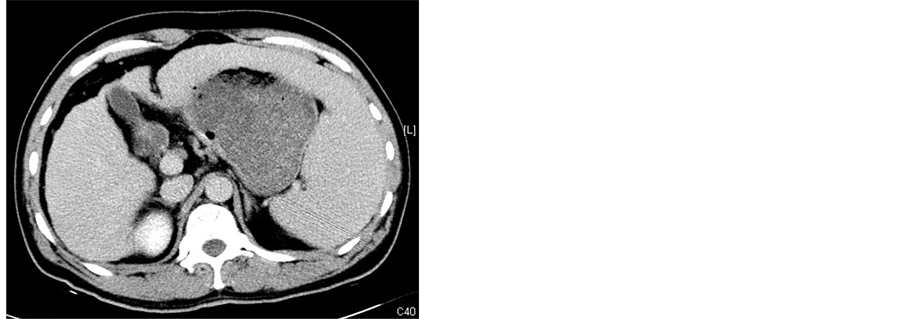
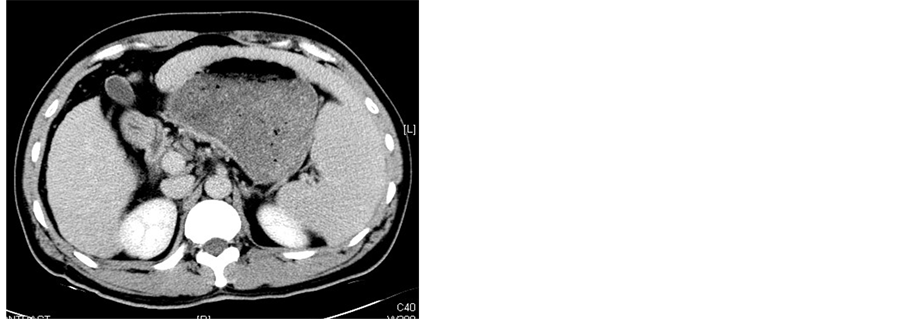
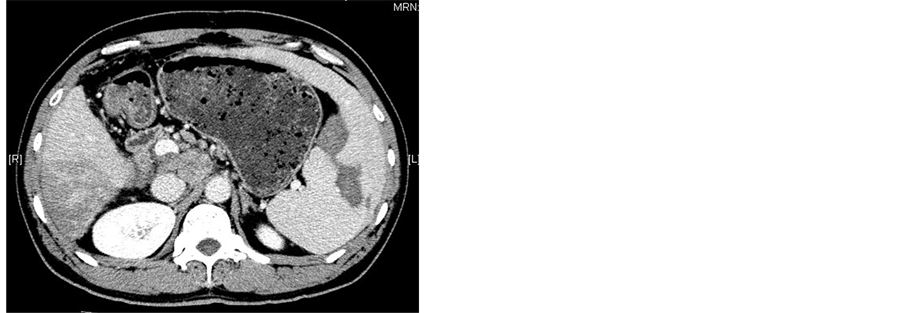
All patients underwent abdominal CT scanning (GE Medical Systems) 1 wk before procedure and 2 wk after procedure to evaluate the necrosis of the spleen (Figure 2). After treatment with MWA combineed with TACE, all patients remained in hospital with their adverse effects or complications observed and were then followed up at outpatient clinic. Peripheral blood cell parameters including white blood cells (WBC), platelets (PLT) and red blood cells (RBC) were obtained 1 wk before procedure and 1 wk, 2 wk, 1 mo, 2 mo, 3 mo after procedure.
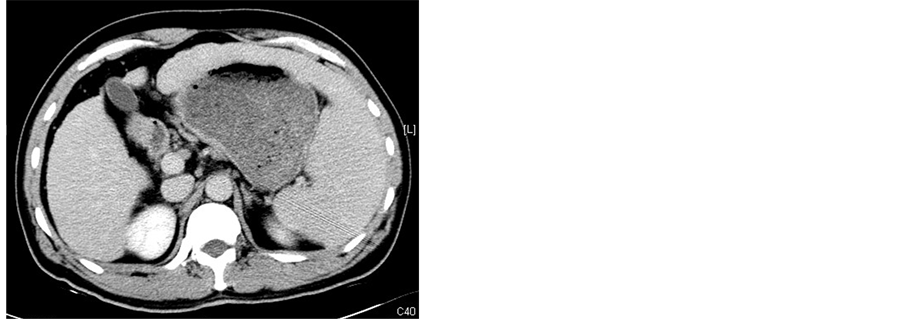
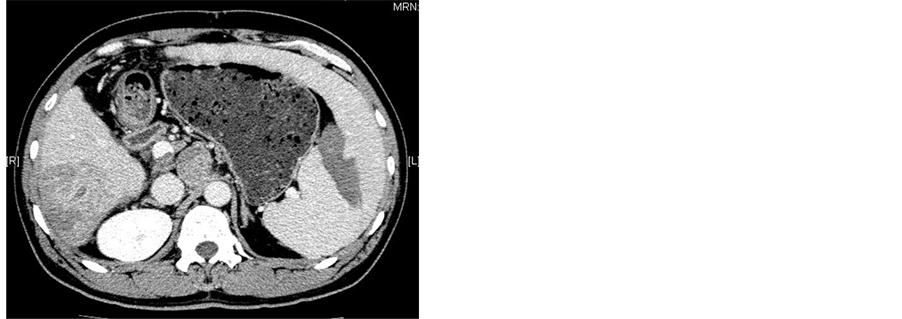
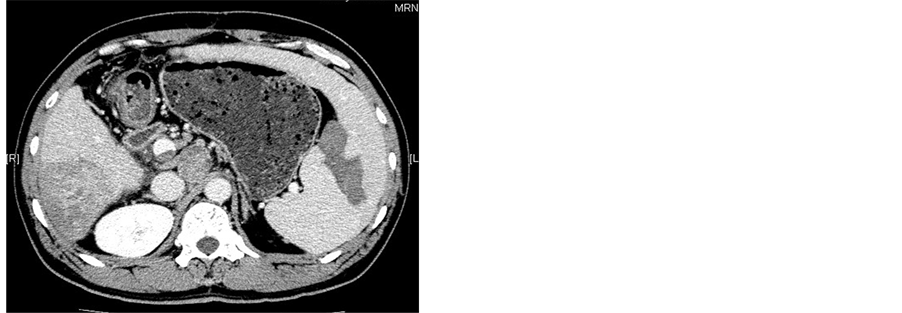
Figure 2. (a)-(c) Contrast-enhanced CT scans of the spleen before splenic MWA; (d)-(f) Two weeks after MWA. Contrast-enhanced CT scans showed unenhanced low attenuation ablated area in inferior part of spleen. The ablation ratio was about 20%.
2.4. Statistical Analysis
All data were analyzed using the SPSS 17.0 software. The paired t-test was used to determine the difference before and after MWA/TACE treatment. Significance was established at P < 0.05.
3. Results
3.1. Chronological Changes in Peripheral Blood Cell Counts
The peripheral blood cell counts before MWA/TACE treatment and from the third day to the forth week after MWA/TACE treatment are listed in Tables 2-4. There were significant differences in WBC and PLT counts before and after treatment (P < 0.01, Table 2 and Table 3). WBC and PLT counts were significantly higher from the first week to the third month after treatment. However, there were no significant differences in RBC counts counts before and after treatment (P > 0.05, Table 4).
3.2. Adverse Effects or Complications
Symptoms of post-embolization syndrome, including right upper quadrant pain, fever and mild nausea and vomiting, occurred in our patients. Right upper quadrant pain was found in 3 patients and was alleviated by durogesic or tramadol. Fever was found in 4 patients and was lowered by salicylic acid drugs. Three patients had mild nausea and vomiting and were alleviated by antanacathartic. Complications such as pleural effusion, ascites, bacterial peritonitis and variceal bleeding did not occur after treatment (Table 5).
Table 2. Follow-up results of WBC counts (×109/L).
Comparison of WBC counts before and after treatment at different time points determined with t-test.
Table 3. Follow-up results of PLT counts (×109/L).
Comparison of PLT counts before and after treatment at different time points determined with t-test.
Table 4. Follow-up results of RBC counts (×1012/L).
Comparison of PLT counts before and after treatment at different time points determined with t-test.
Table 5. Adverse effects or complications observed in 5 patients 2 wk after treatment.
4. Discussion
It is difficult to perform TACE in the cases of HCC associated with hypersplenism because of the high incidence of hemorrhagic complications and/or portal hypertension, as well as poor tolerance of cirrhotic patients to chemotherapeutic drugs [13] . Since PSE is proved as a useful support therapy in reducing episodes of variceal bleeding, improving hematologic parameters, enhancing hepatic protein synthesis, and reducing the severity of hepatic encephalopathy [14] -[16] , combined PSE/TACE treatment can improve the tolerance of HCC patients with advanced/decompensated cirrhosis and hypersplenism to chemotherapeutic drugs. It can also reduce the risk of complications of invasive radiologic procedures and/or portal hypertension [5] . However, it’s hard to accurately evaluate embolism volume of PSE in real time and sometimes it carries a risk of severe complications such as pleural effusion, rupture of spleen and portal vein thrombosis in the cases with excessive embolism volume [5] [8] . So, minimally invasive techniques of thermal ablation methods such as MWA, RFA were developed rapidly for hypersplenism and the destruction volume of the spleen could be easily controlled [9] -[11] . Furthermore, coagulative necrosis, not liquefaction necrosis, occurs in the spleen after thermal ablation and the probability of splenic abscess are obviously decreased. The recurrence rate of hypersplenism is obviously decreased because the heat-sink effect can damage the vascular endothelium and cause thrombogenesis, which can lead to spleen consolidation, interstitial fibrosis and decreased splenic recirculation. Compared with RFA, MWA has the added advantages of a higher rise in temperature, a higher thermal efficiency, more stable and controllable thermal field, better hemostatic effect, and more effective blood vessel coagulation [17] [18] .
Some reports have also shown that MWA improves the peripheral blood conditions [12] [19] . In our study, thrombocytes and leucocytes increased markedly and the increase of PLT count was particularly remarkable. An increase in PLT count probably reflected a decreased sequestration in the ablated spleen. Comparing the changes in WBC count and PLT count, the abrupt increase in WBC count may be regarded as an acute stress response, rather than a decrease in the splenic capture rate. Increased WBC counts may be due to decreased splenic pooling of WBCs, as well as the reaction of hepatic emblization. In this study, we tried to ablate about 30% - 40% of the splenic volume in every case and got satisfied results for the increasing of thrombocytes and leucocytes. Although several studies revealed that the larger the ablation ratio was, the higher the PLT count was comparable to that of partial splenic embolization and radiofrequency [20] [21] , further research should investigate the specific relationship between the destructive proportion of the spleen and the improvement of the peripheral blood conditions.
Severe complications such as pleural effusion, ascites, bacterial peritonitis and variceal bleeding did not occur in this study. According to our experience, appropriate ablation volume in one session and repeated ablation could effectively alleviate the complications which were related to the ablated volume and larger ablated volumes were associated with more severe complications. In this study, about 15% - 20% of splenic MWA was ablated in one session, and repeated MWA was performed until over 30% - 40% of the splenic volume was ablated. Our prior study in porcine spleen in vivo showed that the MWA volume increased with increased ablation time, from 1 to 10 minutes when the power was set at 60 W. Also, the MWA volume in the spleen increased with the increase of ablation power, ranging from 30 to 100 W when the ablation time was set to 3 min. So, by adjusting ablation time and power, MWA can accurately control the destruction volume of the spleen in cases of splenomegalia and hypersplenism and effectively overcome the limitations in existing treatments, such as a reduced therapeutic effect caused by a smaller destruction volume and more complications associated with excess destruction volume [11] . Adverse effects in this study such as fever, pain, mild nausea and vomiting after treatment in some of the patients were mainly due to TACE as reactions of hepatic embolization.
Although our study was limited by a small sample size, it revealed that the combined MWA/PSE treatment improved the tolerance of HCC patients with advanced cirrhosis and hypersplenism to chemotherapeutic drugs and reduced the risk of complications of PSE. As a new and promising minimally invasive alternative for treating hypersplenism, MWA may have advantages over PSE, including a relatively simple procedure, control of the ablation volume, milder complications, shorter recovery time, repeatability and fewer complications. However, a large cohort of patients and a prolonged observation period are required to evaluate its long-term therapeutic effects.
References
- Llovet, J.M., Burroughs, A. and Bruix, J. (2003) Hepatocellular Carcinoma. The Lancet, 362, 1907-1917. http://dx.doi.org/10.1016/S0140-6736(03)14964-1
- Bruix, J., Sala, M. and Llovet, J.M. (2004) Chemoembolization for Hepatocellular Carcinoma. Gastroenterology, 127, S179-S188. http://dx.doi.org/10.1053/j.gastro.2004.09.032
- Han, M.J., Zhao, H.G., Ren, K., et al. (1997) Partial Splenic Embolization for Hypersplenism Concomitant with or after Arterial Embolization of Hepatocellular Carcinoma in 30 Patients. CardioVascular and Interventional Radiology, 20, 125-127. http://dx.doi.org/10.1007/s002709900119
- Ohmoto, K. and Yamamoto, S. (2003) Prevention of Variceal Recurrence, Bleeding, and Death in Cirrhosis Patients with Hypersplenism, Especially Those with Severe Thrombocytopenia. Hepatogastroenterology, 50, 1766-1769.
- Huang, J.H., Gao, F., Gu, Y.K., et al. (2007) Combined Treatment of Hepatocellular Carcinoma with Partial Splenic Embolization and Transcatheter Hepatic Arterial Chemoembolization. World Journal of Gastroenterology, 13, 6593- 6597. http://dx.doi.org/10.3748/wjg.13.6593
- Tajiri, T., Onda, M., Yoshida, H., et al. (2002) Long-Term Hematological and Biochemical Effects of Partial Splenic Embolization in Hepatic Cirrhosis. Hepatogastroenterology, 49, 1445-1448.
- Kimura, F., Ito, H., Shimizu, H., Togawa, A., et al. (2003) Partial Splenic Embolization for the Treatment of Hereditary Spherocytosis. American Journal of Roentgenology, 181, 1021-1024. http://dx.doi.org/10.2214/ajr.181.4.1811021
- Sakata, K., Hirai, K. and Tanikawa, K. (1996) A Long-Term Investigation of Transcatheter Splenic Arterial Embolization for Hypersplenism. Hepatogastroenterology, 43, 309-318.
- Matsuoka, T., Yamamoto, A., Okuma, T., et al. (2007) CT-Guided Percutaneous Radiofrequency Ablation of Spleen: A Preliminary Study. American Journal of Roentgenology, 188, 1044-1046. http://dx.doi.org/10.2214/AJR.06.0641
- Liang, P., Gao, Y., Zhang, H., et al. (2011) Microwave Ablation in the Spleen for Treatment of Secondary Hypersplenism: A Preliminary Study. American Journal of Roentgenology, 196, 692-696. http://dx.doi.org/10.2214/AJR.10.4193
- Gao, F., Gu, Y.K., Shen, J.X., et al. (2011) Experimental Study of Destruction to Porcine Spleen in Vivo by Microwave Ablation. World Journal of Gastroenterology, 17, 5014-5020. http://dx.doi.org/10.3748/wjg.v17.i45.5014
- Gao, Y., Wang, Y., Duan, Y., et al. (2010) 915MHz Microwave Ablation with High Output Power in in Vivo Porcine Spleens. European Journal of Radiology, 75, 87-90. http://dx.doi.org/10.1016/j.ejrad.2009.03.009
- Roversi, R., Ricci, S., Gambari, P.I., et al. (1993) [Splenic Embolization and Hepatic Chemoembolization: Combined Transcatheter Treatment of Hepatocellular Carcinoma in Cirrhosis with Hypersplenism]. La Radiologia Medica, 85, 444-449.
- Koconis, K.G., Singh, H. and Soares, G. (2007) Partial Splenic Embolization in the Treatment of Patients with Portal Hypertension: A Review of the English Language Literature. Journal of Vascular and Interventional Radiology, 18, 463-481. http://dx.doi.org/10.1016/j.jvir.2006.12.734
- Shimizu, T., Onda, M., Tajiri, T., et al. (2002) Bleeding Portal-Hypertensive Gastropathy Managed Successfully by Partial Splenic Embolization. Hepatogastroenterology, 49, 947-949.
- Romano, M., Giojelli, A., Capuano, G., et al. (2004) Partial Splenic Embolization in Patients with Idiopathic Portal Hypertension. European Journal of Radiology, 49, 268-273. http://dx.doi.org/10.1016/S0720-048X(03)00134-7
- Brace, C.L. (2009) Radiofrequency and Microwave Ablation of the Liver, lung, Kidney, and Bone: What Are the Differences? Current Problems in Diagnostic Radiology, 38, 135-143. http://dx.doi.org/10.1067/j.cpradiol.2007.10.001
- Brace, C.L. (2009) Microwave Ablation Technology: What Every User Should Know. Current Problems in Diagnostic Radiology, 38, 61-67. http://dx.doi.org/10.1067/j.cpradiol.2007.08.011
- Duan, Y.Q., Gao, Y.Y., Ni, X.X., et al. (2007) Changes in Peripheral Lymphocyte Subsets in Patients after Partial Microwave Ablation of the Spleen for Secondary Splenomegaly and Hypersplenism: A Preliminary Study. International Journal of Hyperthermia, 23, 467-472. http://dx.doi.org/10.1080/02656730701474533
- Wang, H.Y., Shih, S.C., Lin, S.C., et al. (2008) Partial Splenic Embolization: 12-Month Hematological Effects and Complications. Hepatogastroenterology, 55, 1838-1842.
- Liu, Q., Ma, K., He, Z., et al. (2005) Radiofrequency Ablation for Hypersplenism in Patients with Liver Cirrhosis: A Pilot Study. Journal of Gastrointestinal Surgery, 9, 648-657. http://dx.doi.org/10.1016/j.gassur.2004.11.006
NOTES

*Corresponding author.