Journal of Power and Energy Engineering
Vol.03 No.08(2015), Article ID:58546,3 pages
10.4236/jpee.2015.38003
Feasibility Study of Melon Seed Oil as a Source of Biodiesel
Kennedy Izuchukwu Ogunwa1, Samuel Ofodile2, Ozioma Achugasim2*
1World Bank African Centre of Excellence in Oilfield Chemicals, Institute Of Petroleum Studies, University of Port Harcourt, Port Harcourt, Nigeria
2Department of Pure and Industrial Chemistry, University of Port Harcourt, Port Harcourt, Nigeria
Email: *ozioma.achugasim@uniport.edu.ng
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 26 May 2015; accepted 1 August 2015; published 4 August 2015
ABSTRACT
Melon seed oils were extracted at a very high yield of 52.2%. The extracted oil was subjected to oil quality tests and subsequently transesterified to give fatty acid methyl esters or biodiesel. The biodiesel was also subjected to fuel quality tests. The results showed that the extracted oil had specific gravity of 0.91 and moisture content of 0.90% indicating that the oil is a very good energy source, a good candidate for transesterification and will not be easily susceptible to microbial attack and autooxidation. The fuel quality parameters of the produced biodiesel showed that it conforms to standards for biodiesel and compares well with a standard petrodiesel.
Keywords:
Biodiesel, Melon, Petrodiesel, Transesterification

1. Introduction
The need for alternatives to fossil fuel sources has become more obvious today than ever and the reasons are not far-fetched. They include increasing cost of fossil fuels, their unrenewable or closed carbon cycle nature, emissions of combustion-generated pollutants, etc [1] . The alternatives are found in biofuels. Among the different biofuels, biodiesel seems to be the most studied and the most promising competitor to the currently used petrodiesel.
Biodiesel has been described as a fuel composed of monoalkyl esters of long-chain fatty acids derived from renewable vegetable oils and fats [2] . Biodiesel production apart from providing a good alternative to petrodiesel, will encourage the market for increased production of vegetable oils and animal fats, reduce global warming and improve the lubricating properties of diesel engines [3] [4] .
Chemically, biodiesels are produced from the transesterification of triglycerides found in vegetables oils and fats to form the monoalkyl esters which are the primary molecules of biodiesel [5] [6] .
The formation of the methyl esters (ME) involves a step-wise reaction with intermediate formation of diglycerides (DG) and monoglycerides (MG) from the starting triglyceride (TG) with the production of glycerol (GL) in the final step.
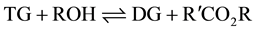 (1)
(1)
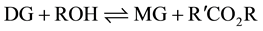 (2)
(2)
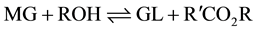 (3)
(3)
The overall reaction is therefore given as:
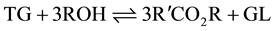
Of course the reaction is catalyzed by either a base or an acid [7] -[9] .
The hydrocarbon moiety of the methyl esters are of comparable carbon number with that of the petrodiesel. Also the cetane number, energy content, viscosity and phase changes of biodiesel are akin to that of petrodiesels [10] .
The vegetable oils that have been utilized as sources of biodiesel include those from rape seed, cotton seed, soybean seed, fluted pumpkin seed, sunflower seed, palm kernel seed and fruit, corn, linseed etc. [11] [12] .
Melon seed (Citrullus lanatus. thumb. mausf) oil provides another useful source of biodiesel production. The melon seed (Citrullus lanatus. thumb. mausf) popularly known as egusi in Nigeria is from the family cucurbifaceae and mainly cultivated as a soup thickener and snack. The seed is rich in oil, low in cholesterol and contains essential and unsaturated fatty acids [13] [14] .
The Local utilization of this seed in biodiesel production will no doubt improve its production/market, generate employment and may earn Nigeria the much needed foreign exchange.
2. Materials and Method
2.1. Oils Extraction
The melon seed samples used for this study were obtained from Ariaria market, Aba in Abia State, Nigeria. The samples were air-dried, weighed, ground and extracted in a soxhlet extractor using hexane as the solvent. The extracted oil was subsequently analyzed for some oil quality parameters as shown in Table 1.
2.2. Biodiesel Production
50 ml of methanol was added to 3.0 g of potassium hydroxide KOH in a 100 ml conical flask. The mixture was stirred with gentle heating. The KOH/methanol mixture was subsequently added to 150 ml of the melon seed oil in 1000 ml conical flask and stirred gently for more than 10 hrs. The entire mixture was then transferred to a separatory funnel and allowed to stand overnight to give two distinct layers of lower glycerol and upper methyl esters (biodiesel). The separated biodiesel was subsequently washed severally with water and subjected to fuel
Table 1. Oil quality parameter of oil extracted from melon seed.
quality tests to determine its suitability or otherwise, as an alternative to petro-diesel. The results are presented in Table 2.
3. Result and Discussion
The result of the oil quality parameters of the extracted melon seed oil is presented in Table 1.
The result of the oil quality parameters shows that melon seed oil could serve as an economic feedstock for biodiesel production given the percentage oil yield of 52.2. The iodine value is not too high showing that biodiesel obtained from melon seed oil may not be too susceptible to oxidation and quick rancidity.
Given the threshold acid value of 1.0 mg KOH/g for fresh oils, it is obvious that melon seed oil (acid value 4.18) will have catalysis problem during esterification (catalyst deactivation) and formation of soaps as side reaction. These problems can be taken care of with the use of heterogeneous catalysts or the addition of bases like sodium hydroxide.
The specific gravity of 0.91 shows that the oil is a good energy source. The very low moisture content of the melon seed oil of 0.90 is an indication that hydrolysis of the produced esters during esterification and its consequent soap formation is not likely to occur if melon seed oil is used in transesterification reaction. The low moisture content will also prevent microbial attack and autoxidation which leads to oil rancidity.
The result of the fuel quality parameters of the melon seed oil methyl esters are shown in Table 2.
It is obvious that transesterification has drastically reduced the moisture content and acid value of melon seed oil giving the produced biodiesel better fuel characteristics. There is also a sharp reduction of the kinematic viscosity of the melon seed oil from 21.65 to about 5.8 mm2/s in the biodiesel, a value that is comparable to that of the petrodiesel.
The suitability of the produced methyl esters as a biodiesel is better appreciated when these parameters are compared with that of a standard petrodiesel (ASTM D975) and a standard biodiesel (ASTM D6751) as shown in Table 3.
The specific gravity of the produced biodiesel compares well with that of the standard biodiesel (D6751) and
Table 2. Fuel quality parameters of methyl esters produced from melon seed oil.
Table 3. Fuel quality parameters of a standard biodiesel, a petrodiesel and melon seed oil methyl esters.
petrodiesel (D975). The flash point is higher than that of a standard biodiesel making it safer for storage and transportation. The acid value and moisture content is highly reduced and the diesel index similar to that of the standard biodiesel and petrodiesel.
4. Conclusions
The high percentage oil yield of melon seed shows that it is a viable oil source. The oil quality parameters show that the oil from melon seed with a moderate degree of unsaturation is not susceptible to oxidative rancidity. The low moisture content makes it a good candidate for transesterification reaction.
The fuel quality parameters of the methyl esters derived from the melon seed oil show that it conforms to standards for biodiesel and compares very well with a standard petrodiesel. So melon seed oil can serve as a good feedstock for biodiesel production.
Acknowledgements
The authors are grateful to Nigerian national Petroleum Cooperation (NNPC) for assistance in carrying out some of the analysis in the work.
Cite this paper
Kennedy IzuchukwuOgunwa,SamuelOfodile,OziomaAchugasim, (2015) Feasibility Study of Melon Seed Oil as a Source of Biodiesel. Journal of Power and Energy Engineering,03,24-27. doi: 10.4236/jpee.2015.38003
References
- 1. Ali, Y. and Hanna, M.A. (1994) Alternative Diesel Fuels from Vegetable Oils. Bioresource Technology, 50, 153-163.
http://dx.doi.org/10.1016/0960-8524(94)90068-X - 2. Canaka, M. and Sanli, H. (2008) Biodiesel Production from Various Feedstocks and their Effects on Fuel Properties. Journal of Industrial Microbiology & Biotechnology, 35, 431-441.
http://dx.doi.org/10.1007/s10295-008-0337-6 - 3. Abayeh, O.J., Ismail, A. and Abayeh, O.M. (2012) Characterization of Pumpkin (Cucurbita Pero) Seed Oil as a Biodiesels Produced through KOH-Catalzed Transesterification Process. Journal of the Chemical Society of Nigeria, 37, 81-86.
- 4. Math, M.C. (2007) Optimization of Restaurant Waste Oil-Methyl Ester Yield. Journal of scientific and Industrial Research, 66, 772-776.
- 5. Romanski, J., Nowak, P., Kosinski, K. and Jurczak, J. (2012) High Pressure Transesterification of Sterically Hindered Esters. Tetrahedron Letters, 53, 5287-5289.
http://dx.doi.org/10.1016/j.tetlet.2012.07.094 - 6. Sidhara, R. and Mathai, I.M. (1974) Transesterification Reaction. Journal of Scientific and Industrial Research, 33, 178-187.
- 7. Damoko, D. and Munir, C. (2000) Kinetics of Palm Oil Transesterification in a Batch Reactor. Journal of American Oil Chemical Society, 77, 1263-1267.
http://dx.doi.org/10.1007/s11746-000-0198-y - 8. Freedom, B., Buterfield, R.O. and Pryde, E.H. (1986) Transesterification Kinetics of Soyabean Oil. Journal of the American Oil Chemists’ Society, 63, 1375-1380.
http://dx.doi.org/10.1007/BF02679606 - 9. Noureddini, H., and Zhn, D. (1997) Kinetics of Transesterification of Soyabean Oil. Journal of the American Oil Chemists’ Society, 74, 1457-1463.
http://dx.doi.org/10.1007/s11746-997-0254-2 - 10. Muniyappa, P.R., Brammer, S.C. and Noureddini, H. (1996) Improved Conversation of Plant Oils and Animal Fat into Biodiesel and Co-Product. Bioresource Technology, 56, 19-24.
http://dx.doi.org/10.1016/0960-8524(95)00178-6 - 11. Eckey, E.W. (1954) Vegetable Fats and Oils. Reinhold, New York, 75-79.
http://dx.doi.org/10.1097/00010694-195407000-00026 - 12. Akintayo, E.T. (1997) Chemical Composition, Physiochemical Properties of Fluted Pumpkin Seed and Seed Oils. Rivista Italiana Sostanze Grasse, 74, 13-16.
- 13. Lazos, E.S. (1986) Nutritional Fatty Acid and Oil Characterization of Pumpkin and Melon Seeds. Journal of Food Science, 51, 1382-1384.
http://dx.doi.org/10.1111/j.1365-2621.1986.tb13133.x - 14. Gushini, G., Wehner, T.C. and Jarret, R.L. (2004) Inheritance of Egusi Seed Type in Water Melon. Journal of Heredity, 94, 268-270.
http://dx.doi.org/10.1093/jhered/esh031
NOTES

*Corresponding author.


