Journal of Materials Science and Chemical Engineering
Vol.02 No.12(2014), Article ID:52340,6 pages
10.4236/msce.2014.212003
Enhanced High-temperature cycling Stability of LiMn2O4 by LiCoO2 Coating as cathode material for Lithium Ion batteries
Jing Yan1, Haohan Liu2,3, Yuelei Wang1, Xinxin Zhao1, Yiming Mi1*, Baojia Xia3
1College of Chemistry and Chemical Engineering, Shanghai University of Engineering Science, Shanghai, China
2Shanghai Nanotechnology Promotion Center, Shanghai, China
3Shanghai Institute of Microsystem and Information Technology, Chinese Academy of Sciences, Shanghai, China
Email: *biluo1873@163.com
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 27 November 2014; revised 10 December 2014; accepted 17 December 2014
ABSTRACT
LiCoO2 surface layer is proposed and prepared through sol-gel method. The physical and electrochemical performances of pristine LiMn2O4 and LiCoO2-coated LiMn2O4 cathode materials were investigated by X-ray diffraction, scanning electron microscopy, transmission electron microscopy, electrochemical measurements respectively. Comparing with the pristine LiMn2O4, the LiCoO2- coated LiMn2O4 phase significantly improved cycling stability, especially at 55˚C. Additionally, the thermal safety of LiMn2O4 is greatly enhanced after being coated by LiCoO2. ICP-AES measurement, structural analysis, and impedance experiments indicate that the improved electrochemical property of LiCoO2-coated LiMn2O4 should be attributed to the alleviated dissolution loss of manganese, strengthened structural stability.
Keywords:
LiMn2O4, Sol-gel Method, Surface Coating, Electrochemical performance

1. Introduction
Lithium ion battery (LIB) has become the most widely used power supply for electronics. Safer electrode material has been pursued by researchers for years [1] . Spinel lithium manganese oxide (LiMn2O4), with the advantages of abundant, nontoxic, and inexpensive, is a promising cathode material for power lithium ion battery mainly [2] . Especially, the good stability may ensure its large-scale usage in the batteries for electric vehicle or energy storage [3] . However, LiMn2O4 shows obvious capacity fade when high-temperature working condition is applied (50˚C - 60˚C) [4] . The cause for the capacity degradation of LiMn2O4 is Jahn-Teller distortion and accepted as electrolyte decomposition, Mn2+ ions dissolution [5] , oxygen deficiency [6] [7] . In order to solve this problem, earlier studies have been focused on a chemical modification of LiMn2O4 by a partial substitution of Mn with some metal ions to obtain LiMxMn2−xO4 (M = Co, Mg, Cr, Ni, Fe, Al, Ti and Zn) [8] - [12] . Another effective way is surface coating on LiMn2O4 by oxide with high thermal and structural stability. ZrO2, SiO2, Al2O3 and MgO [13] - [16] have been used to coat LiMn2O4 by some chemical processes. LiCoO2 coating may suppress the dissolution of Mn and with de-intercalation and intercalation of Li ions, which will enhance the capacity of LiMn2O4. Therefore, it is expected that the LiMn2O4 by coating LiCoO2 will show an excellent cycle performance at high-temperature. In this study, the effect of LiCoO2 layer on the morphology and electrochemical performances of LiMn2O4 cathode materials were examined in detail.
2. Experimental
LiMn2O4 powder was purchased from Hebei Strong-Power Li-ion Battery Technology Co. Ltd. (D98, China). LiCH3COO∙2H2O (1.03 g), Co (CH3COO)2∙4H2O (2.53 g) with a stoichiometric ratio (1:1) were dissolved in distilled water. An aqueous solution of ethylene glycol and citric acid (1:4) as a chelating agent was added to the mixtures. pH value at 7.0 - 7.5 was achieved by Ammonium hydroxide. Then slowly add the LiMn2O4 powders (50 g) to the sol and vigorously stirred at 85˚C for 5 h. As the evaporation of water proceeding, the sol was turned into a viscous transparent gel. After drying and sieving, the powder was sintering in air at 350˚C for 3 h and 650˚C for 3 h to obtain LiCoO2-coated LiMn2O4. For a comparison, pristine LiMn2O4 was also heat-treated in the same condition.
2.1. Structure and morphology Characterization
X-ray diffraction patterns were recorded on a DX-2700 diffract meter (Siemens D-5000, Mac Science MXP 18) equipped with Cu Kα radiation of λ = 0.154145 nm. The diffraction patterns were recorded between scattering angles of 15˚ and 80˚ at a step of 4˚/min. The morphology was studied by a scanning electron microscopy (S4700, Hitachi) and transmission electron microscope (JEOL-1200EX). After cycling, the batteries were disassembled in glove box and the electrodes and membrane were washed by EC/DMC for several times. The cathode was used to examine the changes in structure by XRD and the obtained solution was diluted to suitable concentration to detect the content of Mn element. Inductively coupled plasma atomic emission spectrometry analysis was conducted on IRIS Intrepid П XSP inductively coupled plasma emission spectrometer (THERMO).
2.2. Electrochemical and thermal characteristics
To obtain working electrode, 85 wt% active materials, 6 wt% polyvinylidene fluoride and 9 wt% acetylene black were homogeneously mixed in NMP. Then the resulting slurry was spread on an Al foil and thoroughly dried. The electrodes were punched in the form of 14 mm diameter disks, and the typical active material mass loading was about 6 mg/cm2. The electrolyte was 1 M LiPF6 dissolved in a mixture of ethylene carbonate and dimethylene carbonate with the volume ratio of 1:1. The anode of the battery is Li electrode. The assembly process was conducted in an argon-filled glove-box with the content of H2O and O2 less than 1 ppm.
Before electrochemical tests, the batteries were aged for 24 h to ensure good soakage. The cells were charged and discharged on a battery tester (CT-3008W, NEWARE) between 3.3 and 4.35 V at the rate of 2C at elevated temperatures (55˚C ± 2˚C) in dry oven (A201113, Shanghai).
3. Results and discussion
Figure 1 shows the XRD patterns. The peaks of both samples could be indexed to a cubic spinel structure with the space group Fd3m. There is no substantial difference between XRD patterns for LiMn2O4 and modified sample. The crystal lattice parameters were calculated by using the software of Jade, are 8.245 and 8.246 Å for the pristine and LiCoO2-coated LiMn2O4, indicating that the bulk structure of LiMn2O4 unchange after surface modification. The characteristic peaks corresponding to LiCoO2 are not observed because of low content (about 2.0 wt%).
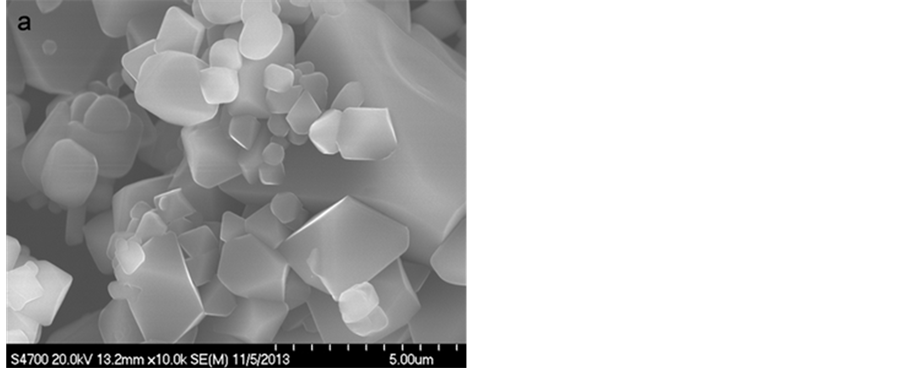
Scanning electron microscopy has been shown in Figure 2 which reveals the pristine and modified samples
Figure 1. X-ray diffraction patterns of (a) Pristine and (b) LiCoO2-coated LiMn2O4.
Figure 2. SEM figures of (a) Pristine and (b) LiCoO2-coated LiMn2O4.
present a uniform particle distribution, ranging from 3 to 6 μm. The pristine spinel crystals are smooth with well-defined facets, as observed in Figure 2(a). It can be seen that the morphology and particle diameter of the LiCoO2-coated LiMn2O4 powders in Figure 2(b), are similar to the pristine sample. No LiCoO2 agglomerations and obscured facets of spinel LiMn2O4 are observed.
The further investment of the surface of LiMn2O4 by transmission electron microscope is displayed in Figure 3. Compared to the pristine sample (Figure 3(a)), about 3 - 5 nm thick layer of LiCoO2 is uniformly formed on the surface of the LiMn2O4 (Figure 3(b)). The coating layer is clearly distinguishable from the crystalline LiMn2O4. This result demonstrates that sol-gel method is an effective way to coat the LiCoO2 layer on the surface of LiMn2O4.
To further identify the homogeneity of coating layer, the element distribution is determined by EDS mapping, which is displayed in Figure 4. The dense accumulation of Mn element is attributed to the host material of LiMn2O4 and there is no significant agglomeration of Co. These results indicate that LiCoO2 is homogeneously dispersed on the surface of the LiMn2O4 particles.
The XPS is shown in Figure 5, for pristine LiMn2O4 sample, there is no Co2p peaks. For LiCoO2-coated LiMn2O4 sample, the Co2p region shows a Co2p3/2 main peak at 780.4 eV with satellite peak at 796.8 eV. It is concluded that Co3+ have deposited on the surface of LiMn2O4. This result is in good agreement with the
Figure 3. TEM figures of (a) Pristine and (b) LiCoO2-coated LiMn2O4.
Figure 4. EDS mappings of Co and Mn elements of modified LiMn2O4 sample.
Figure 5. The Co2p X-ray photoelectron spectra of the pristine and LiCoO2-coated LiMn2O4.
observation in TEM and EDS element mapping.
The structure of pristine LiMn2O4 and LiCoO2-coated LiMn2O4 cathodes after cycling 100 times (55˚C) was examined. The results are given in Figure 6. It can be seen that, the diffraction peaks of cycled LiMn2O4 cathode are widened and the peak intensity declined compared with the pristine LiMn2O4 cathode. In addition, some extra peaks appear in LiMn2O4 cathode XRD pattern after cycling, which should be assigned to Li2Mn2O4. Usually, tetrahedral Li2Mn2O4 can be generated at the final discharge stage of LiMn2O4 because of more Mn3+ and more significant Jahn-Teller effect. However, for the LiCoO2-coated LiMn2O4 cathode, the diffraction peak width changed insignificantly before and after cycling. Comparing with cycled LiMn2O4 cathode, in the XRD
Figure 6. XRD patterns of (a) Pristine, (b) LiCoO2-coated LiMn2O4.
pattern of the cycled LiCoO2-coated LiMn2O4, the peak intensity declines a polarization, we still should ascribe them to the LiCoO2 on the surface of LiMn2O4.
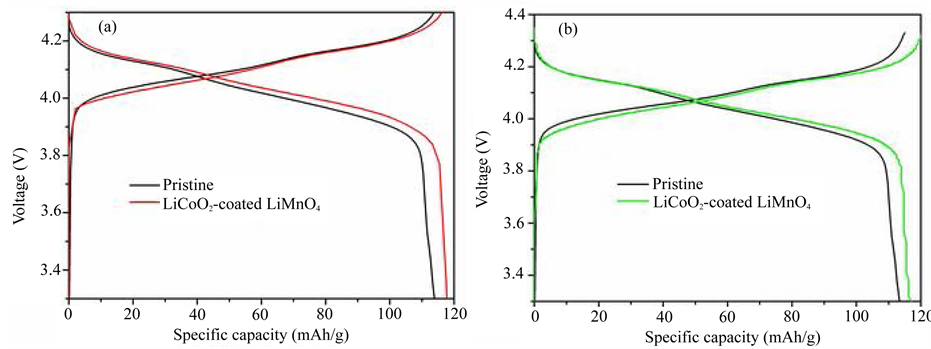
In Figure 7, the galvanostatic charge-discharge curves under a current rate of 2C were conducted at (a) room temperature and (b) elevated temperatures in drying oven. They shows two discharge plateaus, which should be attributed to orderly intercalating of lithium ions in the tetrahedral (8a) sites at 4.1 V and disorderly intercalating lithium ions at 3.9 V which substantially maintains the intercalation feature of LiMn2O4 substrate [17] , indicating LiCoO2 surface layer rather than Ni-doped LiMn2O4 because LiMn2O4 with Ni-doped spinel surface showed two ambiguously resolved discharging plateaus [18] . LiCoO2-coated LiMn2O4 shows a higher discharge capacity compares to the pristine sample. The reason of high initial discharge capacity is that LiCoO2 has capacity at this voltage range.
Figure 8 shows the cycling performance of electrodes with and without LiCoO2 coating at (a) room temperature and (b) elevated temperatures. After 100 cycles at room temperature, the capacity retention of pristine sample (94.3%) is similar to that of modified sample (94.4%), as shown in Figure 8(a). However, after 100 cycles at elevated temperature, the discharge capacity of the pristine LiMn2O4 drops from 115.3 to 100.6 mAh/g. In contrast, the discharge capacity of modified sample changes from 117.2 to 110.1 mAh/g. The capacity retention increases from 87.5% to 93.6% after LiCoO2 coating. Compared with other coating materials such as Al2O3 [19] , La2O3 [20] , AlPO4 [21] , In these paper, surface modification by sol-gel method can improve the high-tempera- ture cycling stability of LiMn2O4, because oxide layer can reduce the contact area of LiMn2O4 and electrolyte. However, the covering layer is not very uniform, the highest capacity retention is about 89%, coupled with the oxide itself do not have de-intercalation and intercalation of Li ions, it will result in a decrease in initial capacity.
To further verify the effects of surface coating on manganese ions dissolution, the quality of the manganese element was directly determined by using ICP-AES. Li metal anode was washed by dilute hydrochloric acid after 100th cycle at 55˚C ± 2˚C. It can be seen in Table 1, the dissolved quality of Mn2+ ions of the pristine and LiCoO2-coated LiMn2O4 electrode was 22.54 and 10.17 μg/cm2, respectively. It can be concluded that after coating by LiCoO2 layer, the dissolution of the manganese ions was significantly reduced. Therefore, LiCoO2- coated LiMn2O4 electrode had better cycle stability at elevated temperature. The reason is that the coating material will reduce the contact area of LiMn2O4 and electrolyte, which may decrease the dissolution of Mn. The reactivity between LiCoO2 and electrolyte has not yet clear, which need further research in future.
4. Conclusion
In summary, the surface of LiMn2O4 sample was modified by LiCoO2 using a sol-gel method. TEM and XPS results confirm the existence of LiCoO2 layer. A uniform and dense layer about 3 - 5 nm was coating on the
Figure 7. The first charge-discharge curves at (a) Room temperature (25˚C ± 2˚C); (b) Elevated temperature (55˚C ± 2˚C).
Figure 8. Cycling behaviors at (a) Room temperature (25˚C ± 2˚C); (b) Elevated temperature (55˚C ± 2˚C).
Table 1. The amount of Mn ions deposited on Li anode after 100 cycles at 55˚C ± 2˚C.
surface of pristine LiMn2O4. The LiCoO2-coated LiMn2O4 sample exhibits much better cycling stability at elevated temperature (55˚C) compared with the pristine sample. These results demonstrated that this is an effective way to improve the high-temperature cyclic performance of spinel LiMn2O4.
Acknowledgements
This work was supported by National Science Foundation of China (No. 50672026). This work was also supported by Shanghai Nano Technology Promotion (No. 12ZR1448800).
References
- Pitchai, R., Thavasi, V., Mhaisalkar, S.G. and Ramakrishna, S. (2011) Nanostructured Cathode Materials: A Key for Better Performance in Li-Ion Batteries. Journal of Materials Chemistry, 21, 11040-11051. http://dx.doi.org/10.1039/c1jm10857c
- Zhao, S., Bai, Y., Ding, L.H., Wang, B. and Zhang, W.F. (2013) Enhanced Cycling Stability and Thermal Stability of YPO4-Coated LiMn2O4 Cathode Materials for Lithium Ion Batteries. Solid State Ionics, 247, 22-29. http://dx.doi.org/10.1016/j.ssi.2013.05.022
- Lee, M.J., Lee, S., Oh, P., Kim, Y. and Cho, J. (2014) High Performance LiMn2O4 Cathode Materials Grown with Epitaxial Layered Nanostructure for Li-Ion Batteries. Nano Letters, 14, 993-999. http://dx.doi.org/10.1021/nl404430e
- Ming, H., Yand, Y.R., Ming, J., Adkins, J., Li, X.W., Zhou, Q. and Zheng, J.W. (2014) Gradient V2O5 Surface-Coated LiMn2O4 Cathode towards Enhanced Performance in Li-Ion Battery Applications. Electrochimica Acta, 120, 390-397. http://dx.doi.org/10.1016/j.electacta.2013.12.096
- Baba, M., Kumagai, N., Fujita, H., Ohta, K., Nishidate, K., Komaba, S., Kaplan, B., Groult, H. and Devilliers, D. (2003) Multi-Layered Li-Ion Rechargeable Batteries for a High-Voltage and High-Current Solid-State Power Source. Journal of Power Sources, 119, 914-917. http://dx.doi.org/10.1016/S0378-7753(03)00223-4
- Kim, D., Park, S., Chae, O.B., Ryu, J.H., Kim, Y.U., Yin, R.Z. and Oh, S.M. (2012) Re-Deposition of Manganese Species on Spinel LiMn2O4 Electrode after Mn Dissolution. Journal of the Electrochemical Society, 159, A193-A197. http://dx.doi.org/10.1149/2.003203jes
- Li, X.F., Xu, Y.L. and Wang, C.L. (2009) Novel Approach to Preparation of LiMn2O4 Core/LiNixMn2-xO4 Shell Composite. Applied Surface Science, 255, 5651-5655. http://dx.doi.org/10.1016/j.apsusc.2008.10.055
- Song, J., Hong, B.L., Zheng, J., Lin, P., Zheng, M.S., Wu, Q.H., Dong, Q.F. and Sun, S.G. (2010) Electronic Properties of LiMn2-xTixO4. Physics A, 98, 455-460.
- Wu, X.L. and Kim, S.B. (2002) Improvement of Electrochemical Properties of LiNi0.5Mn1.5O4 Spinel. Journal of Power Sources, 109, 53-57. http://dx.doi.org/10.1016/S0378-7753(02)00034-4
- Yuan, A.B., Tian, L., Xu, W.M. and Wang, Y.Q. (2010) Al-Doped Spinel LiAl0.1Mn1.9O4 with Improved High-Rate Cyclability in Aqueous Electrolyte. Journal of Power Sources, 195, 5032-5038. http://dx.doi.org/10.1016/j.jpowsour.2010.01.074
- Arumugam, D., Kalaignan, G.P., Vediappan, K. and Lee, C.W. (2010) Synthesis and Electrochemical Characterizations of Nano-Scaled Zn Doped LiMn2O4 Cathode Materials for Rechargeable Lithium Batteries. Electrochimica Acta, 55, 8439-8444. http://dx.doi.org/10.1016/j.electacta.2010.07.033
- Amaral, F.A., Bocchi, N., Brocenschi, R.F., Biaggio, S.R. and Rocha-Filho, R.C. (2010) Structural and Electrochemical Properties of the Doped Spinels Li1.05M0.02Mn1.98O3.98N0.02 (M = Ga3+, Al3+, or Co3+; N = S2− or F−) for Use as Cathode Material in Lithium Batteries. Journal of Power Sources, 195, 3293-3299. http://dx.doi.org/10.1016/j.jpowsour.2009.12.002
- Thackeray, M.M., Johnson, C.S., Kim, J.S., Lauzze, K.C., Vaughey, J.T., Dietz, N., Abraham, D., Hackney, S.A., Zeltner, W. and Anderson, M.A. (2003) ZrO2- and Li2ZrO3-Stabilized Spinel and Layered Electrodes for Lithium Batteries. Electrochemistry Communications, 5, 752-758. http://dx.doi.org/10.1016/S1388-2481(03)00179-6
- Huang, B., Li, X.H., Wang, Z.X., Guo, H.J., Xiong, X.H. and Wang, J.X. (2014) A Novel Carbamide-Assistant Hydrothermal Process for Coating Al2O3. Journal of Alloys and Compounds, 583, 313-319. http://dx.doi.org/10.1016/j.jallcom.2013.08.157
- Zheng, Z.H., Tang, Z.L., Zhang, Z.T., Shen, W.C. and Lin, Y.H. (2002) Surface Modification of Li1.03Mn1.97O4 Spinels for Improved Capacity Retention. Solid State Ionics, 148, 317-321. http://dx.doi.org/10.1016/S0167-2738(02)00068-1
- Gnanaraj, J.S., Pol, V.G., Gedanken, A. and Aurbach, D. (2003) Improving the High-Temperature Performance of LiMn2O4 Spinel Electrodes by Coating the Active Mass with MgO via a Sonochemical Method. Electrochemistry Communications, 5, 940-945. http://dx.doi.org/10.1016/j.elecom.2003.08.012
- Wu, F., Wang, M., Su, Y.F., Chen, S. and Xu, B. (2009) Effect of TiO2-Coating on the Electrochemical Performances of LiCo1/3Ni1/3Mn1/3O2. Journal of Power Sources, 191, 628-632. http://dx.doi.org/10.1016/j.jpowsour.2009.02.063
- He, X.M., Li, J.J., Cai, Y., Wang, Y.W., Ying, J.R., Jiang, C.Y. and Wan, C.R. (2005) Preparation of Co-Doped Spherical Spinel LiMn2O4 Cathode Materials for Li-Ion Batteries. Journal of Power Sources, 150, 216-222. http://dx.doi.org/10.1016/j.jpowsour.2005.02.029
- Jang, S.B., Kang, S.H., Amine, K., Bae, Y.C. and Sun, Y.K. (2005) Synthesis and Improved Electrochemical Performance of Al(OH)3-Coated Li[Ni1/3Mn1/3Co1/3]O2 Cathode Materials at Elevated Temperature. Electrochimica Acta, 50, 4168-4173. http://dx.doi.org/10.1016/j.electacta.2005.01.037
- Shan, H., Göktepe, H. and Patat, S. (2011) A Novel Method to Improve the Electrochemical Performance of LiMn2O4 Cathode Active Material by CaCO3 Surface Coating. Journal of Materials Science & Technology, 27, 415-420. http://dx.doi.org/10.1016/S1005-0302(11)60084-4
- Fey, G.T.K., Lu, C.Z. and Kumar, T.P. (2003) Preparation and Electrochemical Properties of High-Voltage Cathode Materials, LiMyNi0.5-yMn1.5O4 (M = Fe, Cu, Al, Mg; y=0.0-0.4). Journal of Power Sources, 115, 332-345. http://dx.doi.org/10.1016/S0378-7753(03)00010-7
NOTES
*Corresponding author.