Advances in Materials Physics and Chemistry
Vol.07 No.04(2017), Article ID:76004,15 pages
10.4236/ampc.2017.74011
Elaboration and Characterization of Glasses and Ceramic-Glasses within Theternary Diagram Li2O-Cr2O3-P2O5
Said Aqdim1,2*, Yassine Er-rouissi1, Abdelmjid Cherif1, Radouane Makhlouk1
1Laboratoire de Génie des Matériaux pour Environnement et Valorisation, Département de Chimie, Faculté des Sciences, Université Hassan II Ain Chock, Casablanca, Morocco
2Laboratoire de Chimie Minérale, Département de Chimie, Faculté des Sciences, Université Hassan II Ain Chock, Casablanca, Morocco

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution-NonCommercial International License (CC BY-NC 4.0).
http://creativecommons.org/licenses/by-nc/4.0/



Received: February 27, 2017; Accepted: April 27, 2017; Published: April 30, 2017
ABSTRACT
Use of the regular melting-quench method allowed the isolation of a small glass domain within the ternary system Li2O-P2O5-Cr2O3 at 1000˚C. Electrical conductivity and dielectric permittivity measures on sample glasses and ceramic glasses of this domain were performed at a frequency of 10 kHz and at temperatures between 25˚C and 300˚C. The values of dielectric permittivity and electrical conductivity increase with increasing Li2O content. However, increases of Cr2O3 content, even at low concentrations, induce a change in electrical conductivity behaviour from that of a glass to that of a ceramic glass. The introduction of an increasing amount of Li2O content in vitreous phosphorus pentoxide changes its three-dimensional network; rupture of the P-O-P bond then occurs and there is formation of easily polarisable entities with quite high values of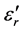 . The vibrational spectroscopy technique I.R has allowed an efficient investigation of the structural change versus composition within the above indicated ternary diagram. The maximal dielectric permittivity obtained at 300˚C, both for the glasses and for the ceramic glasses varied in the order 104 to 3 × 105, while the maximum electrical conductivity obtained at 300˚C for the ceramic glasses was in the order of 10−3 Ωcm−1.
. The vibrational spectroscopy technique I.R has allowed an efficient investigation of the structural change versus composition within the above indicated ternary diagram. The maximal dielectric permittivity obtained at 300˚C, both for the glasses and for the ceramic glasses varied in the order 104 to 3 × 105, while the maximum electrical conductivity obtained at 300˚C for the ceramic glasses was in the order of 10−3 Ωcm−1.
Keywords:
Phosphate Glasses, Glass Formation, XRD, IR Spectroscopy, Electrical Conductivity, Dielectric Permittivity

1. Introduction
Several fundamental studies have been made on glasses composed of silica and with oxides of phosphoric anhydrides [1] [2] [3] [4] . Due to their low chemical durability, phosphate glasses have gained less attention. However, several phosphate glasses with high aqueous corrosion resistance have been reported [5] - [12] . The electrical engineering field constitutes one of the numerous fields of application of glasses. This field is particularly interested in materials with high ionic conductivity that can be used as solid electrolytes, or in materials of very high resistivity capable of playing the dielectric role for miniaturised condensers. Historically, ionic conduction has been studied in crystalline compounds for a long time [13] . The interest in ion-conducting glasses is more recent, but has progressed rapidly over the last thirty years due to the use of these materials as solid electrolytes. The transport properties of these electrolytes have been the subject of numerous works, whose main lines have been grouped together in some publications [14] [15] [16] [17] . The notion of point defects is a very useful tool in the interpretation of conduction in crystallised materials, but it is ill-defined for amorphous materials, because they do not have long-range structural periodicity. However, glasses have many spaces (due to their low density), which are capable of containing cations of different sizes [7] [16] [18] [19] . Ions that are weakly bonded to the network can move in the material under the action of a force resulting from an electric potential gradient. The well-known mobility of alkaline cations, and in particular Li+ and Na+, in solid electrolytes has prompted us to explore the electrical conductivity of the revealed vitreous phases [2] [3] [4] [5] . Our aim in the present work is to define the glass area in the ternary system Li2O-P2O5-Cr2O3 for the first time. A second aim is to undertake an electrical study of glasses and glass ceramics of the ternary system Li2O-P2O5- Cr2O3. The study of the conduction mechanisms and their relation to the structure of the phosphorus network illustrate that with increased amounts of the modifier oxide Li2O in the glass network, the mobility of the Li+ carriers is facilitated, due to increased depolymerisation of the phosphate network [7] [18] [19] . On the other hand, the increasing glassy depolymerisation of the network induces an increase in the number of non-bridge oxygens and consequently an increase in the dielectric permittivity [7] [18] [20] [21] .
2. Experimental Procedures
A glass of composition xLi2O-yCr2O3-(100-(x + y)P2O5 (mol %) was obtained by the melting quench method at 1000˚C. Appropriate mixtures of the compounds Li2CO3, Cr2O3 and (NH4)2HPO4 were initially prepared at various temperatures between 300˚C - 500˚C in order to achieve a preliminary preparation before the final glass preparation. The melts were performed in alumina crucibles for about 20 min at 1000˚C ± 10˚C. The isolated glass samples were approximately 10 mm diameter and 1 to 3 mm in thickness. Their vitreous state was first evidenced by their shiny aspect and confirmed by XRD. Annealing of these glasses was realised at increasing temperatures in intervals of 100˚C. The first structural approach was X-ray diffraction, which allowed the crystallisation of the vitreous domain Li2O-P2O5-Cr2O3 to be followed. The dielectric permittivity (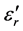 ) and dielectric loss (tnδ) measurements were determined from capacity measurements realised on samples in the form of small cylinders carved with abrasive papers. While depositing a layer of silver lacque on the parallel faces of the samples, we form a condenser plan of which the capacity is measured using a Hewlett Packard 4262 A LCR Meter. The measures of conductivity were determined from resistivity measurements realised on samples. The samples were polished in the form of small cylinders using abrasive papers. Pastilles so obtained have diameters of around 10 mm and thicknesses varying between 1 and 3 mm.
) and dielectric loss (tnδ) measurements were determined from capacity measurements realised on samples in the form of small cylinders carved with abrasive papers. While depositing a layer of silver lacque on the parallel faces of the samples, we form a condenser plan of which the capacity is measured using a Hewlett Packard 4262 A LCR Meter. The measures of conductivity were determined from resistivity measurements realised on samples. The samples were polished in the form of small cylinders using abrasive papers. Pastilles so obtained have diameters of around 10 mm and thicknesses varying between 1 and 3 mm.
3. Results
In the Li2O-P2O5 system, transparent and colourless glasses could be prepared with molar fractions of Li2O between 0 and 0.62. These values are in good agreement with previously published results [18] but are rather superior to those given by the other authors [20] [22] . This is probably caused by different experimental conditions (temperature of melting; speed of tempering, etc.) [23] . In the binary systemCr2O3-P2O5, the substitution of P2O5 by Cr2O3 led to very hygroscopic green glasses with a chromium oxide content between 0 and 7 mol%, see Figure 1. Ternary glasses have also a green colour which becomes more clear and green as the lithium oxide content increases and as the Cr2O3 content increases between 0 £ y < 5; (mol%). The demarcation of the glassy zone within the ternary diagramLi2O-P2O5-Cr2O3 is given by the following limits: 0 £ x £ 62; 0 £ y <7; 36 £ χ £ 100 (mol%) (Figure 1). The localization of analysed compounds is presented inside the ternary diagram given in Figure 2.
3.1. Annealing Temperature
The annealing temperatures of the binary Li2O-P2O5, ternaries glasses and ceramic glasses were tested at increasing temperatures in intervals of 100˚C. Increasing amounts of the lithium oxide modifier lead to an increase of the values for the crystallisation temperature. For the glasses and ceramic glasses of composition xLi2O-2Cr2O3-(98-χ)P2O5 and xLi2O-5Cr2O3-(98-χ) P2O5, Figure 3 shows that the crystallisation temperature increases from (250 ± 10)˚C to (540 ±
Figure 1. Extended from the vitreous field within the ternary diagram Li2O-P2O5-Cr2O3 to 1000˚C.
Figure 2. Localization of the investigatedglass and ceramic glass compositionsin the ternary diagram Li2O-Cr2O3-P2O5.
Figure 3. Variation of crystallization temperature as a function of the Li2O/P2O5 ratio for glasses and ceramic glasses with the composition Li2O-Cr2O3-P2O5.
10)˚C as the Li2O/P2O5 ratio increases from 0 to 2. However, the annealing temperature (Tc) variations for the ceramic glasses are greater than those observed in the glasses when the Li2O content increases.
3.2. Electrical Conductivity
The electrical conductivities were measured at various temperatures between 25˚C and 300˚C. Figure 4 shows the variation of the logarithm of the conductivity as a function of the inverse of the absolute temperature for some compositions. In a large part of the envisaged domain of temperature, the conductivity σ varies according to Arrhenius’s law σ0 Exp Ea/kT where σ0 is the pre-exponential term, Ea is the activation energy of conduction, and k is the Boltzmann constant, 1.38 × 10−23 J/K. The activation energies, Ea, calculated from this relationship are shown in Table 1 as are the conductivities at 300˚C. Examination of this table shows that the activation energy is a decreasing function of the Li2O content. Figure 5 clearly indicates the variations of the conductivity at 300˚C according to the Li2O content for the glasses and glass ceramics with different Cr2O3 contents. We can conclude that the introduction of chromium oxide into the phosphate network seems to change the behaviour from that of a glass to that of a ceramic glass. We note a decrease of σ for glasses, whereas we observe an increase of σ for ceramic glasses. On the other hand Figure 6 indicates that the
Figure 4. The Arrhenius plot for some glasses and glass-ceramics, (mol%) in the ternary diagram Li2O-Cr2O3-P2O5.
Table 1. Glasses and ceramic glasses composition in mol% and selected properties as electrical conductivity and activation energies.
Figure 5. Variation of the conductivity logarithm at 300˚C as a function of Li2O content for some glasses and ceramic glasses in the ternary diagram Li2O-Cr2O3-P2O5.
Figure 6. Variation of the activation energy at 300˚C as a function of Li2O content for some glasses and ceramic glasses in the ternary diagram Li2O-Cr2O3-P2O5.
Figure 7. Variation of dielectric permittivity with temperature at 10 KHz of binary glasses with the composition x Li2O-(100-x)P2O5.
activation energies, for different lines, varies inversely proportionnel with the increase of the Li2O content [7] [18] [20] [24] .
3.3. Dielectric Permittivity
Figures 7-9 show the thermal variations of log 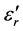 for three families of composition: xLi2O?(100-x)P2O5; xLi2O-2Cr2O3?(98-x)P2O5; and xLi2O-5Cr2O3-(95-x) P2O5. Examination of these figures shows that when the temperature and the composition are modified, the variation in the dielectric values is proportionally greater than those of silicate glasses [1] [2] [3] . However the values of
for three families of composition: xLi2O?(100-x)P2O5; xLi2O-2Cr2O3?(98-x)P2O5; and xLi2O-5Cr2O3-(95-x) P2O5. Examination of these figures shows that when the temperature and the composition are modified, the variation in the dielectric values is proportionally greater than those of silicate glasses [1] [2] [3] . However the values of 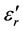 remain very modest below a certain temperature (Tt) after which the log
remain very modest below a certain temperature (Tt) after which the log 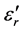 slope increases more and more quickly with increased Li2O content. Figure 10 shows that the curve (
slope increases more and more quickly with increased Li2O content. Figure 10 shows that the curve (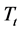 ) changes according to the Li2O content
) changes according to the Li2O content 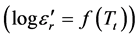 for all different lines studied, manifested as an increase in
for all different lines studied, manifested as an increase in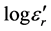 , and a negative slope. The results are regrouped in Table 2. The study of the
, and a negative slope. The results are regrouped in Table 2. The study of the 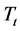 in relation to the Li2O content shows that when the Li2O content increases for various compositions, the temperature decreases. Similar results have been shown in previous studies [18] [20] .
in relation to the Li2O content shows that when the Li2O content increases for various compositions, the temperature decreases. Similar results have been shown in previous studies [18] [20] .
3.4. Dielectric Loss
Figures 11-13 show the curves representing thermal variations of the dielectric
Figure 8. Variation of dielectric permittivity with temperature at 10 KHz for glasses with the composition -xLi2O-2Cr2O3(98-x)P2O5.
Figure 9. Variation of dielectric permittivity with temperature at 10 KHz for ceramic glasses with the composition xLi2O-5Cr2O3-(95-x)P2O5.
Figure 10. Variation of Tt as a function of the Li2O content for glasses and ceramic glasses with the composition x Li2O-yCr2O3-(100-(x + y))P2O5.
Table 2. Glasses and ceramic glasses composition in mol% and selected properties as Permittivity and Tt.
Figure 11. Thermal variation of dielectric losses at 10Khz of glasses with the composition xLi2O-(100-x)P2O5.
losses of glasses and vitreous ceramics of the ternary system Li2O-P2O5-Cr2O3. These curves show a more or less Gaussian behaviour with maxima of tnδ, which appear at decreasing temperatures as the Li2O content increases. The temperatures of these maxima seem to coincide with the Tt observed on the log
Figure 12. Thermal variation of dielectric losses at 10 Khz of ceramic glasses with the composition xLi2O-2Cr2O3-(98-x)P2O5.
Figure 13. Thermal variation of dielectric losses at 10Khz of ceramic glasses with the composition Li2O-5Cr2O3-(95-x)P2O5.
Figure 14. Thermal variation of dielectric losses at 10 Khz of glasses and ceramic glasses with the composition (60-y)Li2O-yCr2O3-40P2O5.
influence on the position of the maxima of tnδ (T) for the glasses and the vitreous ceramics, but specially induces the decrease of the amplitude of the dielectric losses.
3.5. Infrared Spectroscopy
The infrared spectra of the glasses of the binary system Li2O-P2O5 are shown in Figure 15. Increase of the lithium oxide content in the phosphate glasses causes an important depolymerisation in the vitreous network, and induces the formation of small units in accordance with the results of M. Ouchetto et al. [18] [19] [25] . The analysis of infra-red spectra shows that the band at 455 - 500 cm−1 is assigned to the deformation of the skeleton δ ske (P-O-P while the band at 735 - 775 cm−1 and the bands at 900 - 910 cm−1 are assigned respectively to the vibration of symmetric stretching νsym (P-O-P and to the asymmetric stretching νasym (P-O-P) [9] [11] . The band υasPO2 that appears about 1250 - 1275 cm−1, attributed to Q2 phosphate tetrahedron, decreases while we move away from point P2O5. Whereas the band υsPO2 that appears about 1090 - 1100 cm−1attributed to Q1 phosphate tetrahedron, increases with the increasing of Li2O content and inducts a break links P-O-P and an increase of the number of non bridge-oxygen. In the other hand, the IR spectra with the compositions xLi2O-2Cr2O3?(98-x)P2O5 and xLi2O-5Cr2O3-(95-x)P2O5, observed in the Figure 16 and Figure 17 respectively, have similar behaviours to the spectra of the binary system xLi2O-(100-x) P2O5 family, which can be explained by a similar structural evolution [7] . We note the presence of a small band uas P-O-P at 995 - 998 cm−1, attributed to isolated orthophosphate units, when the Li2O content exceeds 55 mol%. This last one can be attributed to the Cr?O?P bond in CrPO4 units [26] . Also, the band υsP = 0 that appears about 1360 cm−1 diminishes while on moves away from point P2O5, that seems to be attributed to the basic glass matrix P2O5 [18] .
Figure 15. I.R spectra of phosphate glasses with the composition xLi2O-(100-x)P2O5.
Figure 16. I.R spectra of chromium phosphate glasses with the composition xLi2O- 2Cr2O3-(98-x)P2O5.
Figure 17. I.R spectra of chromium phosphate ceramic glasses with the composition xLi2O-5Cr2O3-(95-x)P2O5.
4. Discussion
The regular melting-quench method allowed the isolation of a small vitreous domain within the ternary system Li2O-P2O5-Cr2O3 at 1000˚C. Conductivity measurements were performed on glasses and vitreous ceramics isolated within the ternary systemLi2O-P2O5-Cr2O3. Dielectric permittivity and dielectric loss were measured at a frequency of 10 kHz and in the temperature range of 298 to 573 K. An increase in the Li2O content in the glass network results at the same time in an increase in 

5. Conclusions
The regular melting-quench method allowed the isolation of a small vitreous domain within the ternary system Li2O-P2O5-Cr2O3 at 1000˚C. Conductivity measurements were realised on glasses and vitreous ceramics isolated within the ternary system Li2O-P2O5-Cr2O3. Dielectric permittivity and dielectric loss were measured at a frequency of 10 MHz and in the temperature range 298 to 573 K. An increase in the Li2O content in the glass network results at the same time in an increase in 
Increase in the Cr2O3 content seems to have caused different behaviour from glasses to vitreous ceramics. The conductivity decreases in the chromium glasses while it increases in glass ceramics due to network defects, which are probably more numerous and in a favorable position for mobile species. On the other hand, an increase in the chromium oxide content induces a decrease dielectric loss and an increase of Tt temperature in the glass. Hence we can say that an increase of Cr2O3 content induces an increase of the rigidity of the glass network. The highest conductivity obtained at 300˚C was in the order of 2 × 10−3 (Wcm)−1 with an activation energy of 0.45 e.v; however, this remains low compared with that of the crystalline compound LiAl11O17 (σ =10−2 (Ωcm)−1) at 25˚C. The obtained conductivities are probably responsible for large dielectric losses, which mean that our glasses are currently not suitable for possible application as dielectric condensers.
Cite this paper
Aqdim, S., Er- rouissi, Y., Cherif, A. and Makhlouk, R. (2017) Elaboration and Characterization of Glasses and Ceramic-Glasses within Theternary Diagram Li2O-Cr2O3-P2O5. Advan- ces in Materials Physics and Chemistry, 7, 123-137. https://doi.org/10.4236/ampc.2017.74011
References
- 1. Zarziky, J. (1982) Les verres et l’état vitreux; Masson Paris.
- 2. Van Ass, H. and Stevels, J.M. (1974) Internal Friction of Mixed Alkali Metaphosphate Glasses. Journal of Non-Crystalline Solids, 15, 215.
- 3. Stevels, J.M. (1957) The Electrical Properties of Glass. Handbuch der PhysiK, Berlin, 20, 350.
- 4. Panier, T., Foultier, M. and Souquet, J.L. (1983) Electrochemical Properties of Phosphate Based Semi-Conductive Glasses. Solid State Ionics, 9-10, 649-654.
- 5. Liu, H.S., Chin, T.S. and Yung, S.W. (1997) FTIR and XPS Studies of Low-Melting PbO-ZnO-P2O5 Glasses. Materials Chemistry and Physics, 50, 1.
- 6. Liu, H.S. and Chin, T.S. (1997) Low Melting PbO-ZnO-P2O5 Glasses. Part 2. A Structural Study by Raman Spectroscopy and MAS-NMR. Physics and Chemistry of Glasses, 38, 123.
- 7. Aqdim, S. (1990) Identification et Etude Thermique et Electrique des Phases Vitreuses des Systèmes Ternaires Li2O-M2O3-P2O5 (M = Cr, Fe). Diplome d’Etude Supérieur de 3ème Cycle, Faculté des Science Rabat.
- 8. Aqdim, S. and Ouchetto, M. (2013) Elaboration and Structural Investigation of Iron (III) Phosphate Glasses. Advances in Materials Physics and Chemistry, 3, 332-339.
https://doi.org/10.4236/ampc.2013.38046 - 9. Aqdim, S., Sayouty, El.H. and Elouadi, B. (2008) Structural and Durability Investigation of the Vitreous Part of the System (35-z)Na2O-zFe2O3-5Al2O3-60P2O5. Eurasian Chemico-Technological Journal, 10, 9.
- 10. Aqdim, S., Sayouty, El.H., Elouadi, B. and Greneche, J.M. (2012) Chemical Durability and Structural Approach of the Glass Series (40-y)Na2O-yFe2O3-5Al2O3-55P2O5. Materials Science and Engineering, 27, Article ID: 012003.
- 11. Makhkhas, Y., Aqdim, S. and Sayouty, El.H. (2013) Study of Sodium Chromium-Iron-Phosphate Glass by XRD, IR, Chemical Durability and SEM. Journal of Materials Science and Chemical Engineering, 1, 1-6.
https://doi.org/10.4236/msce.2013.13001 - 12. Beloued, N., Chabbou, Z. and Aqdim, S. (2016) Correlation between Chemical Durability Behaviour and Structural Approach of the Vitreous Part of the System 55P2O5-2Cr2O3-(43-x)Na2O-xPbO. Advances in Materials Physics and Chemistry, 6, 149-156.
https://doi.org/10.4236/ampc.2016.66016 - 13. Réau, J.-M, Portier, J., Levasseur, A., Villeneuve, G. and Pouchard, M. (1978) Characteristic Properties of New Solid Electrolytes. Materials Research Bulletin, 13, 1415-1423.
- 14. Van Gool, W. (1973) Fast Ion Transport in Solids. North Holland, Amsterdam.
- 15. Hagenmuller, P. and Van Gool, W. (1978) Solid Electrolytes. General Principles, Characterization, Materials, Applications. Academic Press, New York.
- 16. Choudhary, C.B., Maiti, HS. and Subbarao, E.C. (1980) Solid Electrolytes and Their Applications. Plenum Press.
- 17. Rao, R.B., Gopal, N.O. and Veeraiah, N. (2004) Studies on the Influence of V2O5 on Dielectric Relaxation and Ac Conduction Phenomena of Li2O-MgO-B2O3 Glass System. Journal of Alloys and Compounds, 368, 25-37.
- 18. Ouchetto, M. (1983) Caractérisation et Approche Structural de la région vitreuse du system ternaire Li2O-CdO-P2O5 Diplome d’Etude Supérieur de 3ème Cycle, Faculté Des Sciences, Rabat.
- 19. Doreau, M., El Anouar, A.A. and Robert, G. (1980) Domainevitreux, structure et conductivité électrique des verres du système LiCl/1b Li2O/1b P2O5. Materials Research Bulletin, 15, 285-294.
- 20. Amraoui, N. (1990) Thèse de 3ème cycle, Faculté des Sciences, Rabat.
- 21. Arbib, H. (1987) Diplome d’Etude Supérieure de 3ème Cycle Université, Faculté des Sciences, Rabat.
- 22. Imoka, M. (1962) Advances in Glass Technologies. Plenum Press, New York.
- 23. Poulain, M., Cohnthansinh, M. and Lucas, J. (1977) Nouveaux verres fluorés. Materials Research Bulletin, 12, 151-156.
- 24. Leclaire, A., Ben Moussa, A., Borel, M.M., Grandin, A. and Raveau, B (1988) Two Forms of Sodium Titanium(III) Diphosphate: α-NaTiP2O7 Closely Related to β-Cristobalite and β-NaTiP2O7 Isotypic with NaFeP2O7. Journal of Solid State Chemistry, 77, 299-305.
- 25. Levasseur, A., Brethous, J.C., Reau, J.M. and Hagenmuler, P. (1979) Etude comparee de la conductivite ionique du lithium dans les halogenoborates vitreux. Materials Research Bulletin, 14, 912-927.
- 26. Zhu, H., Liao, Q., Wang, F., Dai, Y. and Lu, M. (2016) The Effects of Chromiumoxide on the Structure and Properties of Iron Borophosphate Glasses. Journal of Non-Crystalline Solids, 437, 48-52.
- 27. Durville, F. (1984) Doctorat de 3ème cycle, Université Claude Bernard Lyon I.
- 28. Santic, A., Kim, C.W., Day, D.E. and Mogus-Milankovic, A. (2010) Electrical Properties of Cr2O3-Fe2O3-P2O5 Glasses. Part II. Journal of Non-Crystalline Solids, 356, 2699-2703.



















