Advances in Materials Physics and Chemistry
Vol.4 No.2(2014), Article ID:42837,4 pages DOI:10.4236/ampc.2014.42005
Triton Facilitated Spherical TiO2 Nanoparticles and Their Advantage in a Dye-Sensitized Solar Cell
1Chemistry Department, College of Science for Girls, University of Dammam, Dammam, KSA
2Chemistry Department, Faculty of Science, Benha University, Benha, Egypt
3King Fahd University of Petroleum and Minerals, Dhahran, KSA
Email: *safenazr@yahoo.com
Copyright © 2014 N. Al-Omair et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In accordance of the Creative Commons Attribution License all Copyrights © 2014 are reserved for SCIRP and the owner of the intellectual property N. Al-Omair et al. All Copyright © 2014 are guarded by law and by SCIRP as a guardian.
Received December 27, 2013; revised January 30, 2014; accepted February 6, 2014
KEYWORDS
TiO2 Nanoparticles; Triton; Orange IV; Dye-Sensitized Solar Cell
ABSTRACT
Spherical TiO2 particles (60 nm) were obtained by using a Triton X-100. The surfactant was employed in two stages, i.e., in the hydrolysis of TiCl4 and then in the precipitation of the corresponding Ti (IV) polymers. The advantages of such spherical TiO2 particles were examined in terms of photovoltaic characteristics of a dye-sensitized solar cell (DSSC) using Orange IV dye as sensitizer. Significantly higher overall solar energy conversion efficiency was obtained for a DSSC using the film of these spherical TiO2 particles, compared with that of a cell using a TiO2 film prepared without surfactant.
1. Introduction
The dye-sensitized nanocrystalline solar cell (DSC) invented by O’Reagan and Gràtzel [1] is a cheap and versatile alternative to silicon based solar cells and has already achieved solar-to-electricity conversion efficiency as high as 11.5% [2,3]. The main stream of the research has been focusing on development of materials which would enhance the conversion efficiency, simplify the production of DSSC and assure their long-lifetime. Titanium dioxide is an n-type wide band gap semiconductor that absorbs in the UV region and is transparent for visible light. In a typical DSSC, the dye molecules are adsorbed on the surface of the TiO2 to assure photoelectric conversion over a broad spectral range of the solar spectrum. When sunlight radiates onto the DSSC, the electrons in the dye molecules (HOMO level) absorb photons that become excited and jump to an unoccupied upper level (LUMO level). The electrons from the LUMO level are then injected into the TiO2 semiconductor conduction band and pass through the TiO2 layer to the transparent conductive oxide (TCO) coated glass and to a load. Further, the vacant “HOMO” level is then filled with electrons supplied by the iodide (I−) ions in the electrolyte while iodide is oxidized to tri-iodide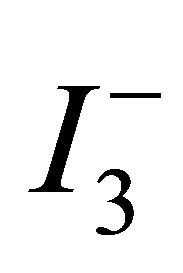 . Meanwhile the platinum counter electrode acts as a catalyst for the redox reaction of the ions in the electrolyte solution and reduces the tri-iodide back to iodide [4]. To realize high-efficiency DSSCs, a high surface area of the nanostructured TiO2 layer is essential, because it allows adsorption of sufficiently large number of dye molecules needed for efficient light harvesting. Beside the high surface area of the TiO2 layer, the morphology and structure of a TiO2 film is one of the strategies adopted for improving the efficiency of a dye-sensitized solar cell (DSSC) [5-8], because the optical, electrical, conductive and other characteristics of a TiO2 film vary greatly with the size and shape of the particles and the clusters comprising the film. A variety of TiO2 films and materials have been produced using different types of surfactants as templates, e.g., alkylphosphate [9], amphiphilic triblock copolymer [10], laurylamine hydrochloride/tetraisopropylorthotitanate [11], self-designed amphiphilic compounds containing cationic charge moieties [12], poly (alkylene oxide) block copolymer [13]. As can be seen above, primary surfactants were rarely used to improve the morphology, crystallinity or particle size of TiO2, thereby to improve the photovoltaics of a DSSC.
. Meanwhile the platinum counter electrode acts as a catalyst for the redox reaction of the ions in the electrolyte solution and reduces the tri-iodide back to iodide [4]. To realize high-efficiency DSSCs, a high surface area of the nanostructured TiO2 layer is essential, because it allows adsorption of sufficiently large number of dye molecules needed for efficient light harvesting. Beside the high surface area of the TiO2 layer, the morphology and structure of a TiO2 film is one of the strategies adopted for improving the efficiency of a dye-sensitized solar cell (DSSC) [5-8], because the optical, electrical, conductive and other characteristics of a TiO2 film vary greatly with the size and shape of the particles and the clusters comprising the film. A variety of TiO2 films and materials have been produced using different types of surfactants as templates, e.g., alkylphosphate [9], amphiphilic triblock copolymer [10], laurylamine hydrochloride/tetraisopropylorthotitanate [11], self-designed amphiphilic compounds containing cationic charge moieties [12], poly (alkylene oxide) block copolymer [13]. As can be seen above, primary surfactants were rarely used to improve the morphology, crystallinity or particle size of TiO2, thereby to improve the photovoltaics of a DSSC.
This paper essentially deals with the formation of spherical TiO2 particles by means of the introduction of Triton X-100. This technique has been well known in order to provide simple and inexpensive alternative routes to synthesize nanoparticulate materials.
2. Experimental
Titanium tetrachloride was used as the starting material to prepare the TiO2 films. All the chemicals were of analytical grade and used without further purification. The influence of Triton X-100 was examined in two steps: (1) the preparation of an aqueous solution of TiCl4 (hydrolysis step), and (2) the precipitation of Ti (IV) polymers from the hydrolyzed TiCl4 solution onto a conducting substrate (precipitation step) [14]. In the hydrolysis step, 22 mL of TiCl4 was added dropwise to 78 mL of distilled water containing 1.3 mM Triton X-100 to produce a 2.0 M Ti (IV) solution. Before the precipitation process, fluorine-doped tin oxide (FTO) conducting glass plates (TCO glass, fluorine-doped SnO2 overlayer, transmission >70% in visible and sheet resistance 20 W/square) were first cleaned with water and ethanol. In the precipitation step, 1.0 ml of the hydrolyzed 2.0 M Ti (IV) solution were pipetted into the conducting glass plates. The plates were dried at 353 K in order to allow the Ti (IV) polymers to deposit onto the conducting glass plates. Subsequently, the glass plates were annealed at 623 K for 1 h. The TiO2 films thus obtained were coated with 0.3 mM of Orange IV in absolute ethanol for 24 h at room temperature. Carbon counter electrode was prepared by sputtering a thin layer of carbon on a transparent conducting glass support (TCO glass, fluorine-doped SnO2 overlayer, transmission >70% in visible and sheet resistance 20 W/square) using a graphite rod. The carbon electrode was placed over the dye-coated electrode, and the edges of the cell were sealed. A redox electrolyte consists of a mixture of iodide/triiodide in acetonitrile was injected by a syringe into the space between the two electrodes. The resulting cell had an active area of 1 × 2 cm2. A schematic diagram showing the construction of the cell is represented in Figure 1.
The photocurrent-voltage (I - V) curves were obtained using a digital source-meter (Keithley 2400). Direct sun light was used to illuminate the working electrode. The surface morphology was examined using SEM (Jeol JXA-840 electron probe microanalyzer). The XRD measurements were carried out with Diano Corporation USA diffractometer using Co radiation radiation. The surface areas were measured using a conventional or classical
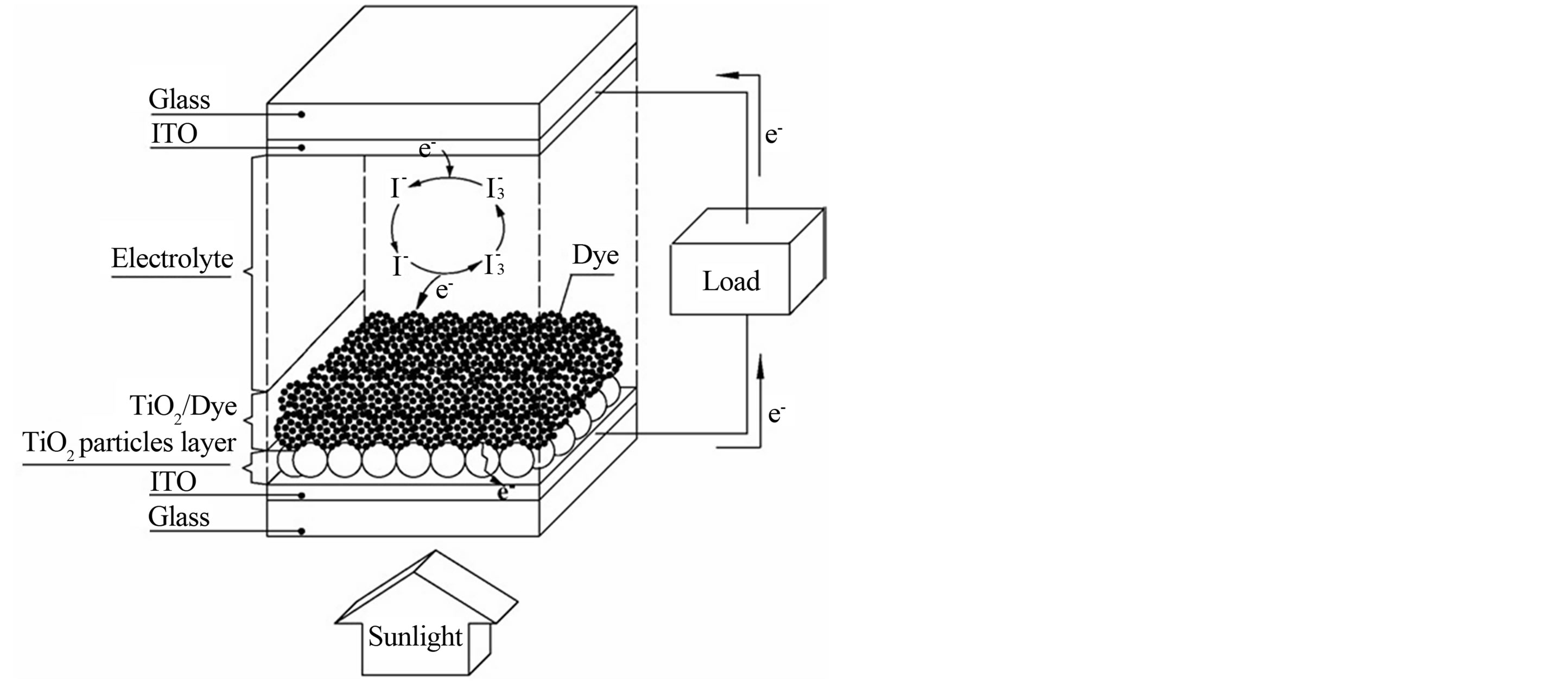
Figure 1. Schematic of the dye-sensitized solar cell with a sandwich TiO2 thin-film.
volumetric apparatus. FTIR spectra were taken using Thermo Avtar 370 spectrometer.
3. Results and Discussion
Figure 2 shows the influence of Triton X-100 on the XRD patterns of the TiO2 films, deposited on FTO. It can be seen that the TiO2 film formed with Triton X-100 completely crystallize as anatase (DB card, No. 01–070- 7348).
The average crystallite size of the anatase TiO2 was calculated using the Scherrer equation [15]. The determined average size of the prepared TiO2 was found to be 60 nm.
FT-IR studies of the TiO2 film show the characteristics of the formation of high-purity material. The FT-IR spectrum of Figure 3 clearly shows the peaks corresponding to TiO2. Peaks located in the area of 400 - 650 cm−1 correspond to the vibration of Ti-O and Ti-O-O bonds [16]. It is also clear from data of Figure 3 the presence of peak at 2900 cm−1 corresponds to –CH2 and at 1500 cm−1 corresponds to C-O-C due to organic moiety of Triton X- 100.
Figure 4 shows the top view SEM image of clusters of the film. The Triton X-100 influenced clusters have a uniform size and consist of well defined spherical TiO2 particles. The clusters composed of these particles tended to fuse together to form large aggregates.
The BET surface areas were 31.1 and 44.6 m2∙g−1 for the TiO2 powders (without and with Triton) and TiO2 formed with Triton, respectively. This increase in surface area was further evidenced by the observation that the amount of dye desorbed from the Triton influenced TiO2 film surface higher than that from the film without the Triton. This observation is consistent with the observation derived from XRD pattern that Triton facilitates
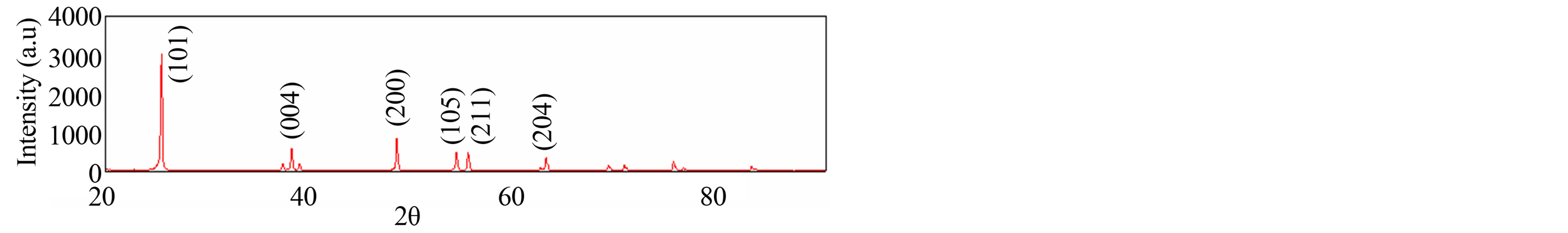
Figure 2. X-ray diffraction patterns of TiO2 electrode.
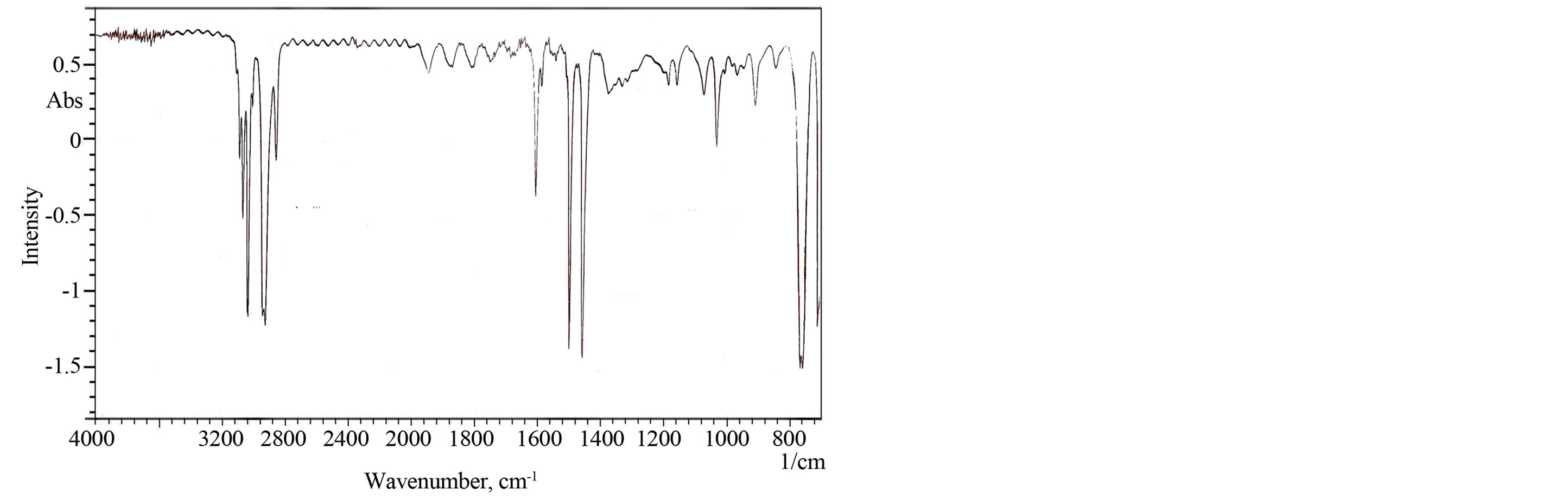
Figure 3. FTIR spectra of TiO2.

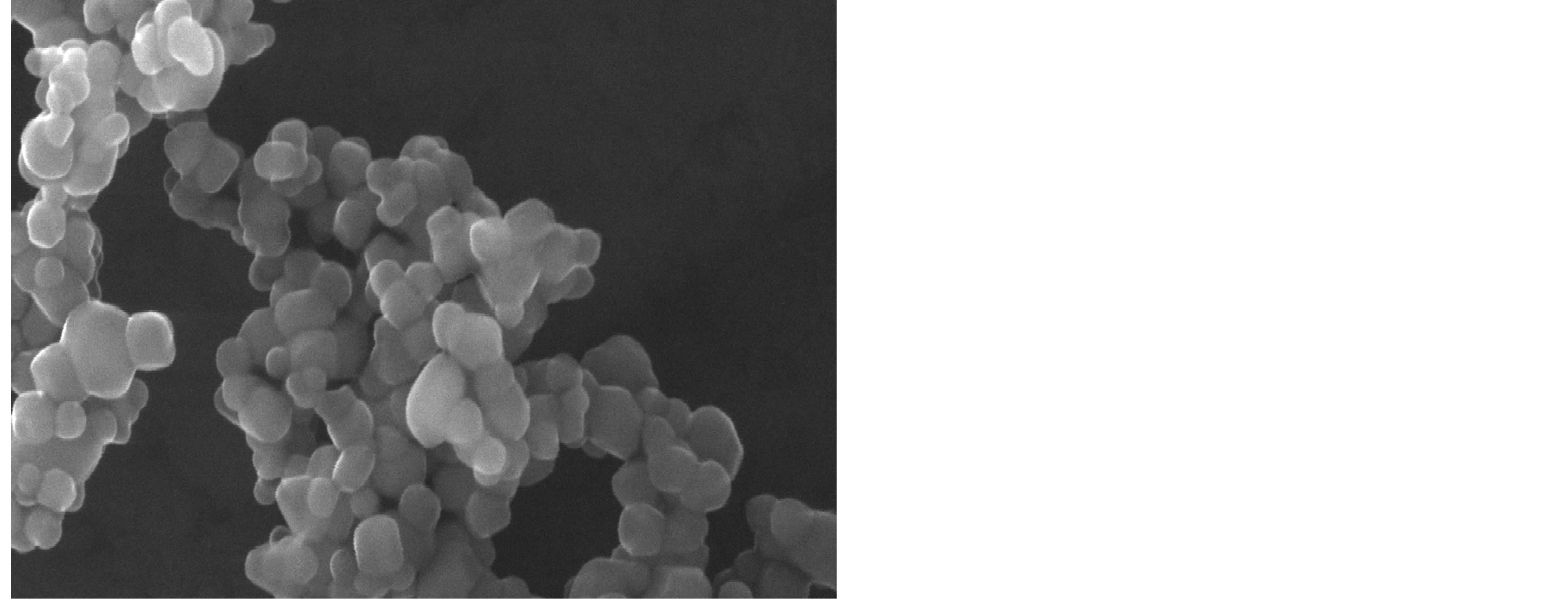
Figure 4. SEM images of the prepared TiO2.
smaller TiO2 particles.
Form the above results; we expected an enhanced performance of a DSSC fabricated with a Triton-influenced TiO2 film. The rationale behind this expectation lies in the regular structure of the film, composed of spherical particles and clusters. Figure 5 and Table 1 present the photocurrent-voltage (I - V) curves and photovoltaic performance data of DSCs fabricated with the TiO2 film electrodes in the presence and absence of Triton. The DSSC fabricated using the Triton-influenced TiO2 film shows a higher short-circuit photocurrent (Isc), opencircuit voltage (Voc) and solar conversion efficiency than that prepared without Triton. The increase in Isc may be
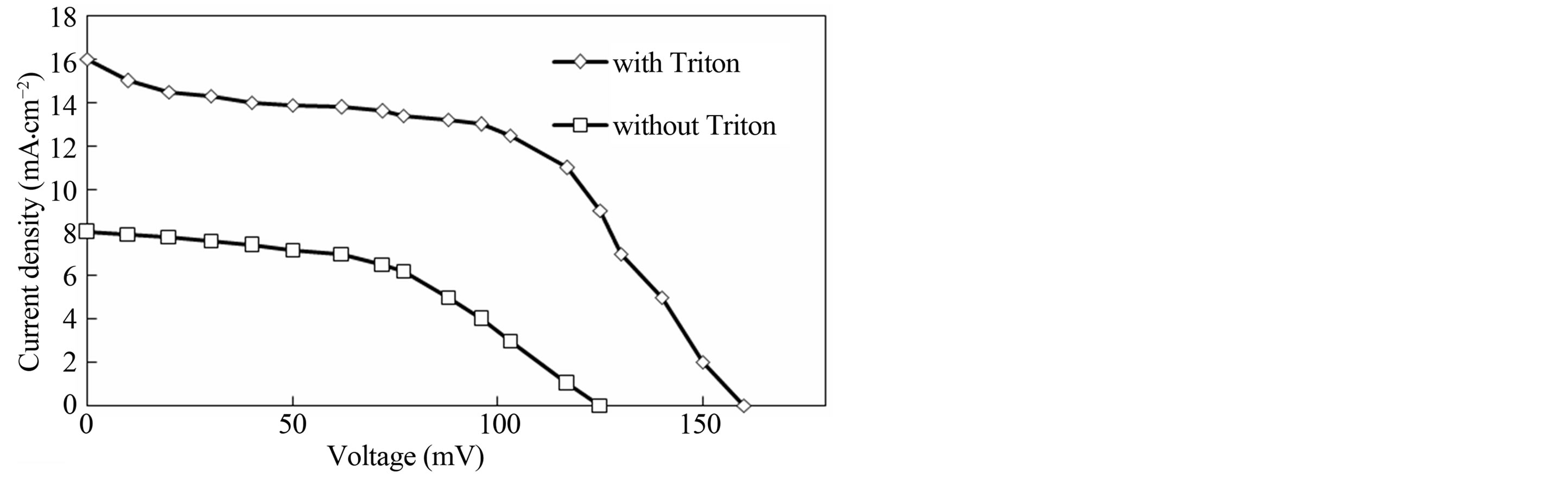
Figure 5. I - V curves of the DSSCs fabricated with TiO2 films and orange IV dye.
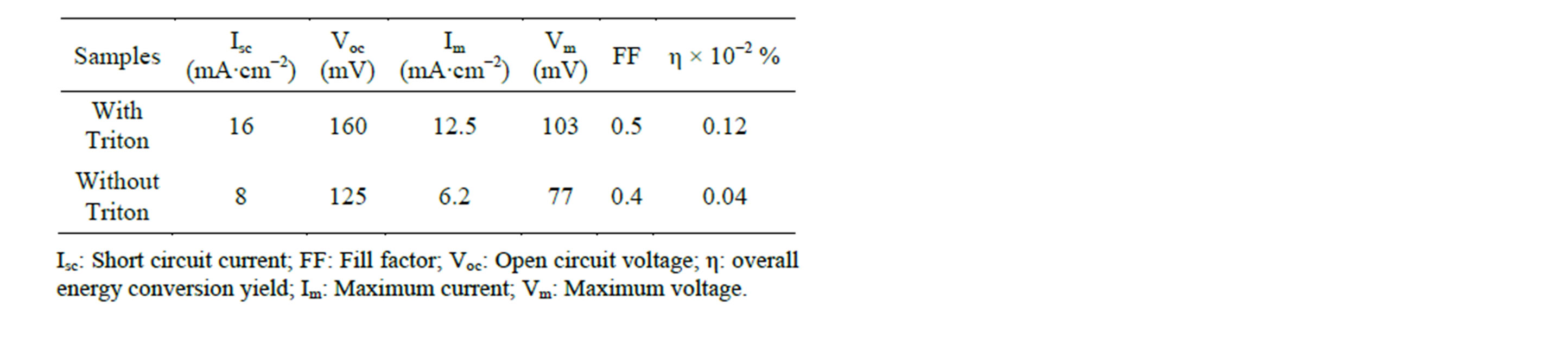
Table 1. Photovoltaic parameters of Orange IV dye-sensitized nanocrystalline TiO2 solar cells.
primarily related to the increased surface area. Furthermore, this increase in Isc can be related to an increase in the number of pathways due to the increased packing of round particles [14]. A more densely packed film improves inter-particle electrical contact. The observed increase in Voc might be due to an increase packing density of the TiO2 particles in the film. Increasing packing of small and round TiO2 particles produce small pores. The effective concentration of 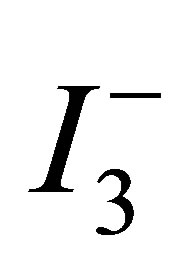 ions in the TiO2 film with smaller pores would be lesser than that in the film with larger pores, because the rate of
ions in the TiO2 film with smaller pores would be lesser than that in the film with larger pores, because the rate of 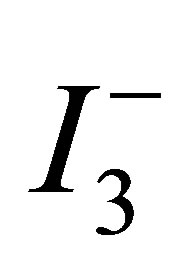 ion replenishment in the TiO2 film with smaller pores becomes slower. This slow replenishment of
ion replenishment in the TiO2 film with smaller pores becomes slower. This slow replenishment of 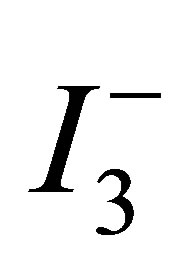 ions leads to the accumulation of photo-injected electrons in the TiO2 conduction band in the open-circuit condition, which in turn leads to an increase in the Voc.
ions leads to the accumulation of photo-injected electrons in the TiO2 conduction band in the open-circuit condition, which in turn leads to an increase in the Voc.
4. Conclusion
Spherical TiO2 nanoparticles were obtained using Triton X-100 in a hydrolyzing of TiCl4 solution. XRD results reveal that Triton generally suppresses the formation of anatase phase of TiO2. The DSSC prepared with TiO2 film using Orange IV as sensitizer showed improved Isc, and Voc as well as substantially improved solar energy conversion efficiency, compared with those of a cell fabricated with TiO2 film obtained without Triton.
Acknowledgements
The authors wish to thank Research Units in Science College at University of Dammam for their support and cooperation through this work.
REFERENCES
- B. O’Reagan and M. Gràtzel, “A Low-Cost, High-Efficiency Solar Cell Based on Dye Sensitized Colloidal TiO2 Films,” Nature, Vol. 353, No. 6346, 1991, pp. 737-740. http://dx.doi.org/10.1038/353737a0
- C. Y. Chen, M. Wang, J. Y. Li, N. Pootrakulchote, L. Alibabaei, C. H. Ngoc-le, J. D. Decoppet, J. H. Tsai, C. Gratzel, C. G. Wu, S. M. Zakeeruddin and M. Gratzel, “Highly Efficient Light-Harvesting Ruthenium Sensitizer for Thin-Film Dye-Sensitized Solar Cells,” ACS NANO, Vol. 3, No. 10, 2009, pp. 3103-3109. http://dx.doi.org/10.1021/nn900756s
- E. V. A. Premalala, N. Dematagea, G. R. A. Kumarab, R. M. G. Rajapakseb, K. Murakamib and A. Konno, “Shorter Nanotubes and Finer Nanoparticles of TiO2 for Increased Performance in Dye-Sensitized Solar Cells,” Electrochimica Acta, Vol. 63, 2012, pp. 375-380. http://dx.doi.org/10.1016/j.electacta.2011.12.127
- M. R. Mohammadi, R. R. M. Louca, D. J. Fray and M. E. Welland, “Dye-Sensitized Solar Cells Based on a Single Layer Deposition of TiO2 from a New Formulation Paste and Their Photovoltaic Performance,” Solar Energy, Vol. 86, No. 9, 2012, pp. 2654-2664. http://dx.doi.org/10.1016/j.solener.2012.06.005
- L. Kavan, M. Gratzel, J. Rathousky and A. Zukal, “Nanocrystalline TiO2 (Anatase) Electrodes: Surface Morphology, Adsorption, and Electrochemical Properties,” Journal of the Electrochemical Society, Vol. 143, No. 2, 1999, pp. 394-400. http://dx.doi.org/10.1149/1.1836455
- K.-H. Jung, J. S. Hong, R. Vittal and K.-J. Kim, “Enhanced Photocurrent of Dye-Sensitized Solar Cells by Modification of TiO2 with Carbon Nanotubes,” Chemistry Letters, Vol. 8, 2000, pp. 864-865.
- N. Papageorgiou, C. Barbe and M. Gratzel, “Morphology and Adsorbate Dependence of Ionic Transport in Dye Sensitized Mesoporous TiO2 Films,” The Journal of Physical Chemistry B, Vol. 102, No. 21, 1998, pp. 4156- 4164. http://dx.doi.org/10.1021/jp980819n
- C. J. Barbe´, F. Arendse, P. Comte, M. Jirousek, F. Lenzmann, V. Shklover and M. Gratzel, “Nanocrystalline Titanium Oxide Electrodes for Photovoltaic Applications,” Journal of the American Ceramic Society, Vol. 80, No., 1997, pp. 3157-3171. http://dx.doi.org/10.1111/j.1151-2916.1997.tb03245.x
- D. M. Antonelli and J. Y. Ying, “Synthesis of Hexagonally Packed Mesoporous TiO2 by a Modified Sol-Gel Method,” Angewandte Chemie International Edition, Vol. 34, No., 1995, pp. 2014-2017. http://dx.doi.org/10.1002/anie.199520141
- H.-S. Yun, K. Miyazawa, H. Zhou, I. Honma, M. Kuwabara, “Synthesis of Mesoporous Thin TiO2 Films with Hexagonal Pore Structures Using Triblock Copolymer Templates,” Advanced Materials, Vol. 13, No. 18, 2001, pp. 1377-1380. http://dx.doi.org/10.1002/1521-4095(200109)13:18<1377::AID-ADMA1377>3.0.CO;2-T
- M. Adachi, Y. Murata, I. Okada and S. Yoshikawa, “Formation of Titania Nanotubes and Applications for DyeSensitized Solar Cells,” Journal of The Electrochemical Society, Vol. 150, No. 8, 2003, pp. G488-G493. http://dx.doi.org/10.1149/1.1589763
- S. Kobayashi, K. Hanabusa, N. Hamasaki, M. Kimura and H. Shirai, “Preparation of TiO2 Hollow-Fibers Using Supramolecular Assemblies,” Chemistry of Materials, Vol. 12, No. 6, 2000, pp. 1523-1525. http://dx.doi.org/10.1021/cm0000907
- L. Kavan, J. Rathousky´, M. Gra¨tzel, V. Shklover, A. Zukal, “Surfactant-Templated TiO2 (anatase): Characteristic Features of Lithium Insertion Electrochemistry in Organized Nanostructures,” The Journal of Physical Chemistry B, Vol. 104, No. 50, 2000, pp. 12012-12020. http://dx.doi.org/10.1021/jp003609v
- D.-U. Lee, S.-R. Jang, R. Vittal, J. Lee and K.-J. Kim, “CTAB Facilitated Spherical Rutile TiO2 Particles and Their Advantage in a Dye-Sensitized Solar Cell,” Solar Energy, Vol. 82, No. 11, 2008, pp. 1042-1048. http://dx.doi.org/10.1016/j.solener.2008.04.006
- M. Ristic, M. Ivanda, S. Popvić and S. Musić, “Dependence of Nanocrystalline SnO2 Paticle Size on Synthesis Route,” Journal of Non-Crystalline Solids, Vol. 303, No. 2, 2002, pp. 270-280. http://dx.doi.org/10.1016/S0022-3093(02)00944-4
- E. Stathatos, Y. J. Chen and D. D. Dionysiou, “QuasiSolid-State Dye-Sensitized Solar Cells Employing Nanocrystalline TiO2 Films Made at Low Temperature,” Solar Energy Materials & Solar Cells, Vol. 92, No. 11, 2008, pp. 1358-1365. http://dx.doi.org/10.1016/j.solmat.2008.05.009
NOTES
*Corresponding author.

