Advances in Materials Physics and Chemistry
Vol. 2 No. 1 (2012) , Article ID: 17893 , 6 pages DOI:10.4236/ampc.2012.21003
TPR Study of Core-Shell Fe@Fe3O4 Nanoparticles Supported on Activated Carbon and Carbon Nanotubes
L.V. Pisarzhevsky Institute of Physical Chemistry, The National Academy of Sciences of Ukraine, Kyiv, Ukraine
Email: *kalishyn.yevhen@gmail.com
Received January 24, 2012; revised February 25, 2012; accepted March 4, 2012
Keywords: Iron; Nanoparticles; Carbon Nanotubes; Temperature-Programmed Reduction
ABSTRACT
Core-shell nanoparticles Fe@Fe3O4 supported on activated carbon (AC) and carbon nanotubes (CNTs) have been studied by H2 temperature-programmed reduction (TPR). Nanoparticles with size of 6.5 nm were synthesized by iron(II) oleate thermal decomposition and were supported on activated carbon and carbon nanotubes by colloid deposition method. The nanoparticles Fe@Fe3O4 are characterized by TEM and IR. Reduction of nanoparticles on AC starts at 140˚C, whereas reduction of nanoparticles on CNTs starts at 200˚C. Moreover, gasification of CNTs with methane releasing starts at 450˚C, whereas gasification of AC is negligible at temperatures up to 800˚C. All these findings illustrate a strong difference in the interaction between nanoparticles and the support material for AC and CNTs.
1. Introduction
Iron containing solids are often used as commercially catalysts for various processes, particularly for FischerTropsch synthesis, ammonia synthesis, etc. [1-4]. Performance of these catalysts is affected by numerous factors, one of which is the nature and structure of the support materials. Mostly studies of iron catalysts have been performed with the metal supported on SiO2, Al2O3, MgO, zeolites, and carbon [5]. Particularly, carbon supported iron catalysts give high selectivities to olefins in the Fisher-Tropsh reaction [6].
Discovery of carbon nanotubes (CNTs), followed by extensive studies of their properties, has also resulted in highlighting their catalytic properties [5,7-12]. Particularly, comparison of the catalytic activity of metal catalysts supported on various oxides, amorphous carbon, and CNTs showed that catalytic performance is generally better for CNTs. For example, a CNT-supported platinum catalyst shows superior activity in catalytic oxidation of various organic compounds [5,10]. CNT-supported metals are active in hydrogen generation from ammonia [13].
The main advantages of CNT supports compared to activated carbon are the high purity of the material can avoid self-poisoning, specific metal-support interaction exists that can directly affect the catalytic activity and selectivity. Unfortunately, a lack of systematic compareson with activated carbon based catalytic systems has to be noted. Particularly, it was shown that Co/CNT catalyst generates about 99% of the activity for CO conversion at 250˚C and thermally stability that is superior to Co/AC [14]. The effect of CNTs as cobalt support on CO conversion, product selectivity, and olefin to paraffin ratio of Fisher-Tropsh synthesis was studied and compared with AC [15]. The results indicated C5+ selectivity enhancement was about 77% as compared to Co/AC.
In this paper a core-shell nanoparticles Fe@Fe3O4 supported on activated carbon (AC) and carbon nanotubes (CNTs) are studied by H2 temperature-programmed reduction (TPR). Nanoparticles with size of 6.5 nm were synthesized by iron(II) oleate thermal decomposition and were supported on AC and CNTs by colloid deposition method. The core of nanoparticles consists of elementary Fe and shell consists of Fe2O3 and Fe3O4 that follows from TEM and IR studies.
2. Experimental
All chemicals and solvent were of the highest purity available and were used as purchased without further purification or distillation.
2.1. Sample Preparation
Preparation of Fe@Fe3O4 nanoparticles colloidal solution was performed by a modification of procedure described earlier which is based on iron(II) oleate thermal decomposition [16]. To synthesize Fe@Fe3O4 nanoparticles, 10 mL of diphenyl ether was heated under Ar atmosphere at 100˚C and appropriate amount of Fe(oleate)2 was added into solution. After reflux for 2 h, the solution turned black, indicating that Fe nanoparticles were formed. The solution was then cooled to room temperature. Nanoparticles were separated and redispersed in hexane.
CNTs were synthesized by the catalytic decomposition of ethylene according to procedure described elsewhere [17].
Deposition of Fe@Fe3O4 nanoparticles on CNTs was performed by mixing appropriate amounts of CNTs and colloidal solution of nanoparticles dispersed in hexane under stirring. The obtained samples were dried in air. The obtained solid Fe@Fe3O4/CNT was found to contain 1.3% of Fe. Iron content was determined by oxygen titration method [18]. Synthesis of Fe@Fe3O4/AC and Fe@Fe3O4/ quartz was performed in a similar way. The obtained samples contained 1.0% of iron.
Deposition of Fe@Fe3O4 nanoparticles on quartz was performed for studying reduction properties of pure nanoparticles.
2.2. Sample Characterization
The average particle size was determined from transmission electron spectroscopy (TEM) images. TEM studies were carried out using PEM-125K (Selmi, Ukraine). Samples for TEM analisys were prepared by ultrasonic dispersion of catalysts in hexane, and the suspensions were dropped onto carbon-coated copper grid. At least 500 nanoparticles per sample were analyzed to determine their size and distribution.
The samples were characterized by temperature-programmed reduction (TPR) in flow system using 5% hydrogen/hellium mixture with 5˚C/min temperature ramp with a flow rate 50 mL/min. The samples were first pretreated in a helliun flow up to 350˚C and kept for 2 h to remove the adsorbed water, and then cooling to room temperature. The samples were heated from 100˚C to 800˚C.
3. Results and Discussion
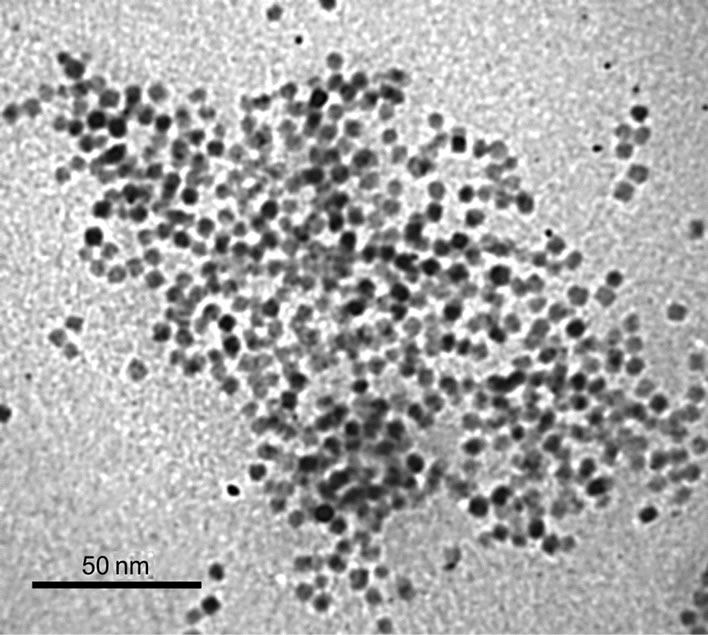
Figure 1(a) shows a typical TEM image of Fe@Fe3O4 nanoparticles from a colloid solution. The presented data indicate that the nanoparticles are almost spherical. The average diameter of prepared Fe@Fe3O4 nanoparticles is 6.5 nm. Corresponding size distribution of Fe@Fe3O4 nanoparticles is presented in Figure 1(b) showing that size distribution is almost gaussian with standard deviation σ = 0.6 nm. Therefore the synthesized Fe@Fe3O4 nanoparticles are characterized by narrow size distribution.
Figure 1(c) shows the representative electron diffracttion patterns of a sample formed from colloidal solution of Fe@Fe3O4 nanoparticles. Two different types of electron diffraction patterns could be detected depending on the position of the electron beam. The diffraction rings are attributed to iron oxides Fe3O4 and γ-Fe2O3 as well as to bcc-Fe.
Figure 1(d) gives IR spectrum of synthesized Fe@Fe3O4 nanoparticles in the range of 450 - 1000 cm–1. Outside this region peaks near 3400 сm–1 and 1650 сm–1 are observed. These bands correspond to stretching and deformation vibrations of OH groups. Peaks at 797 and 690 cm–1 correspond to deformation vibrations of the Fe-OH bond. Peak at 891 cm–1 may be attributed to vibrations of the Fe-O bond for FeO(OH) whereas peaks at 481 and 605 cm–1 correspond to vibrations of the Fe-O bond for Fe2O3. The IR data evidently indicate existence of the surface hydroxyl groups formed by the interaction of iron with water and oxygen.
TEM, electron diffraction and IR data allows one to conclude that the initially formed iron nanoparticles are partially oxidized due to contact with atmospheric oxygen and water and characterized by core-shell structure. Core of nanoparticle consists of elemental iron. The core is surrounded by iron oxide and the surface hydroxyl groups.
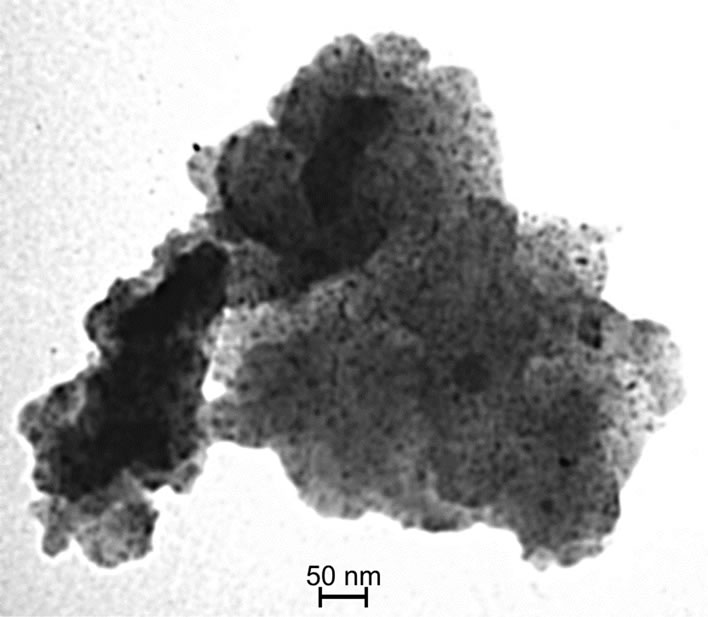
Figures 2(a) and (b) present TEM images of Fe@Fe3O4 nanoparticles deposited on AC and CNT, correspondingly. Obtained solids are marked as Fe@Fe3O4/AC and Fe@Fe3O4/CNT. Distribution of nanoparticles Fe@Fe3O4 over both supports is homogeneous as it follows from the data presented in Figure 2. For both supports the mean diameter of supported Fe@Fe3O4 nanoparticles is 6.7 nm with standard deviation σ = 0.6 nm. The size of supported nanoparticles is the same as in colloid solution. Therefore, there are no changes of nanoparticles morphology or chemical composition during their deposition.
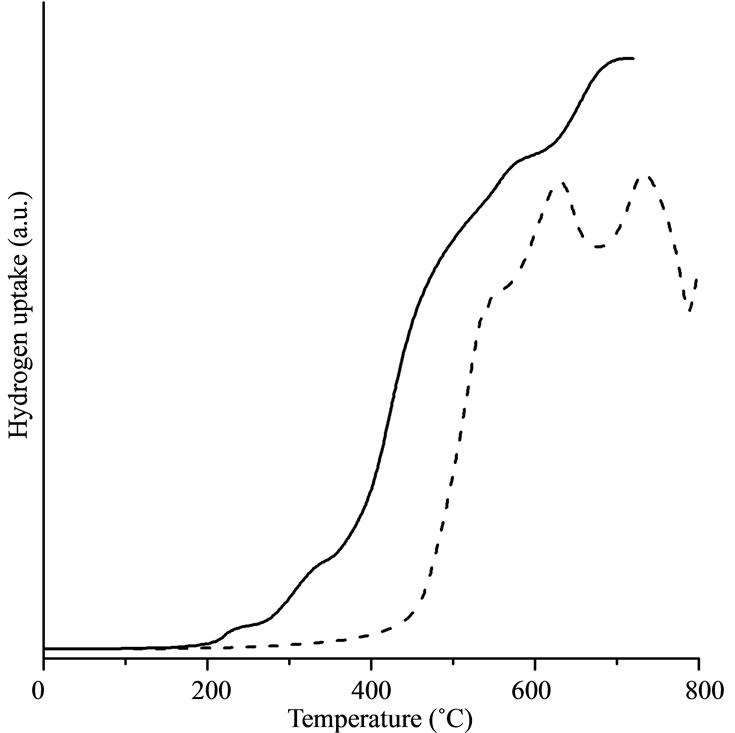
Figure 3 gives the H2-TPR curves as solid lines for Fe@Fe3O4/CNT (Figure 3(a)), Fe@Fe3O4/AC (Figure 3(b)), and Fe@Fe3O4/quartz (Figure 3(c)). For comparison the H2-TPR curves of pure CNTs and AC are presented in these figures by dashed lines.
Reduction of pure CNTs starts at 450˚C. It is characterized by two well-defined peaks which correspond to the gasification of the CNTs [3]. Methane was detected by mass spectrometry as a single product of gasification at temperatures above 550˚C. There are two peaks (at ca. 650˚C and ca. 750˚C) for the H2-TPR profiles for pure CNT. These peaks can be assigned for gasification started as transformation of outer and inner walls of CNT. Reduction of Fe@Fe3O4/CNT starts at 200˚C. Two small peaks near 300˚C may be attributed to the reduction of two different types of oxygen in the shell of nanoparticle. Methane is detected in products at temperatures above 450˚C. Therefore, in temperature range 200˚C - 450˚C only reduction of iron oxides takes place. At higher temperatures nanoparticles catalyze gasification of CNTs reducing temperature of the methane release to from 550˚C to 450˚C.
 (a)
(a)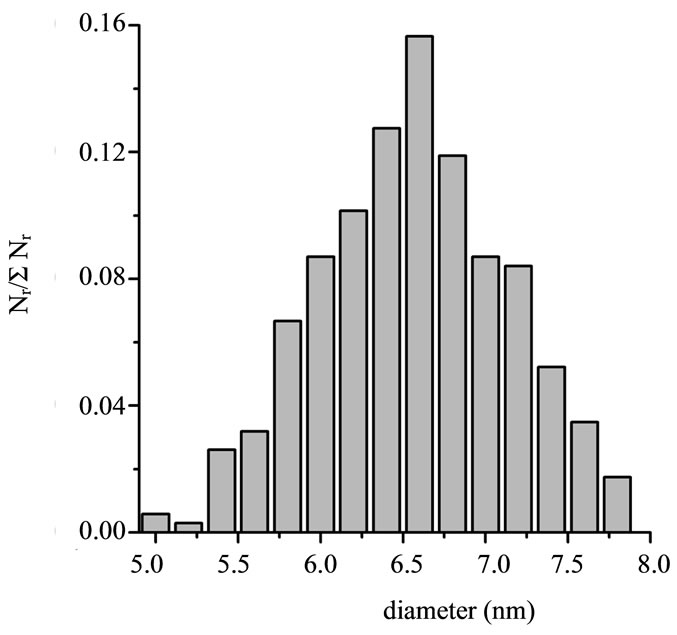 (b)
(b)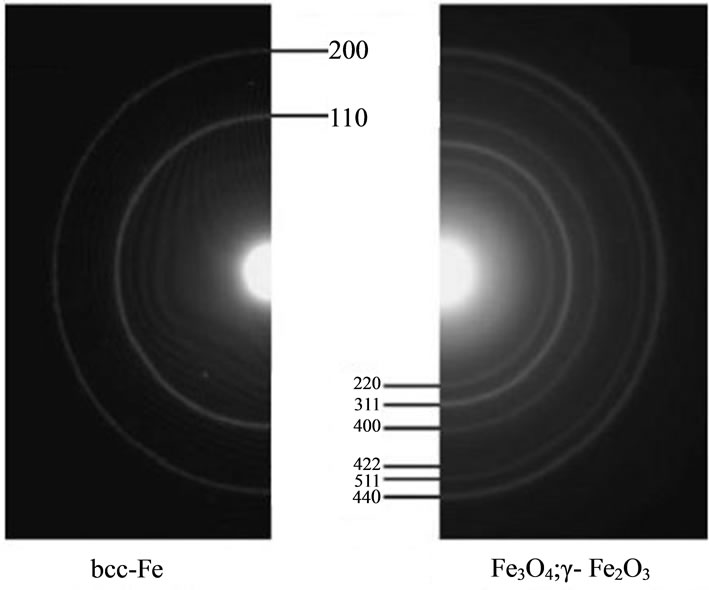 (c)
(c)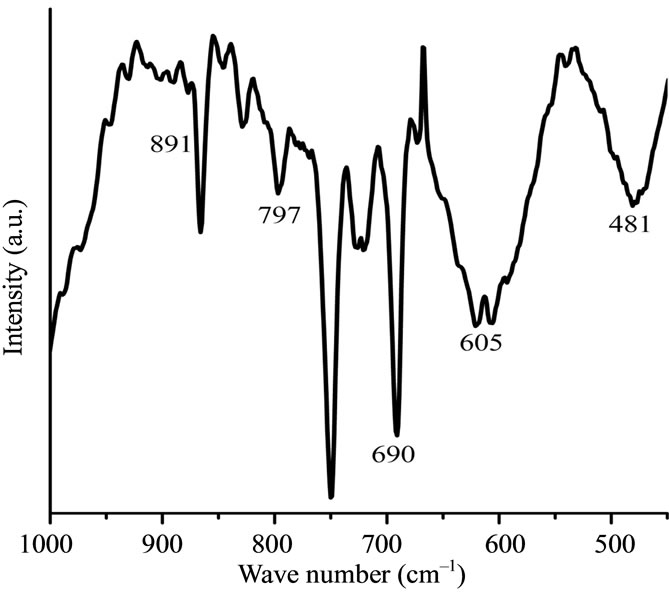 (d)
(d)
Figure 1. TEM images of Fe@Fe3O4 nanoparticles prepared from colloidal solution (a); Their distribution by size (b); Selected area electron diffraction patterns of Fe nanoparticles (c); IR spectrum of Fe@Fe3O4 nanoparticles (d).
 (a)
(a)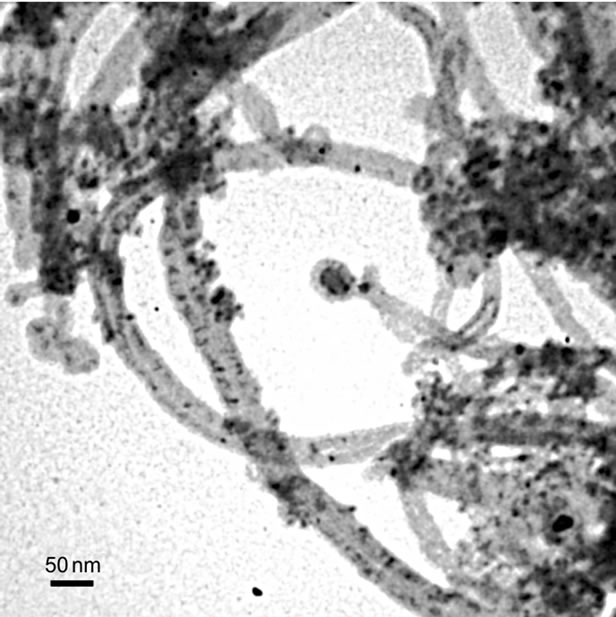 (b)
(b)
Figure 2. TEM images of Fe@Fe3O4 nanoparticles supported on AC (a) and CNTs (b).
 (a)
(a)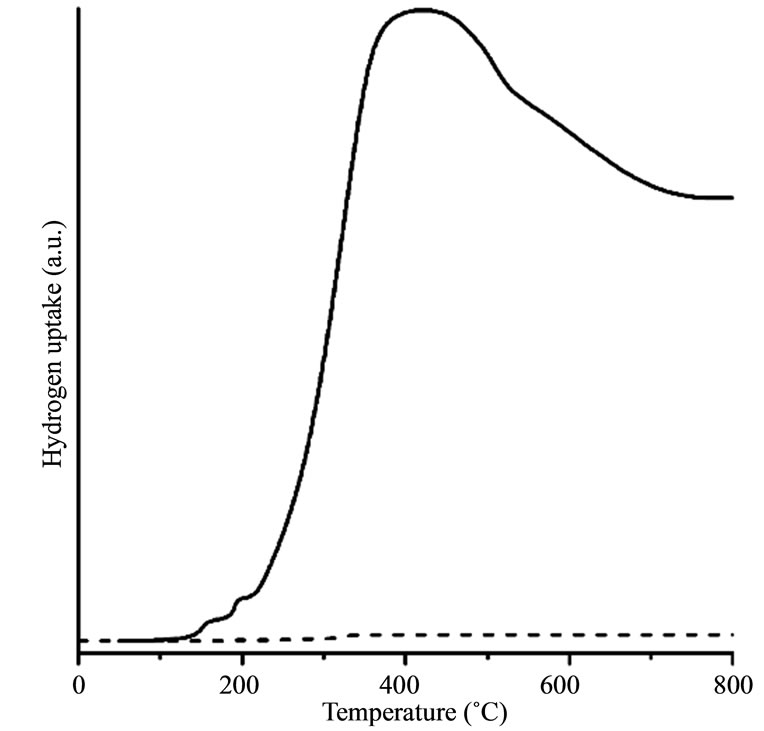 (b)
(b) (c)
(c)
Figure 3. H2-TPR profiles for (a) Fe@Fe3O4/CNT (solid line) and pure CNTs (dashed line); (b) Fe@Fe3O4/AC (solid line) and pure AC (dashed line); (c) Fe@Fe3O4/quartz.
Similar picture is observed for the reduction of Fe@Fe3O4 nanoparticles supported on AC at low temperatures. However, reduction starts at 140˚C. Contrary to CNTs no gasification appears for both AC and Fe@Fe3O4/AC and no methane formation was found. Therefore, broad peak at temperatures above 400˚C may be attributed to the reduction of the shell of Fe@Fe3O4 nanoparticles.
H2-TPR curves for pure Fe@Fe3O4 nanoparticles are presented in Figure 3(c). Reduction of Fe@Fe3O4 nanoparticles starts at 200˚C. It is characterized by four welldefined peaks at 250˚C, 400˚C, 550˚C, and 700˚C. First three peaks correspond to the stepwise reduction of iron oxides as: Fe2O3 → Fe3O4 → FeO → Fe [19,20]. The Fe2O3 → Fe3O4 peak at 250˚C demonstrates that γ-Fe2O3 exists in the nanoparticles shell. However, relatively low intensity of the Fe2O3 → Fe3O4 peak suggests the low fraction of Fe2O3 in the nanoparticles shell. Next reduction step Fe3O4 → FeO is observed at 400˚C. Peak at 550˚C may be considered for conversion of the iron oxide into metallic iron. The last peak is observed at 700˚C. It may be associated with reduction of Fe carbide [21]. Fe carbide is formed during the interaction between iron nanoparticles and organic surfactants that stabilize nanoparticles in colloidal solution.
The H2-TPR curves for pure CNT and AC supports are totally different. Such difference of reduction properties of these supports is expected and agreed with literature data [4,22,23]. It was found that CH4 production is not observed in the temperature until 1000˚C for activated carbon [22]. Contrary, methane production was detected for CNT gasification [4,23]. This difference is associated with chemical structure and properties of AC and CNT.
Comparison of reduction properties of Fe@Fe3O4/CNT and pure Fe@Fe3O4 shows similarity of reduction Fe@Fe3O4 nanoparticles. Reduction of Fe@Fe3O4/CNT gives three small peaks on the H2-TPR curves (Figure 3(a)). Temperature of reduction of Fe@Fe3O4/CNT corresponds to the pure nanoparticles. Therefore, in temperature range 200˚C - 450˚C only reduction of iron oxides takes place. At higher temperatures the reduction of supported nanoparticles and gasification of CNTs appears simultaneously.
Reduction properties of Fe@Fe3O4/AC and pure Fe@Fe3O4 are significantly differed. Reduction of Fe@Fe3O4/AC starts at 140˚C and one well-defined peak is observed at 400˚C. It indicates that total reduction of Fe@Fe3O4/AC occurs in one stage and may be attributed to the reduction of the shell of Fe@Fe3O4 nanoparticles.
Comparison of reduction properties of Fe@Fe3O4/CNT and Fe@Fe3O4/AC indicates a huge difference in an interaction between nanoparticles and support. No gasification appears for Fe@Fe3O4/AC whereas nanoparticles catalyze reduction of CNTs for Fe@Fe3O4/CNT. As a result, H2-TPR curve at temperatures above 400˚C for Fe@Fe3O4/CNT is caused by a superposition of both CNTs gasification with methane formation and reduction of the nanoparticles shell. Reduction of nanoparticles shell starts at lower temperatures for AC. Differences in gasification and shell reduction suggest strong interaction between nanoparticles and support for CNTs. Possibly, it is associated with existence of semiconducting zones in CNTs resulting in a formation of Schottky barrier between CNT and nanoparticle.
Strong interaction between nanoparticles and support for CNTs could be associated with particularities of CNT structure. Tubular morphology of the graphene layers governs difference in properties of CNT and other carbonaceous supports. Exterior surface of the CNT is electron-rich, whereas the interior surface is electron-deficient. That could influence metal and metal oxide particles in contact with CNT surface [24,25]. Interaction between electronic structure of CNT and metal or metal oxide nanoparticles may drastically change properties of these nanoparticles. As a result, that enhances reduction of nanoparticles supported on CNT. Moreover it was found that metal particles supported on CNT are more catalytically active in gasification process comparing with other carbonaceous supports [25].
4. Conclusions
In this study, nanoparticles with size of 6.5 nm were synthesized by iron(II) oleate thermal decomposition and were deposited on AC and CNTs by colloid deposition method. No agglomeration of nanoparticles on these supports was observed. TEM, electron diffraction and IR data allows one to conclude that the initially formed iron nanoparticles are partially oxidized due to contact with atmospheric oxygen and water and characterized by coreshell structure.
H2-TPR study of obtained solids has shown a difference of reduction properties for Fe@Fe3O4/CNT and Fe@Fe3O4/AC. Reduction of shell for Fe@Fe3O4/AC starts at 140˚C and no gasification of AC occurs at temperatures up to 800˚C. Contrary, for Fe@Fe3O4/CNT the reduction starts at 200˚C and gasification appears with methane formation. The difference in H2-TPR behavior for Fe@Fe3O4/CNT and Fe@Fe3O4/AC may indicate a possible difference in catalytic performance of these solids in various heterogeneous catalytic processes.
5. Acknowledgements
The work is supported by the grants of the National Academy of Sciences of Ukraine and the Ministry of Education and Science of Ukraine. Authors are thankful to A. I. Tripolsky for a help in conducting H2-TPR studies.
REFERENCES
- P. Li, D. Miser, S. Rabiei, S. Yadav and M. Hajaligol, “The Removal of Carbon Monoxide by Iron Oxide Nanoparticles,” Applied Catalysis B, Vol. 43, No. 2, 2003, pp. 151-162. doi:10.1016/S0926-3373(02)00297-7
- T. Rostovshchikova, V. Smirnov, O. Kiseleva, V. Yushcenko, M. Tzodikov, Yu. Maksimov, et al., “Acidic and Catalytic Properties of Silica Modified by Iron Oxide Nanoparticles,” Catalysis Today, Vol. 152, No. 1-4, 2010, pp. 48-53. doi:10.1016/j.cattod.2009.10.017
- M. Bahome, L. Jewel, D. Hildebrandt, D. Glasser and N. Coville, “Fischer-Tropsch Synthesis over Iron Catalysts Supported on Carbon Nanotubes,” Applied Catalysis A, Vol. 287, No. 1, 2005, pp. 60-67. doi:10.1016/j.apcata.2005.03.029
- L. Guczi, G. Stefler, O. Geszti, Zs. Koppany, Z. Kónya, É. Molnár, M. Urbán and I. Kiricsi, “CO Hydrogenation over Cobalt and Iron Catalysts Supported over Multiwall Carbon Nanotubes: Effect of Preparation,” Journal of Catalysis, Vol. 244, No. 1, 2006, pp. 24-32. doi:10.1016/j.jcat.2006.08.012
- J. Garcia, H. T. Gomes, P. Serp, P. Kalck, J. L. Figueiredo and J. L. Faria, “Platinum Catalysts Supported on MWNT for Catalytic Wet Air Oxidation of Nitrogen Containing Compounds,” Catalysis Today, Vol. 102-103, 2005, pp. 101-109. doi:10.1016/j.cattod.2005.02.013
- M. Dry, “The Fisher-Tropsh Synthesis,” In: J. R. Anderson and M. Boudart, Eds., Catalysis Science and Technology, Springer, New York, 1981, pp. 159-256.
- P. Serp, M. Corrias and P. Kalck, “Carbon Nanotubes and Nanofibers in Catalysis,” Applied Catalysis A, Vol. 253, No. 2, 2003, pp. 337-358. doi:10.1016/S0926-860X(03)00549-0
- H. Marsh, E. A. Heintz and F. Rodriquez-Reinoso, “Introduction to Carbon Technologies,” Universidad de Alicante, Alicante, 1997.
- F. Rodriguez-Reinoso, “The Role of Carbon Materials in Heterogeneous Catalysis,” Carbon, Vol. 36, No. 3, 1998, pp. 159-175. doi:10.1016/S0008-6223(97)00173-5
- H. T. Gomes, P. V. Samant, P. Serp, P. Kalck, J. L. Figueiredo and J. L Faria, “Carbon Nanotubes and Xerogels as Supports of Well-Dispersed Pt Catalysts for Environmental Applications,” Applied Catalysis B, Vol. 54, No. 3, 2004, pp. 175-182. doi:10.1016/j.apcatb.2004.06.009
- C. N. R. Rao, B. C. Satishkumar, A. Govindaraj and M. Nath, “Nanotubes,” ChemPhysChem, Vol. 2, No. 2, 2001, pp. 75-105. doi:10.1002/1439-7641(20010216)2:2<78::AID-CPHC78>3.0.CO;2-7
- B. Rajesh, K. Ravindranathan, J. M. Bonard, N. X. Xanthopoulos, H. J. Mathieu and B. Viswanathan, “Carbon Nanotubes Generated from Template Carbonization of Polyphenyl Acetylene as the Support for Electrooxidation of Methanol,” The Journal of Physical Chemistry B, Vol. 107, No. 12, 2003, pp. 2701-2708. doi:10.1021/jp0219350
- S.-F. Yin, Q.-H. Zhang, B.-Q. Xu, W.-X. Zhu, C.-F. Ng and C.-T. Au, “Investigation on the Catalysis of COx-Free Hydrogen Generation from Ammonia,” Journal of Catalysis, Vol. 224, No. 2, 2004, pp. 384-396. doi:10.1016/j.jcat.2004.03.008
- C.-Y. Lu and M.-Y. Wey, “The Performance of CNT as Catalyst Support on CO Oxidation at Low Temperature,” Fuel, Vol. 86, No. 7-8, 2007, pp. 1153-1161. doi:10.1016/j.fuel.2006.09.022
- M. Zaman, A. Khodadi and Y. Mortazavi, “Fischer-Tropsch Synthesis over Cobalt Dispersed on Carbon NanotubesBased Supports and Activated Carbon,” Fuel Processing Technology, Vol. 90, No. 10, 2007, pp. 1214-1219. doi:10.1016/j.fuproc.2009.05.026
- D. L. Lee, Y. H. Kim, X.-L. Zhang and Y. S. Kang, “Preparation of Monodisperse Co and Fe Nanoparticle Using Precursor of M2+-Oleate2 (M = Co, Fe),” Current Applied Physics, Vol. 6, No. 4, 2006, pp. 786-790. doi:10.1016/j.cap.2005.04.040
- V. O. Khavrus, N. V. Lemesh, S. V. Gordijchuk, A. I. Tripolsky, T. S. Ivashchenko, M. M. Biliy and P. E. Strizhak, “Chemical Catalytic Vapor Deposition (CCVD) Synthesis of Carbon Nanotubes by Decomposition of Ethylene on Metal (Ni, Co, Fe) Nanoparticles,” Reaction Kinetics and Catalysis Letters, Vol. 93, No. 2, 2008, pp. 295-303. doi:10.1007/s11144-008-5225-6
- S. Storsæter, B. Tøtdal, J. C. Walmsley, B. Steinar Tanem and A. Holmen, “Characterization of Alumina-, Silica-, and Titania-Supported Cobalt Fischer-Tropsch Catalysts,” Journal of Catalysis, Vol. 236, No. 1, 2005, pp. 139-152.
- Y. Wu, H. Yu, F. Peng and H. Wang, “Facile Synthesis of Porous Hollow Iron Oxide Nanoparticles Supported on Carbon Nanotubes,” Materials Letters, Vol. 67, No. 1, 2012, pp. 245-247. doi:10.1016/j.matlet.2011.09.097
- W. K. Jozwiak, E. Kaczmarek, T. P. Maniecki, W. Ignaczak and W. Maniukiewicz, “Reduction Behavior of Iron Oxides in Hydrogen and Carbon Monoxide Atmospheres,” Applied Catalysis A, Vol. 326, No. 1, 2007, pp. 17-27. doi:10.1016/j.apcata.2007.03.021
- B. H. Davis, “Fischer-Tropsch Synthesis Reaction Mechanisms for Iron Catalysts,” Catalysis Today, Vol. 141, No. 1-2, 2009, pp. 25-33. doi:10.1016/j.cattod.2008.03.005
- M. C. Román-Martínez, D. Cazorla-Amorós, A. LinaresSolano and C. Salinas-Martínez de Lecea, “TPD and TPR Characterization of Carbonaceous Supports and Pt/C Catalysts,” Carbon, Vol. 31, No. 6, 1993, pp. 895-902. doi:10.1016/0008-6223(93)90190-L
- M. Trepanier, A. K. Dalai and N. Abatzoglou, “Synthesis of CNT-Supported Cobalt Nanoparticle Catalysts Using a Microemulsion Technique: Role of Nanoparticle Size on Reducibility, Activity and Selectivity in Fischer-Tropsch Reactions,” Applied Catalysis A, Vol. 374, No. 1-2, 2010, pp. 79-86.
- A. Tavasoli, K. Sadagiani, F. Khorashe, A. A. Seifkordi, A. A. Rohani and A. Nakhaeipour, “Cobalt Supported on Carbon Nanotubes—A Promising Novel Fischer-Tropsch Synthesis Catalyst,” Fuel Processing Technology, Vol. 89, No. 5, 2008, pp. 491-498. doi:10.1016/j.fuproc.2007.09.008
- W. Chen, Z. Fan, X. Pan and X. Bao, “Effect of Confinement in Carbon Nanotubes on the Activity of FischerTropsh Iron Catalysts,” Journal of the American Chemical Society, Vol. 130, No. 29, 2008, pp. 9414-9419. doi:10.1021/ja8008192
NOTES
*Corresponding author.

