Open Journal of Marine Science
Vol.07 No.02(2017), Article ID:75491,13 pages
10.4236/ojms.2017.72018
Study of Toxicology Effects of Herbicide Paraquat on Heamatological Parameters of Mesopotamichthys sharpeyi
Rahin Alsadat Hashemi1, Yaghoub Jaddi2, Mohammad Ali Sadeghi3, Samane Ghiamati4, Msuod Motazedi5
1College of Natural Resource, Islamic Azad University, Rasht Unit, Rasht, Iran
2Municipal Maklavan, College of Marine Science, Khorramshahr Marine Science and Technology University, Khorramshahr, Iran
3Master of Geopolitical-Mayor Maklavan, Iran
4Master of Management-Administration Officer, Iran
5Expert Municipal Utilities Valley City of Ilam, Islamic Azad University of Bushehr, Iran

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: January 6, 2017; Accepted: April 16, 2017; Published: April 19, 2017
ABSTRACT
Pesticides are environmental contaminants that after use in agriculture, aquatic ecosystems are entered. These contaminants can enter the food chain and can cause problems for aquatic organisms and even humans. During the acute toxicity of this herbicide paraquat on some blood parameters, Mesopotamichthys sharpeyi (the total number of red blood cells, hemoglobin concentration, and total white blood cell count, hematocrit and blood indices) in fisheries laboratories, Khorramshahr University of Marine Science and Technology were studied. The sub lethal toxicity studies of 40 fish in 4 treated on the basis of three different treatments LC50 96 h (25, 50 and 75 percent LC50 96 h), and a control treatment for 96 hours were exposed to the pesticides paraquat. 10 fish were randomly distributed into plastic containers 25 liters. Tests based on standard methods O.E.C.D were on the fish and the static. The number of red blood cells, hemoglobin concentration, percent hematocrit, white blood cell count, MCV, MCH and MCHC Mesopotamichthys sharpeyi at different concentrations after 96 h with the control treatment significantly reduced (0.05 > P) that was directly affected by different concentrations of the paraquat.
Keywords:
Sub Lethal Acute, Paraquat, Heamatological, Biochemical, Mesopotamichthys

1. Introduction
Nowadays, pollution of natural ecosystems is increasing so that with increasing human activities is becoming a fundamental problem. Determination of the toxic compounds in aquatic environments and their effects on aquatic organisms are a fundamental issue in ecotoxicology science. Toxic substances present in the environment can be determined by chemical analysis, but its effects on aquatic organisms in aquatic ecosystems cannot be determined by chemical analysis. Hence, to assess the environmental impact of toxic compounds, the use of mortality or bioassay experiments is necessary [1] . Water quality is rapidly declining due to the rapid growth of industries, various applications of chemical fertilizers and the use of pesticides. One of the main causes of pollution of aquatic ecosystems is agricultural pesticide, which is used to deal with pests, weeds and agricul- tural diseases and they have adverse effects on the environment [2] . Within a few weeks of using these pesticides in agricultural activities, they entered to aquatic ecosystems through surface runoff and subsurface drainage. 40% of the world’s productions of pesticides are related to herbicides. Herbicides are generally used by farmers to control weeds, remove aquatic plants in rivers, lakes and water reservoirs, which generally have harmful effects on aquatic animal health. Paraquat (1,1-dimethyl-1,4, 4 B Prydynyvm chloride) is a herbicide that is used to destroy the weeds in tropical regions and it enters to aquatic ecosystems [3] . Herbicide paraquat is used in different parts of Iran with different climatic conditions and different agricultural activities, and the proposed amount of this herbicide is three liters per hectare for sugarcane plantations [4] . Herbicide paraquat is toxic to fish and one of these species is Benni with the scientific name Mesopotamichthys sharpeyi [5] that is from the carp family and Barbinae genus. Benni fish is one of the commercial species of Khuzestan wetlands that is very important due to the relatively good growth, tolerance to adverse environmental conditions, high resistance to warm and stagnant waters with low oxygen levels and also high economic value of culture among other native fish [6] . Benni fish has gained popularity among residents of southern and southwestern of Khuzestan Province and can be considered as an important source of protein for them [7] . This species is distributed in limited areas of the world and its main habitats in Khuzestan Province are Horolazim and Shadegan wetlands. Shadegan wetland is threatened with the arrival of drainage of Sugarcane agro-industry companies and their components and also toxins wastewater of these units. The wetland is damaged in recent years under the influence of non-natural agents and entering of pollutants, including agricultural waste along with a variety of pesticides that threaten wetland wildlife [8] . Fish directly associate with the aquatic environment, so the incidence of physical and chemical changes in the aquatic environment quickly leads to measurable physiological changes in fish [9] . In recent years, the incidence of mortality due to pesticides, industrial effluents and sewage contamination has been reported in Iran [10] . One of the most important and reliable indicators for the health assessment and fish physiology is measurement of blood parameters which are influenced by the nutritional and environmental factors. Blood characteristics of fish are one of the most important evidences of their physiological processes and reflect the relationship between aquatic ecosystem characteristics and their health status [11] [12] . For this reason having a normal range of fish blood parameters can be used as a bio-in- dicator. Changes of blood properties in response to environmental conditions are a response to environmental stress and can be considered as an important bio-indicator. Using of hematology methods is expanding in aquaculture activities, particularly is important in toxicology researches, environmental monitoring and evaluation of aquatic animal health [13] . There are few reports about the effects of herbicide Paraquat on the health status of fish. [14] reported a decrease in the growth of rainbow trout in chronic poisoning with the herbicide Paraquat. [15] examined the toxic effects of various concentrations of the herbicide Paraquat on the liver of farmed rainbow trout. Also [16] studied the effects of herbicide Paraquat on the hematological parameters of African catfish and observed that these factors significantly reduced at non-lethal concentrations of Paraquat compared with the control group. The aim of this research was to study the effects of sublethal concentrations of the herbicide Paraquat on Benni hematological parameters including the white blood cells count (WBC), red blood cells count (RBC), hemoglobin concentration, hematocrit, the mean corpuscular volume (MCV), the mean corpuscular hemoglobin (MCH) and the mean corpuscular hemoglobin concentration (MCHC).
2. Materials and Methods
Fish (9.36 ± 0.56 g) were obtained from warm-water fish breeding and cultivation center of martyr Maleki, Ahvaz. 20% emulsion of Paraquat pesticide, made by Partonar Company, Iran was purchased from the shopping center pesticides. For adaptation to the environmental conditions, Fingerlings were kept for one week in tanks of cultivation center of Khorramshahr University of Marine Sci- ence and Technology. Water physico-chemical parameters such as pH, dissolved oxygen and temperature were measured and recorded on a daily basis by a por- table multimeter (WTW, Germany). During this time, in addition to proper aeration, fish feeding was performed by plate (0.02 of body weight per day). Feeding was stopped 24 hours before acute toxicity test [17] . [18] reported 1.49 mg∙L−1 for the LC50-96 h of herbicide Paraquat in Benni fish. Sublethal toxicity studies were conducted by distributing 40 juveniles in the three treatments (25, 50 and 75 percent LC50-96 h) and a control group and exposing them to the pesticide Paraquat for 96 hours. Ten fingerlings were randomly distributed into 25-liter plastic containers. Experiments were carried out based on standard method of O.E.C.D [19] and static technique on the Benni fish. Sampling was done after 96 hours of exposing. For hematological studies of experimental and control groups, ten fish were caught from each group and blood was sampled from the caudal vein using 1 cc syringes and blood samples were transferred to eppendorf tubes coated with heparin. Serum separation from blood cells by centrifuge (Labofuge 200, Sepatech Heraeus, Germany) was conducted at 3000 rpm for 10 minutes [20] . For counting of white blood cells, hemocytometer (neobar) slide was used and white blood cells were counted by blue bulb pipette (melangeur) [21] [22] . For differential count of white blood cells initially need to prepare blood smear slides. For this purpose, blood smears were prepared after blood collection. After fixation of blood smears with methanol, they stained by Giemsa solution. After performing these steps, blood smears were prepared for observation with a microscope [23] . The number of red blood cells was counted using a Neubauer slide after dilution of no-clotting blood by Hime solution [24] . The amount of hematocrit was mea- sured by microhematocrit [25] . Hemoglobin levels were determined by colorimetric method using a spectrophotometer at a wavelength of 560 nm [26] . The blood indices such as MCV, MCH and MCHC were measured by following for- mulas:
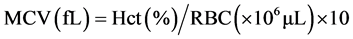
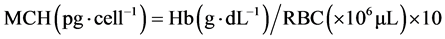
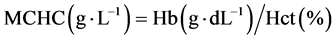
To measure the protein content of the plasma, the refractometer was used [27] . Automatic osmometers were used to measure serum osmolality. Glucose measurement was carried out by an enzymatic method using a spectrophotometer with a wavelength of 546 nm [28] . Data normality was investigated by normality test. One-way analysis of variance (ANOVA) was used to determine significant differences in normal data of blood parameters and in the case of non- normal data, Kruskal-Wallis test was used. Statistical analysis and graphs preparation was carried out through SPSS 16 and Excel 2010, respectively [29] .
3. Results
Water quality parameters including oxygen, temperature and pH were measured and recorded during the experimental period on a daily basis, which are indicated in Table 1.
Blood indices of Benni fish in three experimental groups and the control group are given in Table 2. The results of this study suggest that Benni blood pa- rameters are affected by herbicide Paraquat. All parameters measured in this experiment included the total number of red blood cells, hemoglobin concentration, the total number of white blood cells, hematocrit percent and blood indices such as MCV, MCH and MCHC significantly decreased in the experimental groups (25, 50 and 75 percent of 96 h LC50) compared to the control group (P < 0.05).
Different letters in each row indicates the presence of significant differences
Table 1. Values of physico-chemical parameters of water during experiment.
between the experimental groups (P < 0.05) (S.D. ± Mean). According to Table 3, white blood cell differential count in this study showed that the percentage of lymphocytes, eosinophils, monocytes and basophils in all treatments after 96 hours were less than the control group. After 96 hours of exposure to Paraquat, significant decrease in the percentage of lymphocytes was observed in different treatments (P < 0.05). In this study in addition to decrease in lymphocytes, percentage of eosinophils, monocytes and basophils in all treatments after 96 hours were less than the control group but the difference was not significant between treatments (P > 0.05). Blood neutrophil counts in this study showed that, unlike other white blood cells (lymphocytes, eosinophils, monocytes and basophils), the number of neutrophils had an increasing trend with increasing concentrations of Paraquat. Comparison between the percentages of blood neutrophils showed that after 96 hours of exposure to Paraquat, a significant increase was observed between the percentages of neutrophils (P < 0.05).
According to Table 4, total serum protein and serum osmolality of Benni fish in different concentrations after 96 hours of exposure to Paraquat are shown in
Table 2. The values of Benni blood parameters exposed to various concentrations 96 h LC50 of the herbicide Paraquat.
Table 3. Differential count of white blood cells of Benni fish exposed to different concentrations 96 h-LC50 of herbicide paraquat.
Table 4. Biochemical parameters of Benni fish exposed to various concentrations 96 h LC50 of herbicide paraquat.
Table 4, and comparison between treatments showed that serum total protein of Benni fish after 96 hours of exposure to paraquat, significantly decreased in all treatments (P < 0.05). According to the results it can be suggested that reduction in serum total protein can be seen with increasing concentrations of Paraquat in different treatments. Blood glucose levels of bream fish at different concentrations after 96 hours is given in Table 4. Comparison of average blood glucose level of Benni fish in different treatments showed that after 96 hours of exposure to Paraquat, a significant increase in blood glucose was observed at all treatments (P < 0.05). According to the results it can be stated that an increase in blood glucose levels can be seen by increasing the concentration of toxin in all treatments and it shows a direct correlation between blood glucose levels and increasing concentrations of Paraquat.
4. Discussion
Biochemical and Hematological parameters are used as indicators for evaluating physiological changes in the fish. Age, sex, nutritional status, environmental factors and stress are considered as known factors to cause changes in hematological parameters [30] . The aim of the measurement of blood parameters is that in recent years, this method is used as an easy and useful method to assess the effects of sublethal toxicity of herbicides on fish [31] . The results of this study showed that the herbicide paraquat led to stress effects on teleost fish such as Benni during the experimental period. In these cases, fish are very sensitive to pathogens and may be very vulnerable against predator creatures. White blood cells are the smaller number compared with red blood cells, and they have the defensive role in the body of organisms. As have been pinpointed from the obtained results, the number of white blood cells in all treatments after exposure to paraquat was lower than the control group and the significant reduction in the number of white blood cells in experimental treatments after 96 hours was observed compared with control group (P < 0.05). [32] studied acute toxicity effects of pesticide Endosulfan on blood parameters of tilapia, Oreochromis mossambicus and they reported that white blood cell count was significantly (P < 0.05) lower than the control group. In eel fish, Monopetrus albus exposed to Endosulfan was obtained similar results so that the amount of white blood cells in the control treatment was significantly different from other treatments [33] (P < 0.05). Changes in the levels of white blood cells following exposure to paraquat may be due to disturbances in the process of hematopoiesis and subsequent reduction or non-specific immune weakening is fish [34] . White blood cells include lymphocytes, neutrophils, eosinophils, monocytes and basophils, each of which plays a different role in the body of fish. The centrality of the specific defense mechanism is lymphocytes. Lymphocytes are seen in the blood circulation, lymphoid organs and other tissues, especially during inflammatory reactions. Lymphocytes are involved in the immune response of aquatic animals by antibody production (immunoglobulin). White blood cell differential count in this study showed that the percentages of lymphocytes, eosinophils, monocytes and basophils in all treatments after 96 hours were measured less than the control group. According to results of this study and other researches, changes in white blood cell differential count after exposure to paraquat may be due to disruption in the process of the hematopoietic and subsequent reduction or suppression of non-specific immune in Benni fish and then body strength of fish exposed to Paraquat reduces and they are easily susceptible to pathogens [35] . Decreasing the percentage of lymphocytes, eosinophils, monocytes and basophils exposed to pesticides was reported by different researchers. [36] studied the effects of diazinon on blood parameters of stellate sturgeon, Acipenser stellatus. [37] investigated the effects of sublethal concentrations of diazinon on grass carp, Ctenopharyngodon idella and reported the significant increase (P < 0.05) in percentage of neutrophils and significant reduction (P > 0.05) in the percent of lymphocytes and monocytes compared with the control group. Other researches in this context can be noted to researches by [38] on common carp, [38] on [39] on Persian sturgeon. [40] also studied the impact of pesticide diazinon on some blood parameters of rainbow trout. Neutrophil count results in control and treatments group in 25, 50 and 75 percents of the LC50 show the highest mean at 50 percent. The results showed that there were statistically significant differences in neutrophils among the treatments and diazinon had had a significant effect on the blood parameters, in which an increasing trend at neutrophils levels was observed in 25% and 50% treatments but a significant reduction was seen in 75% treatment along with diazinon increasing. This study revealed that the number of red blood cells in all treatments after exposure to paraquat was less than the control group and a significant reduction in the number of red blood cells was observed at all treatments (P < 0.05). The results of this study are similar to the results [41] . Red blood cells play an important role in oxygen transportation in the body and an insufficient amount of red blood cells has the negative effect on the body of aquatic animals and reduces the total protein in blood plasma [42] measured the effect of herbicide Butachlor on blood parameters of perch fish. The results indicated a significant reduction in red blood cells compared with control group (P < 0.05). The number of red blood cells can have a significant effect on the total energy balance of the body. When the fish has less metabolic activity, a large number of red blood cells are not needed and their number continues to decline [43] . A significant reduction in the number of red blood cells indicates the severe anemia caused by herbicide paraquat in fish that have been exposed to the toxin. Anemia can be the answer to the destruction or inhibition of production of red blood cells, dilution of the blood (increase in plasma volume) and can also be caused by the destruction of intestinal cells by toxic substances [44] . Reducing the number of red blood cells may be because of inflation of red blood cells or lysis of blood that is probably due to the presence of protein-carbon IV oxide in the blood. It is also possible as a result of anemia which is probably due to the dilution of the blood caused by disorder of osmoregulation across the gill epithelium [45] . However, the decline in production of red blood cells in the kidney or dilution of blood due to dysfunction of osmoregulation across the epithelium may be the cause of reducing the number of red blood cells of Benni fish exposed to different concentrations of paraquat [12] . [46] by investigating malathion on Cyprinion wabosoni reported that toxins caused changes in the body of the fish, which reduced the activity of initial hematopoietic tissue and thus caused anemia in the fish. Hematocrit is used to measure the volume of red blood cells and expressed in terms of volume percentage of red blood cells to total blood volume. This test is used today as the separately simplest test for anemia [47] exposed to Paraquat which is less than the control group and a significant decrease is observed in the amount of hematocrit in all treatments with control group after 96 hours (P < 0.05). [48] studied the effect of herbicide Butachlor on some haematological parameters of Kutum (Rutilus frisii kutum Kamenski) and obtained results showed that hematocrit significantly decreased from 51.23 in control group to 33.96 in LC50 75% (P < 0.05). Reducing the hematocrit may be due to hemolysis of red blood cells which result in anemia in fish [49] . This study showed that hemoglobin in all treatments after exposure to paraquat was less than the control group and a significant decrease in the number of blood hemoglobin was observed in all treatments (P < 0.05). The researchers in this study reported hemoglobin reduction due to anemia caused by exposure to diazinon. Also the significant reduction (P < 0.05) of blood hemoglobin was reported in the study of acute toxicity of diazinon on hematological parameters of European catfish, Silurus glanis L. [50] . Reduction in hemoglobin may be due to the destructive effects of pesticides on tissues that produce hemoglobin. Its result is damage to hemoglobin. Regarding the role of hemoglobin in the transmission and distribution of dissolved oxygen to the tissues for metabolism, reduction in hemoglobin leads to respiratory disorders and lack of oxygen in Benni fish [51] .
According to the results of this research, the blood indices including MCV, MCH and MCHC of Benni exposed to paraquat were lower than the control group (P < 0.05). [52] studied the toxicity effects of pesticide Lindane on blood indices (MCV, MCH and MCHC) of common carp, Cyprinus carpio and the significant reduction (P < 0.05) of blood indices (MCV, MCH and MCHC) observed compared with the control group. [53] examined acute toxicity effects of pesticides Endosulfan in concentrations of 2 µg∙L−1, and more on hematological indices (MCV, MCH and MCHC) of Cichlasoma dimerus. The reduction in size and quantity of hemoglobin of red blood cells is measured by the indices MCV, MCH, MCHC which can be a sign of anemia in fish [18] . This anemia is resulting from the stress of bacterial infections (Silveira-Coffigny et al., 2004) and exposure to agricultural pesticides [25] . The presence of a large percentage of immature red blood cells in the bloodstream may be a reason for reduction of MCV and MCH. On the other side, reduction of MCHC in this study may be due to decreased production of hemoglobin after exposure to diazinon [30] . Ano- ther reason for reduction in MCHC is reduction in cell hemoglobin or hypochromic [27] . During the anemia, MCHC values reduced because large cells had less hemoglobin concentration [50] . MCHC reduction resulted from increased production and secretion of reticulocytes that had a larger size but less hemoglobin content compared to mature red blood cells (Lermen, 2004). Changes in serum biochemical parameters can be considered as a suitable factor for detection of sub-lethal toxic effects on target organs and physiological status of fish exposed to toxins [9] . Of major changes in blood biochemical parameters of Benni fish after exposure to paraquat can be noted to the significant reduction in total serum protein in blood serum. Total concentration of plasma proteins compared with the base is used as a clinical indicator in measuring the health, stress and body condition of aquatic organisms [19] . Change in protein synthesis is one of the most common responses to cellular damage, therefore, by measuring the amount of protein can be understood the amount of cell damage [11] . Given that most of the proteins are synthesized in the liver, protein reduction in blood plasma may be related to liver impairment of fish in the vicinity of pesticides. The significant decrease (P < 0.05) was observed in the blood total protein of European eel Anguilla Anguilla that had been exposed to pesticide Malathion [26] . [33] studied the effects of diazinon sublethal concentrations on serum total protein of Persian sturgeon (Acipenser persicus) fingerlings and concluded that with increasing concentration of toxin, a significant reduction could be seen in the amount of total protein at 24 and 96 hours of exposure to pesticide diazinon in comparison with control group (P < 0.05). [36] studied effects of sublethal concentrations of pesticide diazinon on some biochemical parameters of rainbow trout and reported that plasma total protein was significantly lower than the control group and its reason was expressed chronic liver disease. Decrease in plas- ma total protein may have effects on physiological activities of bream and suppresses the immune system, which may have negative effects on the life of this fish [44] .
Finally, after 96 hours of testing of biological effects of Paraquat on Benni fish, it was found that these herbicides had significant effects on hematological and biochemical parameters and could cause such risks in the growth and survival of these species.
Acknowledgements
We appreciate the staff and faculty of Fisheries and Marine Biology Department of Khorramshahr University of Marine Science and Technology which helped us in this project.
Cite this paper
Hashemi, R.A., Jaddi, Y., Sadeghi, M.A., Ghiamati, S. and Motazedi, M. (2017) Study of Toxicology Effects of Herbicide Paraquat on Heamatological Parameters of Mesopotamichthys sharpeyi. Open Journal of Marine Science, 7, 258-270. https://doi.org/10.4236/ojms.2017.72018
References
- 1. Bagheri, F. (2007) Study of Pesticide Residues (Diazinon, Azinphosmethyl) in the Rivers of Golestan Province (Gorganroud and Gharehsou). MSc Thesis, Tehran University of Medical Science, Tehran, Iran, 1-125.
- 2. Bahmani, M., Kazemi, R. and Donskaya, P. (2001) A Comparative Study of Some Hematological Features in Young Reared Sturgeon. Fish Physiology and Biochemistry, 24, 135-140.
https://doi.org/10.1023/A:1011911019155 - 3. Bahmani, M., Kazemi, R., Yousefi, A., Hallajian, A. and Mojazi Amiri, B. (2008) The final Project Report: Studying the Possibility of Artificial Reproduction of Farmed Stellate Sturgeon (Broodstock, Artificial Reproduction and Production of Fingerlings from Farmed Sturgeon Broodstocks). Iranian Fisheries Research Organization Press, 132 p.
- 4. Banaee, M., Mirvagefei, R., Rafei, G. and Majazi Amiri, B. (2008) Effect of Sub-Lethal Diazinon Concentrations on Blood Plasma Biochemistry. International Journal of Environmental Research, 2, 189-198.
- 5. French, B. and Kerper, D. (2004) Salinity Control as a Mitigation Strategy for Habitat Improvement of Impacted Stuaries. 7th Annual EPA Wetlands Workshop, NJ, USA.
- 6. Banaee, M., Sureda, A., Mirvaghefi, A.R. and Ahmadi, K. (2011) Effects of Diazinon on Biochemical Parameters of Blood in Rainbow Trout (Oncorhynchus mykiss). Pesticide Biochemistry and Physiology, 99, 1-6.
- 7. Basak Kahkesh, F., Salehi, H., Amiri, F. and Nikpey, M. (2011) Integrated Culture of Benni (Barbus sharpeyi, Günther, 1874) with Chinese Carp and Its Economic Comparison with Conventional Farming Methods. Fisheries Journal (Islamic Azad University, Azadshahr Branch), 4, 73-85.
- 8. Canli, M. (1996) Effects of Mercury, Chromium, and Nickel on Glycogen Reserves and Protein Levels in Tissues of Cyprinus carpio. Journal of Zoology, 20, 161-168.
- 9. Adhikari, S., Sarkar, B., Chatterjee, A., Mahapatra, C.T. and Ayyappan, S. (2004) Effects of Cypermethrin and Carbofuran on Certain Hematological Parameters and Prediction of Their Recovery in a Freshwater Teleost, Labeo rohita (Hamilton). Ecotoxicology and Environmental Safety, 58, 220-226.
- 10. Agha Mohammadi, M., Farrokhi, F. and Tokmechi, A. (2012) Study the Toxic Effects of Various Concentrations of the Herbicide Paraquat on Liver Tissue of Farmed Rainbow Trout.
- 11. Osman, A.G.M. and Kloas, W. (2010) Water Quality and Heavy Metal Monitoring in Water, Sediments, and Tissues of the African Catfish Clarias gariepinus (Burchell, 1822) from the River Nile, Egypt. Journal of Environmental Protection, 1, 389-400.
- 12. Atamanalp, M. and Yanik, T. (2003) Alternations in Hematological Parameters of Rainbow Trout (Oncorhynchus mykiss) Exposed to Mancozeb. Turkish Journal of Veterinary & Animal Sciences, 27, 1213-1217.
- 13. Verma, P.S. and Agarwal, V.K. (2007) Environmental Biology: Principles of Ecology. 11th Reprinted Edition. S. Chand & Co. Ltd., India.
- 14. El-Shebly, A.A. and El-Kady, M.A.H. (2008) Effects of Glyphosate Herbicide on Serum Growth Hormone (GH) Levels and Muscle Protein Content in Nile Tilapia (Oreochromis niloticus L.). Research Journal of Fisheries and Hydrobiology, 3, 84-88.
- 15. Evans, G.O. (2009) Animal Hematotoxicology. CRC Press, Bocarton, 204 p.
- 16. Fanouraki, E., Divanach, P. and Pavlidis, M. (2007) Baseline Values for Acute and Chronic Stress Indicators in Sexually Immature Red Porgy (Pagrus pagrus). Aquaculture Research, 265, 294-304.
- 17. Farrokhrooz, M., Ghasemi Nejad, A., Falakru, K., Fahim, M. and Rahimi Bashar, M. (2011) The Effect of Herbicide Butachlor on Some Haematological Parameters in Kutum (Rutilus frisii kutum Kamenskii 1901). Journal of Biological Sciences (Lahijan Branch), 4, 57-65.
- 18. Feiz, S. (2010) Effect of Herbicide Butachlor on Blood Parameters of Pikeperch. MSC Thesis, Islamic Azad University, Lahijan Branch, 110 p.
- 19. Feldman, B.F., Zinkl, J.G. and Jain, N.C. (2000) Schalm’s Veterinary Hematology. 5th Edition, Lippincott Williams & Wilkins, 1120-1124.
- 20. Gbore, F.A., Oginni, O., Adewole, A.M. and Aladetan, J.O. (2006) The Effect of Transportation and Handling Stress on Haematology and Plasma Biochemistry in Fingerlings of Clarias gariepinus and Tilapia zillii. World Journal of Agricultural Science, 2, 208-212.
- 21. Groff, J.M. and Zinkl, J.G. (1999) Hematology and Clinical Chemistry of Cyprinid Fish Common Carp and Goldfish. Veterinary Clinics of North America: Exotic Animal Practice, 2, 741-776.
- 22. Da Cuna, R.H., Rey Vázquez, G., Piol, M.N., Guerrero, N.V., Maggese, M.C. and Lo Nostro, F.L. (2011) Assessment of the Acute Toxicity of the Organochlorine Pesticide Endosulfan in Cichlasoma dimerus (Teleostei, Perciformes). Ecotoxicology and Environmental Safety, 74, 1065-1073.
- 23. Lermen, C.L., Lappe, R., Crestani, M., Vieira, V.P., Gioda, C.R., Schetinger, M.R.C., Baldisseretto, B., Moraes, G. and Morsch, V.M. (2004) Effect of Different Temperature Regimes on Metabolic and Blood Parameters of Silver Catfish Rhamdia quelen. Aquaculture Research, 239, 497-507.
- 24. Mikula, P., Modra, H., Nemethova, D., Groch, L. and Svobodova, Z. (2008) Effects of Subchronic Exposure to LASSO MTX® (Alachlor 42% W/V) on Hematological Indices and Histology of the Common Carp, Cyprinus carpio L. Bulletin of Environmental Contamination and Toxicology, 81, 475-479.
https://doi.org/10.1007/s00128-008-9500-z - 25. Mohammadian, T., Kochanian, P., Niku, S., Sheikholeslami, M., Bita, S., Skandari, Gh. and Abhari Segonbad, H. (2010) Comparing the Effects of the GnRH Hormone Analogue Along with Anti-Dopamine Domperidone (Ova-Fact) by Linpe Method, with Common Carp Pituitary Extract (CPE) on Reproductive Indices of Benni (Bar-bus sharpeyi). Iranian Veterinary Journal, 2, 70-80.
- 26. Hashemi, R. (1394) Effects of Super Supplements on Growth, Immune and Osmotic System of Farmed Siberian Sturgeon (Acipenser baerii). MSc Thesis, Islamic Azad University, Rasht Branch, 86 p.
- 27. Hii, Y.S., Lee, M.Y. and Chuah, T.S. (2007) Acute Toxicity of Organochlorine Insecticide Endosulfan and Its Effect on Behaviour and Some Hematological Parameters of Asian. Pesticide Biochemistry and Physiology, 89, 46-53.
- 28. Houston, A.H. (1990) Blood and Circulation. In: Schreck, C.B. and Moyle, P.B., Eds., Methods in Fish Biology, American Fisheries Society, Bethesda, Maryland, 335 p.
- 29. Jadi, Y., Safahiye, A. and Salighe Zade, R. (2015) Determination of Lethal Range and Median Lethal Concentration (96h LC50) of Herbicide Paraquat on Benni Fish (Mesopotamichthys sharpeyi) Fingerlings. Journal of Aquaculture and Fisheries (Islamic Azad University, Bandar Abbas Branch), 5, 21-31.
- 30. Kazemi, R., Pour Dehghani, M., Yousefi Joordehi, A., Yarmohammadi, M. and Nasri Tajan, M. (2011) The Physiology of Circulatory System of Aquatic Animals and Physiology and Techniques of Fish Hematology. Bazargan Press, Rasht, 194 p.
- 31. Khajeh Pour, M., Golabkesh, Sh. and Ghiasy Khayat, M. (2011) Examines the Importance of Shadegan International Wetland (Values, Threats and Ways to Improve It). The National Conference of Wetlands and Their Role in Integrated Water Resources Management.
- 32. Koprucu, S.S., Koprucu, K., Ural, M.S., Ispir, U. and Pala, M. (2006) Acute Toxicity of Organophosphorous Pesticide Diazinon and Its Effects on Behavior and Some Hematological Parameters of Fingerling European Catfish (Silurus glanis L.). Pesticide Biochemistry and Physiology, 86, 99-105.
- 33. Kori-Siakpere, O., Adamu, K.M. and Madukelum, I.T. (2007) Acute Haematological Effect of Sublethal of Paraquat on the African Catfish, Clarias gariepinus (Osteichthyes: Clariidae). Research Journal of Environmental Sciences, 1, 331-335.
https://doi.org/10.3923/rjes.2007.331.335 - 34. Kumar, N., Antony, P., Pal, A.K., Remya, S., Aklakur, M., Rana, R.S., Gupta, S., Raman, R.P. and Jadhao, S.B. (2011) Anti-Oxidative and Immuno-Hematological Status of Tilapia (Oreochromis mossambicus) during Acute Toxicity Test of Endosulfan. Pesticide Biochemistry and Physiology, 99, 45-52.
- 35. Kumar, S., Sahu, N.P., Pal, A.K., Choudhury, D., Yengkokpam, S. and Mukherjee, S.C. (2005) Effect of Dietary Carbohydrate on Haematology, Respiratory Burst Activity and Histological Changes in L. rohita Juveniles. Fish and Shellfish Immunology, 19, 331-344.
- 36. Notes on the Herbicide Paraquat (2011) Research and Development Group (R & D), Plant Company, 80 p.
- 37. Nussey, G., Vanvuren, H.J. and Dupreez, H.H. (1995) Effect of Copper on the Differential White Blood Cell Counts of Mozambique tilapia (Oreochromis mossambicus). Comparative Biochemistry and Physiology, 111, 381-388.
- 38. Padash-Barmchi, Z., Safahieh, A., Bahmani, M., Savari, A. and Kazemi, R. (2010) Immune Responses and Behavior Alterations of Persian Sturgeon Fingerlings Acipenser persicus Exposed to Sublethal Concentrations of Diazinon. Toxicological and Environmental Chemistry, 92, 159-167.
https://doi.org/10.1080/02772240902927577 - 39. Patnaik, L. and Patra, A.K. (2006) Haematopoietic Alterations Induced by Carbaryl in Clarias batrachus (linn). Journal of Applied Sciences and Environmental Management, 10, 5-7.
- 40. Pottinger, T.G. and Carrick, T.R. (2001) A Comparison of Plasma Glucose and Plasma Cortisol as Selection Markers for High and Low Stress-Responsiveness in Female Rainbow Trout. Aquaculture, 175, 351-363.
- 41. Pourgholam, R., Soltani, M., Hassan, M.D., Ghoroghi, A., Nahavandi, R. and Pourgholam, H. (2006) Determination of Diazinon LC50 in Grass Carp (Ctenopharyngodon idella) and the Effect of Sublethal Concentration of Toxin on Some Hematological and Biochemical Indices. Iranian Journal Fisheries Sciences, 5, 67-82.
- 42. Ramesh, M., Srinivasan, R. and Saravanan, M. (2009) Effect of Atrazine (Herbicide) on Blood Parameters of Common Carp Cyprinus carpio (Actinopterygii: Cypriniformes). African Journal of Environmental Science and Technology, 3, 453-458.
- 43. Silveira-Coffigny, R., Prieto-Trujillo, A. and Ascencio-Valle, F. (2004) Effects of Different Stressors in Haematological Variables in Cultured Oreochromis aureus S. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 139, 245-250.
- 44. Svobodova, M., Luscova, V., Drastichova, J. and Habek, V. (2001) The Effect of Diazinon on Haematological Indices of Common Carp (Cyprinus carpio L.). Acta Veterinaria Brno, 70, 457-465.
https://doi.org/10.2754/avb200170040457 - 45. Tabarestani, M. (1985) Medical Hematology. Mashhad University Press, Mashhad, 209 p.
- 46. TRC (1984) O.E.C.D. Guideline for Testing for Chemical Section 2, on Biotic Systemmms. 39 p.
- 47. Yekeen, T.A. and Fawole, O.O. (2011) Toxic Effects of Endosulfan on Haematological and Biochemical Indices of Clarias gariepinus. African Journal of Biotechnology, 10, 14090-14096.
https://doi.org/10.5897/AJB10.2468 - 48. Rezaei, J., Tukmehchi, A. and Nejati, V. (2012) Reducing the Growth of Rainbow Trout in Chronic Poisoning by the Herbicide Paraquat. The National Conference on Climate Change and Its Impact on Agriculture and the Environment.
- 49. Saeidi Far, M., Vahabzadeh Roodsari, H., Zamini, A. and Kazemi, R. (2013) The Effect of Pesticide Diazinon on the Behavior and Some Hematological Indices of Rainbow Trout (Oncorhynchus mykiss). Fisheries Journal (Islamic Azad University, Azadshahr Branch), 1, 95-106.
- 50. Sancho, E., Ferrando, M.D. and Andrew, E. (1997) Sublethal Effects of an Organophosphate Insecticide on the European Eel, Anguilla anguilla. Ecotoxicology and Environmental Safety, 36, 57-65.
- 51. Saravanan, M., Kumar, K.P. and Ramesh, M. (2011) Haematological and Biochemical Responses of Freshwater Teleost Fish Cyprinus carpio (Actinopterygii: Cypriniformes) during Acute Chronic Sublethal Exposure to Lindane. Pesticide Biochemistry and Physiology, 100, 206-211.
- 52. Sarikaya, R. and Yilmaz, M. (2003) Investigation of Acute Toxicity and the Effect of 2,4-D (2,4-Dichlorophenoxyacetic Acid) Herbicide on the Behavior of the Common Carp (Cyprinus carpio L., 1758; Pisces, Cyprinidae). Chemosphere, 52, 195-201.
- 53. Sattari, M. (2003) Ichthyology 1, Anatomy and Physiology. Naghsh Mehr Press in Cooperation with University of Guilan, 659 p.


