Open Journal of Marine Science
Vol.3 No.2A(2013), Article ID:33663,13 pages DOI:10.4236/ojms.2013.32A003
UV-B as a Photoacclimatory Enhancer of the Hermatypic Coral Stylophora pistillata
1The Mina & Everard Goodman Faculty of Life Sciences, Bar-Ilan University, Ramat-Gan, Israel
2The Interuniversity Institute for Marine Sciences, Eilat, Israel
Email: *2itaycohen@gmail.com
Copyright © 2013 Itay Cohen et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received April 11, 2013; revised May 18, 2013; accepted June 8, 2013
Keywords: Corals; Depth Transfer; Photoacclimation; UV-B; Underwater Light
ABSTRACT
Photoacclimation processes are crucial for the survival of all photosynthetic organisms in the photic zone. Changes in photosynthetic active radiation (PAR) are however simultaneous to changes in UV-B radiation. The influence of UV-B levels on bio-optical and physiological parameters of deep (30 m) corals from the species Stylophora pistillata and their symbiotic algae, zooxanthellae, was examined during their gradual, stepwise acclimation to a shallow depth (3 m). Drastic exposure of deeper corals to higher UV-B levels in shallower depths is usually fatal. Hence, the acclimation process lasted 118 days and included 10 intermediate stations with an addition of similar amount of PAR at each depth transfer. Concomitantly, in an on-shore experiment, fragments from the same colonies were acclimated by changing shading nets corresponding in PAR levels to each in situ station. Since UV-B is attenuated more efficiently than PAR in seawater, the PAR: UV-B ratio changes in the depth experiment while remaining constant under the neutral density nets. This provided the opportunity to evaluate the importance of UV-B to photoacclimation. In both experiments all fragments survived, in spite of a four-fold difference in levels of PAR and a 140-fold difference in UV-B flux between the initial and final conditions. Both experimental designs resulted in reduction of zooxanthellae density, photosynthesis rates, and quantum yields of PSII, while cellular chlorophyll content remained unaffected. Zooxanthellae density and maximal photosynthetic rate was found decreased in correlation with UV-B radiation, whether it was elevated logarithmically with reducing depths or linearly with reducing shades. Conversely, quantum yields of PSII were adjusted according to the enhancement of PAR rather than UV-B. We conclude that UV-B enhances the magnitude of photoacclimation to higher PAR. This novel aspect of photoacclimation can provide the basis for our understanding of the underlying mechanisms that result in UV-related bleaching.
1. Introduction
As a response to changes in the intensity of underwater light, algal cells (zooxanthellae) living symbiotically with coral polyps, optimize pigment content in a process called photoacclimation [1]. This process can be observed in nature when coral colonies of the same species growing under different irradiance levels are compared [2-4], or when corals are transplanted experimentally to locations with light intensities different from those in their original location [1,5]. Adjusting to variations in light intensity is crucial for optimal utilization of the arriving photons and minimizing over photosynthetic saturation of the zooxanthellae. Photoacclimation at different depths is usually attributed to changes in the photosynthetically active radiation (PAR, 400 - 700 nm). Whereas, the influence of ultraviolet (UV, 200 - 400 nm) is overlooked, although it contributes 15% of the photons at sea surface in the Red Sea (calculated from data provided by the monitoring program of Eilat, http://www.iui-eilat.ac.il/NM P/).
Small changes in depth have a significant effect on light quantity. This reduction is accompanied by major changes in spectral distribution due the characteristic attenuation coefficients of the different wavelengths. The attenuation of UV in the sea depends on its absorption by the water [6] as well as substances such as organic compounds with ring structures [7]. This result in low levels of UV-B below the first few meters [8] however can be measured also at 30 m (Table 1). On the other hand, the water column in the photic zone is replete with the
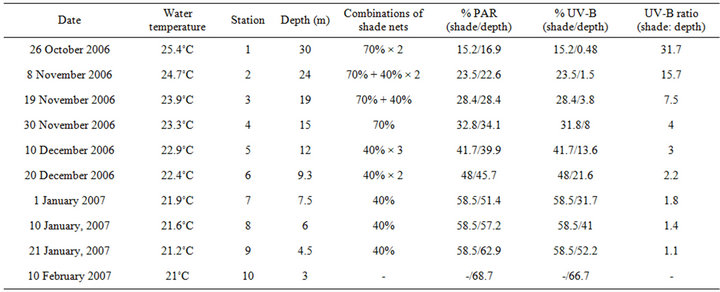
Table 1. Summary of the physical conditions at each of the intermediate stations throughout both of the depth and shading experiments.
lower-energy UV-A radiation (320 - 400 nm), which is biologically less reactive compared to UV-B (280 - 320 nm) [9,10]. Since the UV-C band (200 - 280 nm) falls beyond the portion of the solar spectrum reaching Earth’s surface, its present influence on coral physiology is negligible. Therefore, the various photoacclimation processes should be determined by changes in PAR and UVB levels.
Jokiel [11] was the first to demonstrate that exposure to ambient levels of UV radiation in shallow water can lead to mortality of reef epifauna and cryptofauna. UV-B radiation can induce stress by photodynamic production of cytotoxic reactive oxygen species (ROS) [12,13]. Production of active oxygen during photosynthetic hyperoxia [14] is common in tissues of corals hosting symbiotic alga [12,15], hence corals might be overstressed during intense exposure to UV-B. The activity of antioxidant enzymes, such as superoxide dismutase (SOD), catalase, and ascorbate peroxidase [16] and the presence of UV-absorbing substances, comprising a family of mycosporine-like amino acids (MAAs) [16,17] are in inverse relation to depth, according to the potential for photo oxidative stress. All protective substances are often inducible under conditions of UV exposure [18,19] whereas high mortality is observed in corals when these are not induced [5,18,20]. Such cases elucidate that photoacclimatory responses to fluctuations in ultraviolet radiation (UVR) may be important. The physiological responses of the host-symbiont relationships under intense PAR/UV exposure are of great interest as they might reveal the very basic photoacclimation mechanisms of both (the holobiont) partners to changes in depth. These responses remains poorly documented since deeper corals either failed to survive [5,18,20,21] or became bleached [22,23] when were exposed to shallow depths. The physiological responses of corals to UV-B are hardly predictable since its effects on the photoacclimation processes of zooxanthellae are unclear.
The aim of this study was to assess the physiological responses and the survival of deep Stylophora pistillata exposed gradually to higher ambient light intensity. Translocation of corals from deep (30 m) to shallow (3 m) sites on the reef was combined with a parallel on-shore experiment, in which corals were exposed to the same PAR gradient as the underwater ones. While the PAR/ UV-B ratio decreases towards the surface in the 1st experiment, the PAR/UV-B ratio was kept constant as light increases at the 2nd experiment. From these paired experiments, the relative importance of visible and UV-B portions of the solar spectrum in inducing various photoacclimative processes, was evaluated.
2. Materials and Methods
2.1. Study Site
This study was carried out at the Interuniversity Institute for Marine Sciences (IUI) in Eilat, Israel, in the northern Red Sea (29˚30’N, 34˚56’E). In that location the reef is characterized by a steep slope, resulting in short distances between different radiation niches. This proximity helped to minimize changes in environmental factors other than light levels.
2.2. Light Measurements
A submersible spectral profiling reflectance radiometer (PRR 800, Biospherical Instruments Inc., San Diego, CA) was used to measure PAR and UV-B irradiance. Downwelling PAR was measured directly using the PRR800 cosine quantum collector, and UV-B was measured in three spectral bands (10 nm bandwidth) centered on 305 nm, 313 nm, and 320 nm. UV-B downwelling irradiance was calculated as the trapezoidal integration of these 3 UV-B bands together with the trapezoidal integration of the 280 - 305 nm region, where the irradiance at 280 nm was assigned a 0 value since it is virtually absent at the sea surface.
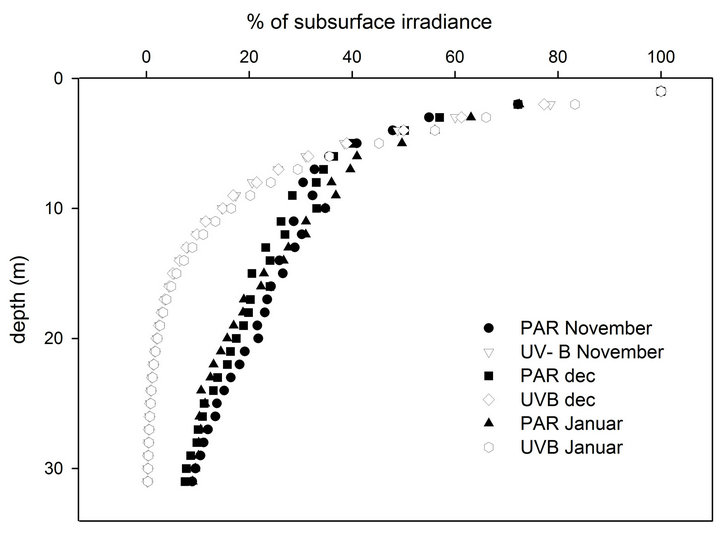
Downwelling irradiance profiles in the PAR and UV-B regions were then used to obtain the attenuation coefficients of these spectral regions with depth (KdPAR, KdUV-B) by fitting linear regressions to the logarithmic transformation of irradiance values. The PRR was set to record light intensity and its spectral distribution five times each second, while being lowered from the surface to a 30-m depth at ca. 1 m·sec–1 descending velocity. We chose to begin the experiment at 30 m since UV-B was undetectable by our instrument below that depth. Once a month (at the beginning of November to January), during a clear day and when the sun was at the zenith, an irradiance profile was measured at a location next to the experimental site. There were no significant differences between the penetration of PAR and UV-B on the three different occasions that data were collected (Figure 1). The PAR channel of the instrument measures photon flux of 400 - 700 nm (µmol quanta per centimeter square per second) whereas the spectral channels measures energy (Watt per centimeter square per nanometer). In order to compare between the relative changes of these light components at each station, changes in UV-B and PAR were calculated as the percentage of their intensity just below the water surface (Figure 1(a)).
2.3. The Acclimation Process in a Depth Experiment and a Shading Experiment
Three S. pistillata colonies were collected from a 30-m depth. Each colony was fragmented into fifty segments of surface areas between 5 and 12 cm2. These fragments were placed on a movable underwater table at their original depth. Twenty days later, half of the fragments from each colony were placed in an outdoor aquarium (400 L) with running seawater system (~10 L·min–1) constantly pumped from 30 m, and under shading that mimics the light intensity at a 30-m depth (Table 1). The fragments were exposed to higher light by upward relocation from 30 to 3 m depth (depth experiment), and by decreasing shades (shading experiment) from dense to a more transparent one (Table 1). For the shading experiment we used two types of neutral density shade nets, 70% and 40%, which are described in the table according to the percentage of light they block. The process in both systems was gradual, with 10 intermediate steps, and lasted 118 days (October 26th to February 10th). To in-
 (a)
(a)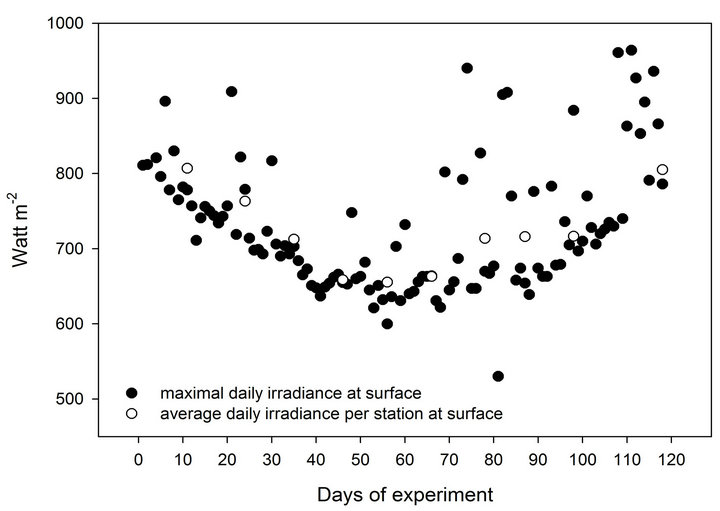 (b)
(b)
Figure 1. (a) Monthly depth profiles of PAR and UV-B (percentage of subsurface values) during both of the experimental acclimation processes. (b) Changes in the ambiant daily energy at sea surface during the 118 days of the experiment.
crease the chances of survival the experiment was executed under mild winter conditions and each upward transfer took place after sunset in order to avoid sudden exposure to increased irradiance. At each station, PAR was increased in parallel in both experiments by 5.8% of its value at the surface and the fragments remained at that light regime 10 - 11 days to acclimate. Since the attenuation of light intensity with depth is exponential, the distance between stations was reduced as the depth of the station approached the surface in order to maintain a linear increase of PAR. Since UV-B levels are attenuated with depth faster than PAR (Figure 1(a)), when ascending to the surface in steps that increase PAR linearly, UV-B levels do increase logarithmically (% of PAR and UV-B, Table 1). Since the plastic shade nets we used were of neutral density, they only attenuate light intensity but do not change its spectral quality. Therefore, unlike the depth experiment, PAR levels in the shading experiment increased while maintaining a constant ratio to UV-B. The ratio of PAR to UV-B was measured with the PRR under all combinations of nets to make sure that the diffusion of light from the nets will not shift the effective transmission for each waveband.
On the first of January 2007, after 7 stations, the PAR/ UV-B ratio between experiments was much closer to 1 compared to the initial conditions (Table 1). Therefore, in the next uppermost three stations of the shading experiment (between 7th and 9th), we maintained the same level of shading, at 58.5% of subsurface irradiance, whereas the depth experiment carried on regularly in order to assess the time scale of stress relaxation.
Throughout the experiment, oceanographic and meteorological data were recorded as part of Israel’s national monitoring of the Gulf of Eilat (http://www.iui-eilat.ac.il/NMP/). Sea temperature, measured underwater (daily) and in the aquaria (weekly) with mercury thermometer, decreased at a steady rate of approximately 0.5˚C with each station from 25.4˚C in October to 21˚C in January (Table 1). Sea-surface irradiance was constantly measured by a CM11B pyranometer (Campbell Scientific, Canada). The presence or absence of clouds and changes in the angle of the sun during the 3 months long experiment slightly diverted the consistency of our irradiance calculation per each 10 days. According to the daily irradiation pattern we calculated that cloud cover was responsible for an average of ~5% variability in light intensity. Figure 1(b) describes some fluctuations in the daily maxima of surface light energy and the variation in average irradiation per station. Although subsurface irradiance decreased during the first 4 stations by 18.4%, the intensity returned to its initial value by the end of the experiments.
2.4. Physiological Assessments (Zooxanthellae Density, Chlorophyll a Concentration, Photosynthesis, Respiration)
Once every 20 days, at the last day of every second station, two fragments from each of the three colonies (n = 3, each n is an average of two fragments representing one colony) were taken from both experiments (depth and shading) to the lab. Each sample was placed in acrylic metabolic chamber (150 ml) containing filtered sea water (using Whatman G/FC 1.2 µm filter). The water was thermostatted according to sea temperature (Table 1) using water bath (NESLAB, RTE 210). Changes in the dissolved oxygen concentrations were measured with Oxi 323 (WTW) connected to a digital data logger. Dark respiretion was measured during 20 minutes at the beginning of the Photosynthesis vs. Energy (P vs. E) curve. Net photosynthetic rates were measured at a sequence of nine increasing illuminations at 10-minute intervals. Both respiration and photosynthesis were normalized to variable surface area of each fragment. The peak of each P vs. E curve was accounted as the Pmax, regardless of its location on the illumination curve. The electrodes were calibrated to saturated O2 levels and the chambers were cleaned each time before placing a new sample.
Coral tissue was removed from the skeleton by a jet of compressed air using an artist’s air brush connected to a SCUBA cylinder. Chlorophyll a was extracted from the tissue slurry collected on a GF/C filter (Whatman) and homogenized in 90% acetone. After incubation in the dark for 24 h at 4˚C, the slurry was filtered and its absorption at 665 nm and 750 nm was determined by a spectrophotometer (Ultrospec 2100 pro, Amersham Pharmacia Biotech, NJ). Final concentrations were calculated following the Lorenzen [24] equations.
Zooxanthellae density per cm2 was determined in triplicates for each sample by inserting with a pipette 250µl of homogenate on a hemocytometer grid and counting under a light microscope (400×) the number of cells in all squares. Surface area was calculated by the weight (using analytic scale METTLER TOLEDO AG245) of aluminum foil covering the skeleton of each fragment.
2.5. Quantum Yield of PSII Fluorescence
fluorescence was measured with a submersible, pulseamplitude, modulated (Diving-PAM, Walz, Germany) chlorophyll fluorometer. The PAM technique allows noninvasive and rapid measurement on the same samples under different depths and light regimes. Initial fluorescence, F0, was determined by a low-intensity measuring light that hardly triggers charge separation at PSII. Maximal fluorescence (Fm) was determined following a saturating pulse of 5000 µmol photons per meter square per second for 0.8 seconds that presumably reduces all PSII reaction centers. The difference between Fm and F0 (Fv) is divided by Fm to achieve quantum yield of PSII (Fv/Fm).
On the last day in each station and at least 20 minutes after sunset, quantum yields of fluorescence of PSII (Fv/ Fm) were measured upon five fragments from each of the three corals in both experiments. Five intact S. pistillata corals growing at the same depths as the transferred ones were also evaluated for their Fv/Fm values. The natural acclimation status and associated parameters of these corals served as a control for the acclimation process and the level of stress exhibited by the fragments in the two experimental treatments.
2.6. Statistical Analysis
The statistical package SPSS 14.0 (SPSS Inc., Chicago, IL) was used for all statistical computations. The influence of the treatment between each individual station was obtained by independent-sample t-test and the influence of depth between two adjacent stations in each experiment was obtained by 1-way ANOVA. In order to test the hypothesis that UV-B has a significant enhancement on photoacclimation, 2-way ANOVA tested the effect of the treatments and depth on variables of both experiments. Linear regression tested the significance of each parameter when plotted against increasing % of PAR and UV-B. Graphs were plotted with SigmaPlot, version 10 (Systat Software Inc., Chicago, IL).
3. Results
The stepwise method used in this study to acclimate S. pistillata fragments originating at 30 m to a 3-m depth proved successful as it resulted in 100% survival of all 150 fragments taken from 3 different colonies. The fragments that were transferred from 30 m depth to the 1st station in the shading experiment remained at the same PAR intensity however experienced a substantial increase in UV-B radiation, from 0.48% of subsurface radiation at 30 m to 15.2% under the shades (Table 1). Twenty days subsequent to that transfer, zooxanthellae cell density per cm2 remained similar in fragments extracted from the two experiments (comparing station 1 between the depth and the shading experiment, Figure 2). Only
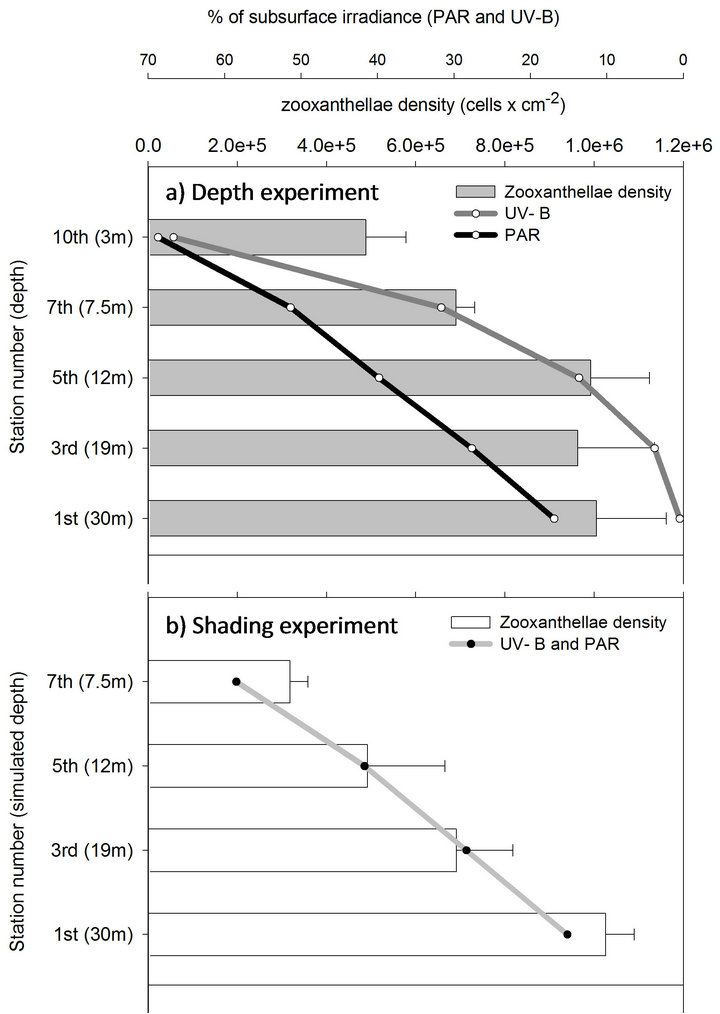
Figure 2. Changes in zooxanthellae density (mean + SE) of deep S. pistillata fragments throughout shallow transfer in a depth and shading experiments.
when PAR was gradually increased (together with further increase in UV-B), by transferring to shallower depths (depth experiment) or to thinner shadings (shading experiment), continuous reduction in the density of zooxanthellae was observed. Cellular-chlorophyll concentration, on the other hand, remained at a constant value throughout the entire process from 30 to 3 m depths, and in the parallel shading experiment (except from increase at the 3rd station which gradually decreased to its initial value by the end of the process, Figure 3). Maximal net photosynthesis (Pmax) of these symbionts also did not decrease after 20 days in the 1st station of the shading experiment (station 1, Figures 4(a) and (b)). Pmax decreased only when both light and UV-B were increased, following the reduction in zooxanthellae density, while respiration did not change during both experiments. The extent by which zooxanthellae density and their photosynthetic activity (Pmax) decreased was not the same between the two experiments although PAR remained similar in parallel stations. Both zooxanthellae density and Pmax were decreasing almost identically at each station of the shading experiment and hence correlated to the constant and proportional enhancement of PAR and
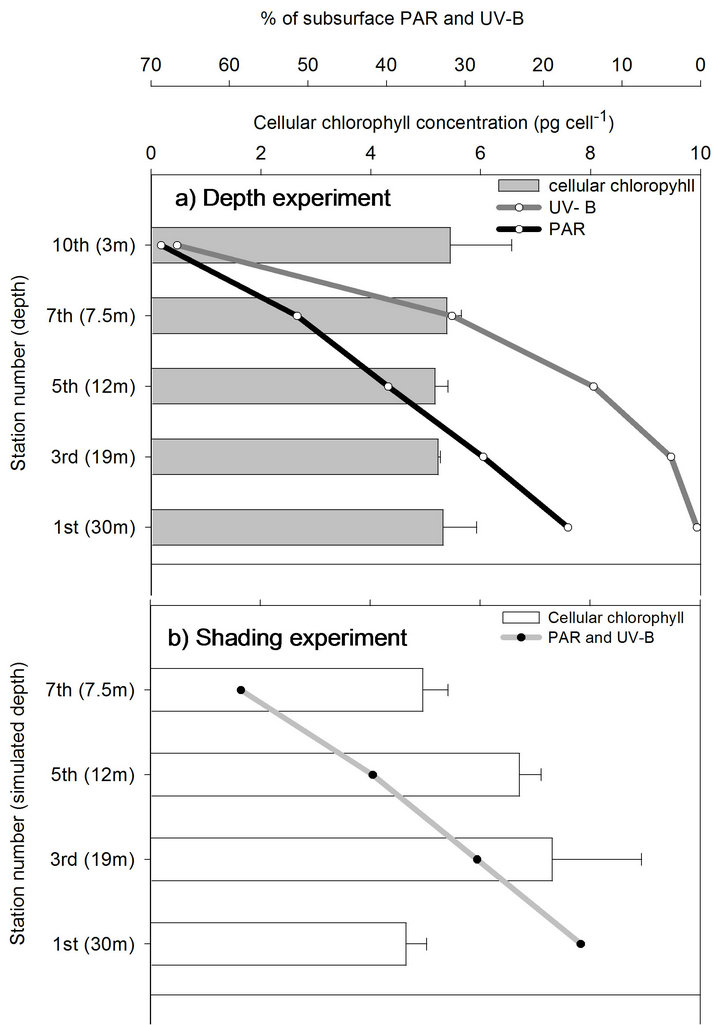
Figure 3. Chlorophyll concentration per zooxanthellae cell (mean + SE) of deep S. pistillata fragments throughout shallow transfer in a depth and shading experiments.
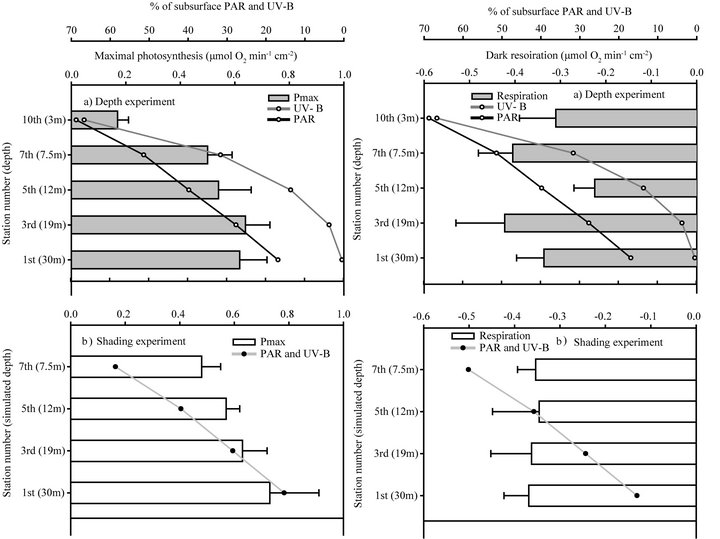
Figure 4. Changes in maximal photosynthesis (mean + SE) and dark respiration (mean − SE) of deep S. pistillata throughout shallow transfer in a depth and shading experiments.
UV-B in that experiment.
In the depth experiment, zooxanthellae density and Pmax also decreased significantly but at an increasing scale with each station, by that corresponded to the logarithmic increase in UV-B levels. Compared to the initial value at the 1st station, zooxanthelae density significantly decreased by the 5th station (P < 0.05) in the shading experiment, and only in the 7th station of the depth experiment. By the 7th station, zooxanthellae density at the shading experiment also became significantly lower than in the parallel fragments in the depth experiment (P < 0.01) corresponding to the higher UV-B levels. According to the linear regression trends, when PAR and UV-B are arriving at similar ratios in the shading experiment, zooxanthellae cells and their photosynthetic capacity (Pmax) are significantly regressed with high R2 (Figures 5(c) and (f), respectively). In order to determine the relative contribution of PAR and UV-B, it was necessary to calculate the regressions of zooxanthellae density and Pmax in the depth experiment against changes in UV-B and separately against changes in PAR. Although zooxanthellae density decreases significantly when plotted against PAR or UV-B in the depth experiment, P value is lower with changes in UV-B (Figure 5). Likewise, linear regression of Pmax is significant only when plotted against UV-B (P = 0.007) and not PAR (P = 0.06). The coefficient of determination (R2) which accounts for the proportion of variability, and hence the strength of linear dependence, is higher when changes in cell density and Pmax are regressed against UV-B (0.94 and 0.93 respectively) rather than PAR levels (0.85 and 0.74 respectively) of the depth experiment. Zooxanthellae density changes significantly different in the two experiments (Table 2) although PAR is similar, further demonstrating the dependence on levels of UV-B. The changes of Pmax on the other hand are not as conspicuous as zooxanthellae density and did not show significant decrease between stations 1 to 7 and between the two experiments (Table 2). Pmax decreased dramatically only in the last station, at 3 m depth.
Quantum yields, unlike zooxanthellae density and Pmax, Fv/Fm was found to vary according to the linear increase in levels of PAR rather than to the logarithmic increase in levels of UV-B with each in situ station (Figure 6). Fv/Fm regression in the depth experiment correlated with changes in UV-B to a lesser extent (R2 =
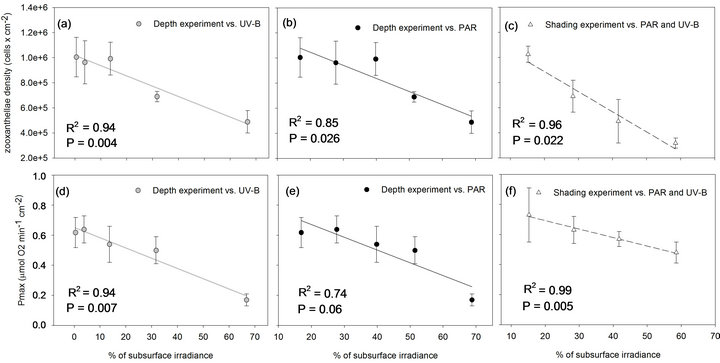
Figure 5. Zooxanthellae densities and net Pmax of S. pistillata in the depth experiment are plotted against the logarithmic increase of UV-B (grey dots) and the linear increase of PAR (black dots) and in the shading experiment against the linear increase in both PAR and UV-B levels (white triangles) which they were exposed to. The statistical parameters, P and R2, extracted from linear regression tests.
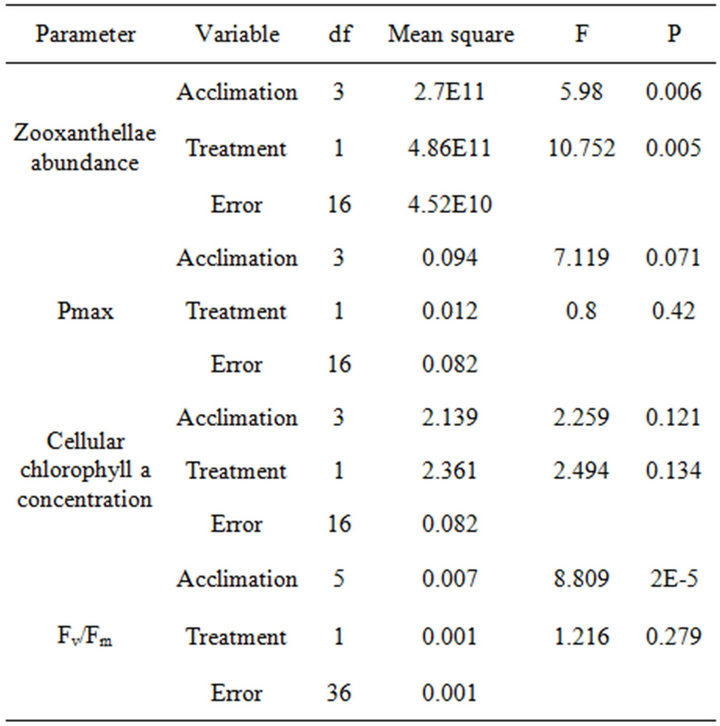
Table 2. Two-way ANOVA tests the effect of acclimation (decreasing depth and shades) and treatment (UV-B) on photosynthetic variables of S. pistillata from 30 m depth.
0.84) compared to changes in PAR (R2 = 0.95) or when both are available equally in the shading experiment (R2 = 0.97) (Figure 7). When comparing changes in Fv/Fm along the first 6 stations, not including the later collapse in the shading experiment, the difference between experiments is not significant (P = 0.28, two-way ANOVA,
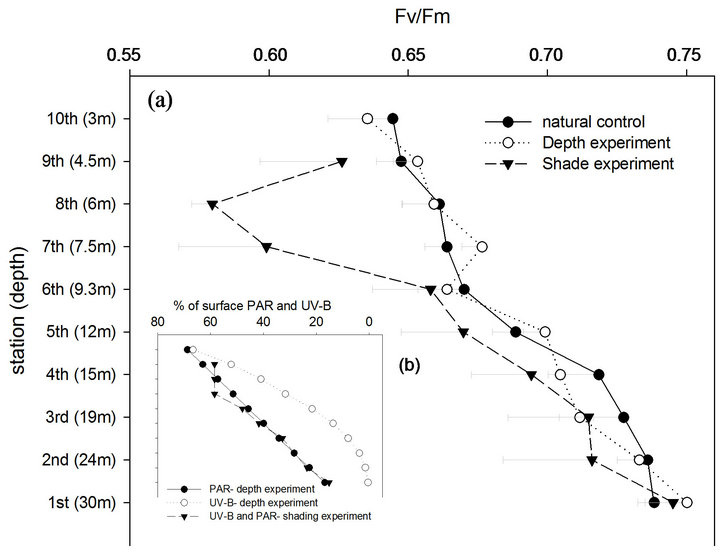
Figure 6. (a) Maximal quantum yield of fluorescence-Fv/Fm (mean-SE) in the depth and shading experiments and in natural control S. pistillata colonies; (b) Levels of UV-B were increased logarithmically in the depth experiment and linearly in the shading experiment while PAR was increased linearly with each station transfer of both experiments.
Table 2) while changes between stations are noticeable in both experimental designs (P < 0.001). Control corals, growing naturally at depths parallel to each station were also found to change their Fv/Fm with higher compatibility to PAR (R2 = 0.93) rather than to UV-B (R2 = 0.79), closely corresponding to most steps of both experiments.
The initial Fv/Fm in the depth-experiment became significantly lower at 15 m depth in the 4th station, like other
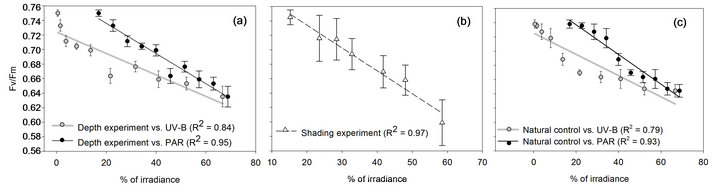
Figure 7. Decreasing Fv/Fm values of S. pistillata fragments in the (a) depth experiment and in (c) control colonies are plotted against the linear increase of PAR (black dots) and the logarithmic increase of UV-B (grey dots) while in the (b) shading experiment Fv/Fm is plotted against the linear increase in both PAR and UV-B levels (white triangles) which they were exposed to. R2, extracted from linear regression tests.
native corals measured on the reef (P < 0.05). Resulting from larger variability, shaded samples attained significant lower Fv/Fm values only at the 7th station (P < 0.01) which resulted from a dramatic decrease in these 10 days of the 7th station (P < 0.05). From that point, the level of shading remained constant for the last 2 stations (20 days) of the shading experiment in order to follow the recovery period. Ten days later, the drop in Fv/Fm values persisted, although not significantly. Only after 20 days of invariable shading, as shown at the 9th station, significant increase was observed (P < 0.05).
4. Discussion
4.1. Mechanism of Photoacclimation to Shallow Depth and the Effect of UV-B
The zooxanthellae abundance and cellular chlorophyll a content in S. pistillata colonies at 30 m depth, as found in this study, is reported in similar values by Mass et al. [3]. Transferring these corals from 30 m to decreasing depth has lowered the areal density of zooxanthellae to values which resembles non-transferred colonies along the same depth gradient [3]. On the other hand, while cellular chlorophyll decreases in S. pistillata growing naturally at shallower depths [3,4], in this study the initial value remained throughout the entire depth experiment, as in Plerogyra sinuosa when transferred from 25 to 5 m depth [5]. The acclimation mechanism observed in this study is also different from the acclimation known for S. pistillata under shaded locations of the shallow reef, which reduce cellular-chlorophyll concentration upon exposure to high light [1]. Shaded corals in the shallow reef experience higher light in similar proportions of wavelengths upon exposure to the sun, whereas upon transfer to shallower depth increasing light is simultaneously with changing spectrum, mainly UV-B. Since fragments in the shading experiment showed similar acclimation mechanism as in the depth experiment we conclude that the difference in photoacclimation mechanism is not due to changes in the spectrum. Rather it could be due to diverse mechanisms evolved from the differences in deeper and shallower corals as the clade of zooxanthellae. Previous studies showed that depth is a distinct “genetic barrier” for clades of zooxanthellae harbored by S. pistillata in the gulf of Eilat [6,25,26]. Zooxanthellae in deep (30 m) S. pistillata harbor clade C, whereas the shallow ones (up to 17 m) were found to harbor clade A. Following that pattern, zooxanthellae density hardly changed in the transfer process up to the 5th station, between 30 and 12 m, whereas a drastic decrease was observed in subsequent stations between 12 to 3 m. It may well be that the significant physiological responses are bathymetrically dependent and might point out UV-B as an important cause for the distribution of clades. Since zooxanthellae density was constantly reduced in this study it is hard to believe that during the time of the experiment zooxanthellae with different clades were acquired.
Several perturbations caused by enhanced UV may influence zooxanthellae density. Indirectly, UV seem to impair nitrate and ammonium transport, and can cause low internal N availability [27], affecting the formation of new zooxanthellae. Indeed, Lesser [28] reported 45% decline in the growth of zooxanthellae cells exposed to UV radiation. Absorption of UV-B and PAR in the presence of oxygen can lead to active oxygen production which could trigger programmed cell-death [29], degradation [30], necrosis and apoptosis [31]. ROS can also affect cellular adhesive proteins [32] which may lead to expulsion of zooxanthellae [31,33] or detachment of gastrodermal host cells [34]. These processes can lead to rapid decrease in zooxanthellae density as was observed in both experiments of this study, and may explain the mechanism of acclimation to shallow waters.
UV is considered in many papers as detrimental to the stability of coral-algal symbiosis [e.g. 7,9] . However, reduction in symbiont densities might actually ameliorate the physiologic conditions of the coral as it is a rapid mechanism to become more eligible to withstand the higher light intensity. ROS production can initiate a negative loop by causing rapid reduction in symbiont cells that would lower the chances for further formation of oxygen which are likely to become toxic. The potential for UV-B damage in deep corals may rise disproportionately as photosynthetic rates increase due to the increasing light at shallower depths. We suggest that by leading to oxidative stress, UV-B might act as an enhancer of photoacclimation to higher light. UV-B was also reported to increase the rate of photoacclimation to high irradiance within species of the macroalgal genus Gelidium from southern Spain and, therefore, helped to reduce the effect of photoinhibition [35].
Under low light conditions, where the potential for oxidative stress is low, augmented UV-B levels may not be sufficient in triggering photoacclimatory responses. This was demonstrated by the relocation of fragments from 30-m depth to a shaded aquarium for the shading experiment. That transfer hardly changed the intensity of PAR, although levels of UV-B were increased by 31.7 fold (from 0.48% to 15.2%, Table 1). Such a change did not initiate any photoacclimation process, as zooxanthellae densities (station 1 in Figures 2(a) and (b)) and cellular chlorophyll (station 1 in Figures 3(a) and (b)) remained as they were 20 days before. It may well be that under the increased UV-B dose, before PAR became increasingly available, a fraction of the chlorophyll became dysfunctional but was not destroyed, as other protein-based pigments described by [36]. We speculate that the synthesis of new chlorophyll in the remaining zooxanthellae, as PAR became increasingly available, caused the appearance of increased cellular chlorophyll in the 3rd station (Figure 3(b)).
The role of UV-B as an enhancer of photoacclimation was further demonstrated by following the physiological responses of the corals in the two experiments. Zooxanthellae density (Figure 2) and Pmax (Figure 4) changed in according to UV-B when increased logarithmically in the depth experiment or linearly in the shading experiment, although PAR was similar between the experiments. When UV-B and PAR increased linearly in the shading experiment acclimation was closely comparable between stations, although irradiance became extremely high in the later stations. This suggests that the level of physiological adjustment correlates to the difference in irradiance between stations regardless of their absolute value. Whether it is at low light or high light, increment of the same amount of irradiance (PAR and UV-B) would modify physiological responses to the same extent.
Mass et al., [3] showed that maximal photosynthetic capacity of S. pistillata is higher in colonies growing at shallower depths although zooxanthellae density is lower. In both experiments of this study however, Pmax cm–2 of deeper corals decreased when transferred to shallower depth (Figure 4). The decrease observed in net O2 production is probably not due to an increase in the respiration rate (Figure 4) or due to inhibition of PSII quantum yield by UV-B as Fv/Fm which decreases similarly in both experiments (Figure 6). Photosynthesis was in correlation with the reduction of zooxanthellae density in both experimental designs. By normalizing to zooxanthellae cell, changes in photosynthesis are hardly noticeable along the depth gradient of both experiments (not shown). Hence, zooxanthellae were not photoinhibitet but also did not utilize irradiance at efficiencies that would be expected from shallow zooxanthellae.
Pmax and Fv/Fm in this study were measured 20 and 10 days, respectively, subsequent to each PAR and UV-B increment. Shorter-term response of deep corals [16] and phytoplankton [37] to increased UV-B does not coincide with our results that both Pmax and Fv/Fm are not inhibited by UV-B. Furthermore, action spectra of photosynthesis in isolated chloroplasts, lacking repair mechanisms typical for whole cells, show marked inhibition mostly by UV-B [38]. In living cells the importance of repair/ recovery mechanism is demonstrated by UV-B was found to enhance the rate of D1 turnover [39]. On the other hand, Hoogenboom et al. [40] suggested that the effect of photoinhibition on corals could last for weeks. The collapse in quantum yields of fluorescence measured at the 7th station of the shaded fragments was surprising since the UV-B levels were only 1.8 times higher than in the parallel station in the depth experiment, contrary to much higher ratios in previous stations that did not exert significant Fv/Fm fluctuations. The lower Fv/Fm values persisted as well during the next 10 days, although irradiance did not further increase for the shading experiment. Only at the 9th station, 30 days under constant shading conditions, photoinhibition seems to be alleviated as fragments regained higher Fv/Fm values. Since the corals in the shading experiment were exposed to higher UV-B levels throughout the experiment, photodamage may have accumulated in the form of dynamic photoinhibition or may have required more energetic resources of the coral for repair processes. In this condition, the threshold by which further elevation of PAR levels could cause longer term inhibition could have been lower than in the shading experiment. Long-lasting decrease in quantum yield synonymous with photodamage was also observed by Gorbunov et al. [41].
The regression in Fv/Fm, measured in both experiments, follows the linear increase in PAR, regardless of the levels of UV-B and zooxanthellae density. Photosynthesis and maximal quantum yield of PSII are therefore slightly decoupled in the depth experiment. These methods were not assessed in parallel as photosynthesis was measured during the day in the lab and Fv/Fm represents the dark adapted state measured in situ after sunset. Lower photosynthesis might result from dynamic inhibition during the day whereas by sunset repair mechanisms of PSII could be significant. Similar to our observation, Fv/Fm in sunflower seedlings is hardly affected by UV-B even though photosynthesis decreased [42]. In corals, rate of photosynthesis is decoupled from the quantum yield of PSII during daily hysteresis [40] and during photoinhibition of both sun and shade-adapted polyps [43]. Furthermore, electron transport through PSII to processes like photorespiration by RuBisCO (Ribulose –1, 5 bisphosphate carboxylase oxygenase) and the Mehler cycle does not affect quantum yields however are pronounced in decreasing CO2 fixation rates and oxygen production [44].
4.2. Seasonal vs. Experimental Influence
This study took place between October and January, when water temperature drops (Table 1) and levels of nitrate increases. Since zooxanthella densities in this study constantly decreased over that time interval, we conclude that the impact of increasing light intensity clearly overrides any possible nutrient increase and temperature drop that would have resulted in zooxanthella proliferation as reported by Winters et al. [4].
4.3. Survival
Irradiance [2,3], as well as UV [8], plays an important role in controlling the bathymetric distribution, diversity, and abundance of benthic coral-reef organisms. Deep corals usually receive lower levels of UV-B compared with PAR levels (0.48% and 16.9% of surface levels, respectively, at 30 m). At the final experimental station, S. pistillata fragments from 30 m had to endure 140 - fold higher levels of UV-B (from 0.48% at 30 m to 66.7% at 3 m, Table 1) and four-fold higher levels of PAR (16.9% at 30 m and 68.7% at 3 m). Nevertheless, no mortality was observed, hence S. pistillata from a 30- m depth can overcome dramatic changes in irradiance and UV-B as long as the rate of exposure does not exceed the rate of photoacclimation. It was essential to minimize the potential for photooxidative damage by balance the absorption and utilization of light energy. Thereby reduction in zooxanthellae density served as a key factor for the survival of all fragments. This mechanism could have been spontaneous, or as a result of controlled cellular modifications to increased UV levels [10]. However the kinetics of this mechanism is one of the factors controlling the survival chances of corals in case of depth relocation.
Hoogenboom et al. [40] showed that with short-term (1 day) exposure to excessive irradiance, respiration does not enhance significantly and concluded that the cost of photoinhibition is negligible. Respiration rates in both experiments of this study (Figure 4) could be a good indication that metabolism of the holobiont remains stable also under long term exposure.
During acclimation of deeper S. pistillata to shallow depth Fv/Fm was similar to control S. pistillata growing at the same depths as the experimental stations (Figures 6 and 7). Since chlorophyll fluorescence can be useful when investigating the extent to which the reaction centers are damaged by excess light [41] we conclude that the stress elicited by the translocation coincides with the customary environmental constraints.
As opposed to Gattuso [20], Shick [45] found that UV-B can stimulate the accumulation of MAAs in S. pistillata despite a decrease, such as in this study, in its population of symbiotic dinoflagellates. This accumulation is proportional to irradiation and is strongly apparent within 7 days [45], allowing the corals to well prepare between each station transfer. Furthermore, the experimental exposure of corals from a 30-m depth to a wider spectrum by reducing their residential depth can increase the host UV tolerance [46] by triggering the activation of two antioxidant enzymes, catalase and SOD [47]. However, the effects of UV radiation can vary even among genotypes within a coral species [48]. Therefore, the results of this study should be applied with care when studying community response to increased UV-B.
5. Conclusion
Zooxanthellae density and Fv/Fm change with acclimation of deep S. pistillata to shallow depth while cellular chlorophyll content, Pmax and dark respiration are less affected (Table 2). The effects of UV-B on photoacclimation are more conspicuous through alteration of the abundance of zooxanthellae rather than their quantum yields (Table 2). We conclude that the oxidative stress caused by UV-B may serve as a signal for corals to enhance acclimation rate while PAR increases. However we do not rule out the option that corals can sense UV-B through photoreceptors which can produce the same signal. Disposing zooxanthellae can be a fast process which is usually required in cases of rapid elevation of irradiance in order to avoid over production of ROS. Physiological changes occur in similar steps in the shading experiment, hence we conclude that photoacclimation depends on the level of variation in irradiance and not on the absolute value of the irradiance, even if irradiance is extremely high.
6. Acknowledgements
We are grateful to Dr. Y. Kamenir for his assistance with the statistical analyses, Ms. S. Victor for the meticulous editing, Mr. D. Tobies for the help with many dives, Ms. D. Nahum Cohen for assistance with analyzing the data, the staff of the IUI and the members of Israel’s national monitoring program for the logistic support. This study was supported by Israel Science Foundation grant number 408/03-17.3, NATO grant number SFP 981883 and the European Research Council 2009-ADG-249930. It is based on part of the M.Sc. thesis of IC at the Mina & Everard Goodman Faculty of Life Sciences, Bar-Ilan University.
REFERENCES
- P. G. Falkowski and Z. Dubinsky, “Light-Shade Adaptation of Stylophora pistillata, a Hermatypic Coral from the Gulf of Eilat,” Nature, Vol. 289, No. 5794, 1981, pp. 172-174. doi:10.1038/289172a0
- Z. Dubinsky, P. G. Falkowski, J. W. Porter and L. Muscatine, “Absorption and Utilization of Radiant Energy by Lightand Shade-Adapted Colonies of the Hermatypic Coral Stylophora pistillata,” Proceedings of the Royal Society of London, Vol. 222, No. 1227, 1984, pp. 203- 214.
- T. Mass, S. Einbinder, E. Brokovich, N. Shashar, R. Vago, J. Erez and Z. Dubinsky, “Photoacclimation of Stylophora pistillata to Light Extremes: Metabolism and Calcification,” Marine Ecology Progress Series, Vol. 334, 2007, pp. 93-102. doi:10.3354/meps334093
- G. Winters, S. Beer, B. Ben Zvi, I. Brickner and Y. Loya, “Spatial and Temporal Photoacclimation of Stylophora pistillata: Zooxanthella Size, Pigmentation, Location and Clade,” Marine Ecology Progress Series, Vol. 384, 2009, pp. 107-119. doi:10.3354/meps08036
- E. Vareschi and H. Fricke, “Light Responses of a Scleractinian Coral (Plerogyra sinuosa),” Marine Biology, Vol. 90, No. 3, 1986, pp. 395-402. doi:10.1007/BF00428563
- J. T. O. Kirk, “Optics of UV-B Radiation in Natural Waters,” Archive of Hydrobiology, Vol. 43, No. 1, 1994, pp. 1-16.
- D. Iluz, R. Vago, N. E. Chadwick, R. Hoffman and Z. Dubinsky, “Seychelles Lagoon Provides Corals a Refuge from Bleaching,” Research Letters in Ecology, 2008, Article ID: 281038. doi:10.1155/2008/281038
- N. G. Jerlov, “Ultraviolet Radiation in the Sea,” Nature, Vol. 166, No. 4211, 1950, pp. 111-112. doi:10.1038/166111a0
- D. F. Gleason and G. M. Wellington, “Ultraviolet-Radiation and Coral Bleaching,” Nature, Vol. 365, No. 6449, 1993, pp. 836-838. doi:10.1038/365836a0
- A. Quesada, J.-L. Mouget and W. F. Vincent, “Growth of Antarctic Cyanobacteria under Ultraviolet Radiation: UVA Can Counteract UVB Inhibition,” Journal of Phycology, Vol. 31, No. 2, 1995, pp. 242-248. doi:10.1111/j.0022-3646.1995.00242.x
- P. L. Jokiel, “Solar Ultraviolet Radiation and Coral Reef Epifauna,” Science, Vol. 207, No. 4435, 1980, pp. 1069- 1071. doi:10.1126/science.207.4435.1069
- J. A. Dykens and J. M. Shick, “Oxygen Production by Endosymbiotic Algae Controls Superoxide Dismutase Activity in Their Animal Host,” Nature, Vol. 297, No. 5867, 1982, pp. 579-580. doi:10.1038/297579a0
- J. A. Dykens, J. M. Shick, C. Benoit, G. R. Buettner and G. W. Winston, “Oxygen Radical Production in the Sea- Anemone Anthopleura elegantissima and Its Endosymbiotic Algae,” Journal of Experimental Biology, Vol. 168, 1992, pp. 219-241.
- K. Asada and M. Takahashi, “Production and Scavenging of Active Oxygen in Photosynthesis,” In: D. J. Kyle, C. B. Osmond and C. J. Arntzen, Eds., Photoinhibition Topics in Photosynthesis, Vol. 9, Elsevier, Amsterdam, 1987, pp. 227-287.
- M. Kühl, Y. Cohen, T. Dalsgaard, B. B. Jorgensen and N. P. Revsbech, “Microenvironment and Photosynthesis of Zooxanthellae in Scleractinian Corals Studied with Microsensors For O2, pH, and Light,” Marine Ecology Progress Series, Vol. 117, No. 1-3, 1995, pp. 159-172. doi:10.3354/meps117159
- J. M. Shick, M. P. Lesser, W. C. Dunlap, W. R. Stochaj, B. E. Chalker and J. W. Won, “Depth Dependent Responses to Solar Ultraviolet Radiation and Oxidative Stress in The Zooxanthellae Coral Acropora microphthalma,” Marine Biology, Vol. 122, No. 1, 1995, pp. 41-51. doi:10.1007/BF00349276
- W. C. Dunlap, B. E. Chalker and J. K. Oliver, “Bathymetric Adaptation of Reef Building Corals at Davies Reef, Great Barrier Reef, Australia. III. UV-Absorbing Compounds,” Journal of Experimental Marine Biology and Ecology, Vol. 104, No. 1-3, 1986, pp. 239-248. doi:10.1016/0022-0981(86)90108-5
- G. M. Scelfo, “Relationship between Solar Radiation and Pigments of the Coral Montipora verrucosa and Its zooxanthellae,” In: P. L. Jokiel, R. H. Richmond and R. A. Rogers, Eds., Coral Reef Population Biology, Tech. Report 37, Hawaii Institute of Marine Biology, Honolulu, 1986.
- R. A. Kinzie, “Effects of Ambient Levels of Solar Ultraviolet-Radiation on Zooxanthellae and Photosynthesis of the Reef Coral Montipora verrucosa,” Marine Biology, Vol. 116, No. 2, 1993, pp. 319-327. doi:10.1007/BF00350022
- J. P. Gattuso, “Ecomorphology, Metabolism, Growth and Calcification of the Zooxanthellate Scleractinian Coral Stylophora pistillata (Gulf of Aqaba, Red Sea)—Effects of Lighting,” PhD Thesis, University of Marseille, Marseille, 1987.
- H. T. Yap, R. M. Alvarez, H. M. Custodio and R. M. Dizon, “Physiological and Ecological Aspects of Coral Transplantation,” Journal of Experimental Marine Biology and Ecology, Vol. 229, No. 1, 1998, pp. 69-84. doi:10.1016/S0022-0981(98)00041-0
- A. C. Baker, “Reef Corals Bleach to Survive Change,” Nature, Vol. 411, No. 6839, 2001, pp. 765-766. doi:10.1038/35081151
- S. Richier, J. M. Cottalorda, M. M. M. Guillaume, C. Fernandez, D. Allemand and P. Furla, “Depth-Dependant Response to Light of the Reef Building Coral, Pocillopora verrucosa: Implication of Oxidative Stress,” Journal of Experimental Marine Biology and Ecology, Vol. 357, No. 1, 2008, pp. 48-56. doi:10.1016/j.jembe.2007.12.026
- C. J. Lorenzen, “Determination of Chlorophyll and PheoPigments—Spectrophotometric Equations,” Limnology and Oceanography, Vol. 12, No. 2, 1967, pp. 343-346. doi:10.4319/lo.1967.12.2.0343
- S. Lampert-Karako, N. Stambler, D. J. Katcoff, Y. Achituv, Z. Dubinsky and N. Simon-Blecher, “Effects of Depth and Eutrophication on the Zooxanthella Clades of Stylophora pistillata from the Gulf of Eilat (Red Sea),” Aquatic Conservation: Marine and Freshwater Ecosystems, Vol. 18, No. 6, 2008, pp. 1039-1045 doi:10.1002/aqc.927
- K. A. Byler, M. Carmi-Veal, M. Fine and T. L. Goulet, “Multiple Symbiont Acquisition Strategies as an Adaptive Mechanism in the Coral Stylophora pistillata,” PLoS ONE, Vol. 8, No. 3, 2013, Article ID: e59596. doi:10.1371/journal.pone.0059596
- G. Dohler, E. Hagmeier, E. Grigoleit and K. D. Krause, “Impact of Solar UV Radiation on Uptake of N-15-Ammonia and N-15-Nitrate by Marine Diatoms and Natural Phytoplankton,” Biochemical Physiology, Vol. 187, 1991, pp. 293-303.
- M. P. Lesser, “Responses of Phytoplankton Acclimated to UV-B Radiation: Ultraviolet Radiation Absorbing Compounds Do Not Provide Complete Protection in the Dinoflagellate Prorocentrum micans,” Marine Ecology Progress Series, Vol. 132, 1996, pp. 287-297. doi:10.3354/meps132287
- D. J. Franklin, P. Hoegh-Guldberg, R. J. Jones and J. A Berges, “Cell Death and Degeneration in the Symbiotic Dinoflagellates of the Coral Stylophora pistillata during Bleaching,” Marine Ecology Progress Series, Vol. 272, 2004, pp. 117-130. doi:10.3354/meps272117
- M. D. A. Le Tissier and B. E. Brown, “Dynamics of Solar Bleaching in the Intertidal Reef Coral Goniastrea aspera at Ko Phuket, Thailand,” Marine Ecology Progress Series, Vol. 136, 1996, pp. 235-244. doi:10.3354/meps136235
- S. R. Dunn, J. C. Bythell, M. D. A. Le Tissier, W. J. Burnett and J. C. Thomason, “Programmed Cell Death and Cell Necrosis Activity During Hyperthermic StressInduced Bleaching of the Symbiotic Sea Anemone Aiptasia sp.,” Journal of Experimental Marine Biology and Ecology, Vol. 272, No. 1, 2002, pp. 29-53. doi:10.1016/S0022-0981(02)00036-9
- K. J. A. Davies, “Protein Damage and Degradation by Oxygen Radicals. 1. General Aspects,” Journal of Biological Chemistry, Vol. 262, No. 20, 1987, pp. 9895-9901.
- E. A. Titlyanov, J. Tsukahara, T. V. Titlyanova, V. A. Leletkin, R. Van Woesik and K. Yamazato, “Zooxanthellae Population Density and Physiological State of the Coral Stylophora pistillata Curing Starvation and Osmotic Shock,” Symbiosis, Vol. 28, 2000, pp. 303-322.
- R. D. Gates, G. Baghdasarian and L. Muscatine, “Temperature Stress Causes Host Cell Detachment in Symbiotic Cnidarians—Implications for Coral Bleaching,” Biological Bulletin, Vol. 182, No. 3, 1992, pp. 324-332. doi:10.2307/1542252
- I. Gómez and F. L. Figueroa, “Effects of Solar UV Stress on Chlorophyll Fluorescence Kinetics of Intertidal Macroalgae from Southern Spain: A Case Study in Gelidium Species,” Journal of Applied Phycology, Vol. 10, No. 3, 1998, pp. 285-294. doi:10.1023/A:1008021230738
- K. Q. Lao and A. N. Glazer, “Ultraviolet-B Photodestruction of a Light-Harvesting Complex,” Proceedings of the National Academy of Science, Vol. 93, No. 11, 1996, pp. 5258-5263. doi:10.1073/pnas.93.11.5258
- A. U. Bracher and C. Wiencke, “Simulation of the Effects of Naturally Enhanced UV Radiation on Photosynthesis of Antarctic Phytoplankton,” Marine Ecology Progress Series, Vol. 196, 2000, pp. 127-141. doi:10.3354/meps196127
- L. W. Jones and B. Kok, “Photoinhibition of Chloroplast Reactions II: Multiple Effects,” Plant Physiology, Vol. 41, No. 6, 1966, pp. 1044-1049. doi:10.1104/pp.41.6.1044
- B. M. Greenberg, V. Gaba, O. Canaani, S. Malkin, A. K. Mattoo and M. Edelman, “Separate Photosensitizers Mediate Degradation of the 32-kDa Photosystem II Reaction Center Protein in the Visible and UV Spectral Regions,” Proceedings of the National Academy of Science, Vol. 86, No. 17, 1989, pp. 6617-6620. doi:10.1073/pnas.86.17.6617
- M. O. Hoogenboom, K. R. N. Anthony and S. R. Connolly, “Energetic Cost of Photoinhibition in Corals,” Marine Ecology Progress Series, Vol. 313, 2006, pp. 1-12. doi:10.3354/meps313001
- M. Y. Gorbunov, Z. S. Kolber, M. P. Lesser and P. G. Falkowski, “Photosynthesis and Photoprotection in Symbiotic Corals,” Limnology and Oceanography, Vol. 46, No. 1, 2001, pp. 75-85. doi:10.4319/lo.2001.46.1.0075
- M. Tevini, U. Mark, G. Fieser and M. Salle, “Effects of Enhanced Solar UV-B Radiation on Growth and Function of Selected Crop Plant Seedlings,” In: E. Riklis, Ed., Photobiology, Plenum, New York, 1991, pp. 635-649.
- K. E. Ulstrup, P. J. Ralph, A. W. D. Larkum and M. Kuhl, “Intra-Colonial Variability in Light Acclimation of Zooxanthellae in Coral Tissues of Pocillopora damicornis,” Marine Biology, Vol. 149, No. 6, 2006, pp. 1325- 1335. doi:10.1007/s00227-006-0286-4
- F. L. Figueroa, R. Conde-Alvarez and I. Gomen, “Relations between Electron Transport Rates Determined by Pulse Amplitude Modulated Chlorophyll Fluorescence and Oxygen Evolution in Macroalgae under Different Light Conditions,” Photosynthesis Research, Vol. 75, No. 3, 2003, 259-275. doi:10.1023/A:1023936313544
- J. M. Shick, S. Romaine-Lioud, C. Ferrier-Pages and J. P. Gattuso, “Ultraviolet-B Radiation Stimulates Shikimate Pathway-Dependent Accumulation of Mycosporine-Like Amino Acids in the Coral Stylophora pistillata Despite Decreases in Its Population of Symbiotic Dinoflagellates,” Limnology and Oceanography, Vol. 44, No. 7, 1999, pp. 1667-1682. doi:10.4319/lo.1999.44.7.1667
- O. Siebeck, “Experimental Investigation of UV Tolerance in Hermatypic Corals (Scleractinia),” Marine Ecology Progress Series, Vol. 43, 1988, pp. 95-103. doi:10.3354/meps043095
- O. Levy, Y. Achituv, Y. Z. Yacobi, N. Stambler and Z. Dubinsky, “The Impact of Spectral Composition and Light Periodicity on the Activity of Two Antioxidant Enzymes (SOD and CAT) in the Coral Favia favus,” Journal of Experimental Marine Biology and Ecology, Vol. 328, No. 1, 2006, pp. 35-46. doi:10.1016/j.jembe.2005.06.018
- D. F. Gleason, “Differential Effects of Ultraviolet Rdiation on Green and Brown Morphs of the Caribbean Coral Porites astreoides,” Limnology and Oceanography, Vol. 38, No. 7, 1993, pp. 1452-1463. doi:10.4319/lo.1993.38.7.1452
NOTES
*Corresponding author.

