Open Journal of Marine Science
Vol.3 No.1(2013), Article ID:27168,9 pages DOI:10.4236/ojms.2013.31003
Dispersal Ability and Environmental Adaptability of Deep-Sea Mussels Bathymodiolus (Mytilidae: Bathymodiolinae)
1Faculty of Education and Human Sciences, University of Yamanashi, Kofu, Japan
2Institute of Biogeosciences, Japan Agency for Marine-Earth Science and Technology, Yokosuka, Japan
Email: *miyazaki@yamanashi.ac.jp
Received October 12, 2012; revised November 17, 2012; accepted November 29, 2012
Keywords: Chemosynthesis-Based Community; Vent; Seep; Mitochondrial DNA; Stepping Stone
ABSTRACT
Dispersal ability and environmental adaptability are profoundly associated with colonization and habitat segregation of deep-sea animals in chemosynthesis-based communities, because deep-sea seeps and vents are patchily distributed and ephemeral. Since these environments are seemingly highly different, it is likely that vent and seep populations must be genetically differentiated by adapting to their respective environments. In order to elucidate dispersal ability and environmental adaptability of deep-sea mussels, we determined mitochondrial ND4 sequences of Bathymodiolus platifrons and B. japonicus obtained from seeps in the Sagami Bay and vents in the Okinawa Trough. Among more than 20 species of deep-sea mussels, only three species in the Japanese waters including the above species can inhabit both vents and seeps. We examined phylogenetic relationships, genetic divergences (Fst), gene flow (Nm), and genetic population structures to compare the seep and vent populations. Our results showed no genetic differentiation and extensive gene flow between the seep and vent populations, indicating high dispersal ability of the two species, which favors colonization in patchy and ephemeral habitats. Our results also indicate that the environmental type (vent or seep) is not the primary factor responsible for habitat segregation in the two species.
1. Introduction
Deep-sea vents and seeps flourish dense biological assemblages, i.e., chemosynthesis-based communities, which contain endemic animals such as vesicomyid clams, Provaniid gastropods, and vestimentiferan tubeworms whose primary production is based on bacterial chemosynthesis [1]. Deep-sea vents and seeps are patchily distributed and separated from each other by a few hundred kilometers in vent sectors and by hundreds of meters to a few kilometers in vent fields within the vent sector [2]. Vents and seeps are ephemeral, because they are located in active geological settings. Especially vents persist for only a few decades [3]. Therefore, one of the topics in deep-sea biology is how those animals can disperse to maintain the communities and avoid extinction and how they can be isolated to accumulate genetic differentiations leading to consequent speciation. The dispersal ability of deep-sea animals is profoundly associated with the evolutionary process of deep-sea animals.
Some studies have suggested that the dispersal ability of deep-seaanimals is high, favoring colonization in patchy and ephemeral habitat, as in Escarpia and Lamellibrachia tubeworms [4,5], Calyptogena clams [6,7], and ventendemic Alviniconcha gastropods [8].
Deep-sea mussels of the genus Bathymodiolus (Mytilidae, Bathymodiolinae) are one of the dominant macroorganisms in the chemosynthesis-based community. Since the original description of the genus [9], 22 extant Bathymodiolus species have been described [10-21]. We showed by sequencing mitochondrial DNA that mussels in the subfamily Bathymodiolinae comprised four groups (Groups 1 to 4) [22,23]. The relationships were corroborated based on the concatenated sequences of COI and 28 S rRNA [24]. Only three Bathymodiolus species in the Japanese waters are capable of inhabiting both vents and seeps [11], although the other species are restricted to either. Bathymodiolus japonicus and B. platifrons are distributed in seeps of the Sagami Bay and vents of the Okinawa Trough, which are separated by approximately 1500 km. Bathymodiolus aduloides is distributed in seeps of Sagami Bay, the Nankai Trough and the subduction zone of the Nansei-shoto Trench, and vents of the Okinawa Trough (however, our genetic analyses have not confirmed its existence in the Nankai Trough). Some B. aduloides specimens have been obtained so far from vents in the Izu-Ogasawara Island-arc [25].
The dispersal ability of Bathymodiolus mussels was suggested to be high based on the larval shell morphology [26] and small egg size [27] that are indicative of planktotrophic (actively feeding planktonic larval) development. The East Pacific deep-sea mussel B. thermophilus was estimated to have dispersal capabilities of at least 2370 km [7]. Our previous study showed that there existed high gene flow between B. septemdierum from the Myojin Knoll and the Suiyo Seamount and B. brevior from the North Fiji Basin and low but not negligible gene flow between B. marisindicus from the Kairei field and B. septemdierum or B. brevior, although their habitats are 5000 to 10,000 km away, indicating a high dispersal ability of Bathymodiolus mussels [28].
We examined genetic population structures of B. platifrons from seeps in the Sagami Bay and vents in the Okinawa Trough in our previous study [28]. Comparisons of the genetic population structures showed no significant genetic differentiation between the seep and vent populations, indicating high dispersal ability and also high adaptability to the abyssal environments albeit vents and seeps provide deep-sea animals with different habitats. Our results, however, were not well supported statistically probably due to genetic heterogeneity in our samples. At that time we could not obtain enough specimens from a single locality in the Okinawa Trough and used combined specimens from three different localities. In this study, in order to elucidate dispersal ability and environmental adaptability of deep-sea mussels, we reevaluate genetic population structures of B. platifrons by use of more than 20 specimens collected since then for each locality. We also presented the first data of genetic population structures for B. japonicus. We analyzed sequences of the mitochondrial ND4 (NADH dehydrogenase subunit 4) gene, because the gene has relatively fast evolutionary rate and has been used for studies of population genetics in diverse animals such as bivalves, fishes, and snakes [29-31].
2. Materials and Methods
2.1. Materials
We used specimens of B. platifrons and B. japonicus obtained from the Off Hatsushima seep population in the Sagami Bay and the North Iheya Ridge population in the Okinawa Trough in addition to those of B. platifrons from Hatoma Knoll population in the Okinawa Trough. Bathymodiolus japonicus has not been found in Hatoma Knoll. The specimens are listed in Table 1, and the collection sites are mapped in Figure 1. All deep-sea mussels of the genus Bathymodiolus were collected during dives by submersibles, Shinkai 2000, Dolphin 3K, and Hyper Dolphin, from the Japan Agency for Marine-Earth Science and Technology (JAMSTEC) in 1993 to 2011. All the mussels collected for this study were frozen and preserved at −80˚C or preserved in 100% ethanol, and deposited in JAMSTEC.
2.2. Sequencing of Mitochondrial Genes
DNA preparation and sequencing were carried out as described previously [28]. Total DNA was prepared from the foot muscle, gill, or mantle. To amplify the 710-bp fragment including tRNAs and ND4, PCR was performed using a reaction mixture containing the template DNA and KOD dash (TOYOBO Co., Osaka) under the following conditions: initial denaturation for 2 mins at 94˚C, 30 cycles of denaturation for 30 s at 94˚C, annealing for 10 s at 50˚C, and extension for 30 s at 74˚C, final extension for 7 mins at 72˚C. We used the ND4 primers described previously for amplification of fish ND4, sense ArgBL (5’-caagacccttgatttcggctca-3’) and antisense NAP2H (5’-tggagcttctacgtgrgcttt-3’). Direct sequencing was performed by using an ABI PRISM BigDye™ Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems Inc., California) and the primers for PCR on a Model 377 DNA sequencer (Applied Biosystems Inc., California) according to the manufacturer’s instructions. The new sequence data were deposited in the DDBJ, EMBL, and GenBank databases under accession numbers AB762012-762066 and AB762466.
2.3. Analysis
The 423-bp mitochondrial ND4 sequences were edited
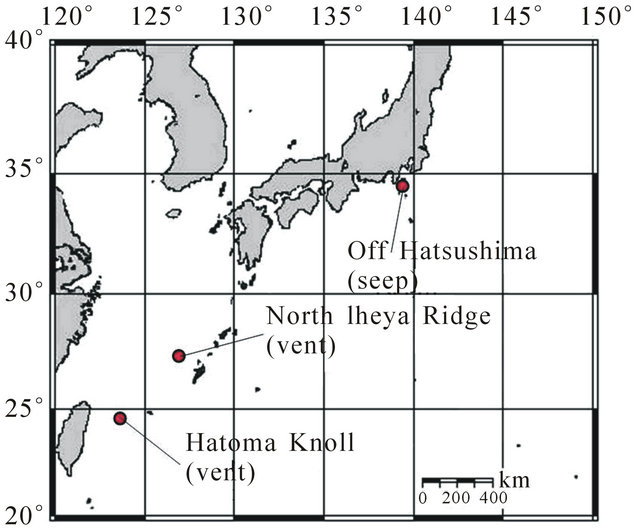
Figure 1. Sampling sites for B. platifrons and B. japonicus. Both species were obtained from seeps of Off Hatsushima in the Sagami Bay and vents of North Iheya Ridge in the Okinawa Trough. Additionally, B. platifrons was obtained from vents of Hatoma Knoll in the Okinawa Trough, where B. japonicus has not been found.
Table 1. Sample list of Bathymodiolus platifrons and Bathymodiolus japonicus.
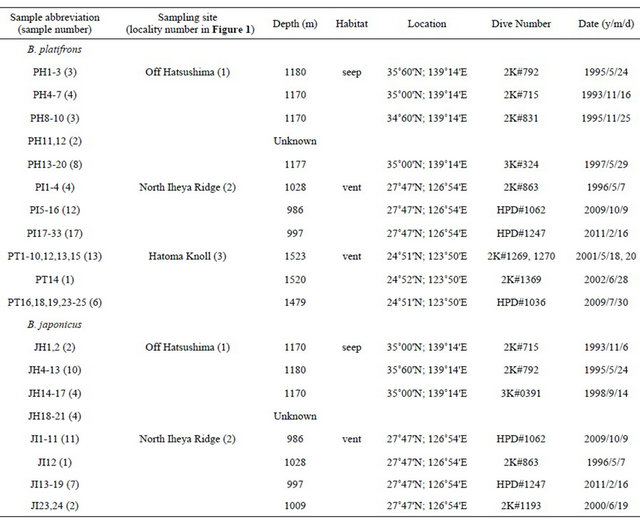
Sample information is presented. A total of 73 samples (20 from Off Hatsushima, 33 from North Iheya Ridge, and 20 from Hatoma Knoll) were used for B. platifrons. A total of 41 samples (20 from Off Hatsushima and 21 from North Iheya Ridge) were used for B. japonicus. 2K, Shinkai 2000; 3K, Dolphin 3K; HPD, Hyper Dolphini in Dive Number.
and aligned using DNASIS (Hitachi Software Engineering Co., Ltd., Tokyo) and MEGA 3.1 [32] and were corrected by visual inspection. We constructed the neighborjoining (NJ) tree using PAUP*4.0 beta10 [33]. Genetic distances were computed according to Kimura’s twoparameter method [34]. The reliability of the tree was evaluated by producing 1000 bootstrap replicates. The majority-rule consensus maximum parsimony (MP) tree was constructed by conducting a heuristic search based on the 1000 bootstrap replicates with an unweighted ts/tv ratio. The Bayesian tree was constructed using MrBayes version 3.1 software [35] based on the model evaluated by the Mrmodel test 2.2 [36]. The Monte Carlo Markov chain (MCMC) length was 5 × 106 generations, and we sampled the chain after every 100 generations. MCMC convergence was assessed by calculating the potential scale reduction factor, and the first 2.5 × 104 generations were discarded.
Based on 423-bp ND4 sequences, we estimated the genetic divergences (Fst) and the bi-directional mean rates of gene flow (Nm; the virtual average number of migrants exchanged per generation) between the populations using Arlequin 3.11 [37]. We evaluated the significance of Fst by calculating 1 × 104 values. We also calculated the mismatch distribution [38] and constructed the minimum spanning tree [39]. We performed goodnessof-fit tests to evaluate discrepancy between the observed and model values of the mismatch distribution. The age of demographic expansion (τ = 2ut) is proportional to the number of generations (t) since a population at equilibrium of size entered a demographic expansion phase, although the mutation rate (u) of mytilid mussels is unknown.
3. Results
In the phylogeny of bathymodioline and related mussels constructed based on the concatenated sequences of mitochondrial ND4 and COI [23], the four species, B. childressi, B. platifrons, B. japonicus, and B. mauritanicus, formed a marginally supported cluster in Group 1. Thus, the phylogenetic relationships of the four species with an outgroup species B. securiformis was depicted (Figure 2) based on the ND4 sequences including all the specimens of B. platifrons and B. japonicus used in this study. Specimens from vents of the Okinawa Trough were not separated from, but intermingled with those from seeps of the Sagami Bay both in B. platifrons and B. japonicus, indicating no clear genetic differentiation between the vent and seep populations.
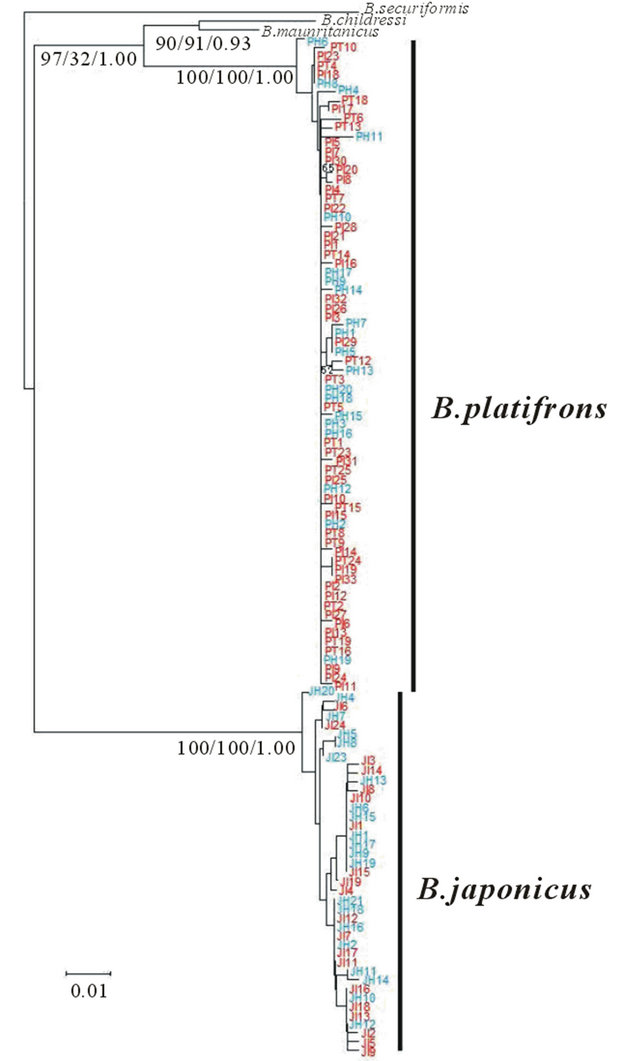
Figure 2. Phylogenetic relationships of the four Bathymodiolus species including all the specimens of B. platifrons and B. japonicus used in this study. Based on the phylogeny described previously, we choose the four species, B. childressi, B. platifrons, B. japonicus, and B. mauritanicus, which formed the marginally supported cluster in Group 1, to construct the NJ, MP, and Bayesian trees using B. securiformis as an outgroup species. Only the NJ (left) and MP (middle) bootstrap values ≥ 90 and the Bayesian (right) posterior probabilities ≥ 0.90 are specified. The scale bar indicates 0.01 substitutions per site. Seep populations in the Sagami Bay are colored blue. PH, Off Hatsushima in B. platifrons; JH Off Hatsushima in B. japonicus. Vent populations in the Okinawa Trough are colored red. PI, North Iheya Ridge in B. platifrons; JI North Iheya Ridge in B. japonicus; PT, Hatoma Knoll in B. platifrons.
The minimum spanning trees were constructed based on the ND4 sequences of a total of 73 specimens (20 Off Hatsushima, 33 North Iheya Ridge, and 20 Hatoma Knoll) in B. platifrons (Figure 3) and a total of 41 specimens (20 Off Hatsushima and 21 North Iheya Ridge) in B. japonicus (Figure 4). The haplotype of the greatest major ity was shared by 41 (56.2%) specimens of B. platifrons from seeps of the Sagami Bay and vents of the Okinawa Trough. The four haplotypes were shared by 24 (40%) specimens of B. japonicus from seeps of the Sagami Bay and vents of the Okinawa Trough. These results also showed no significant genetic differences between the seep and vent populations.
In B. platifrons, the values of Fst were estimated to be −0.01233 between the two vent populations in the
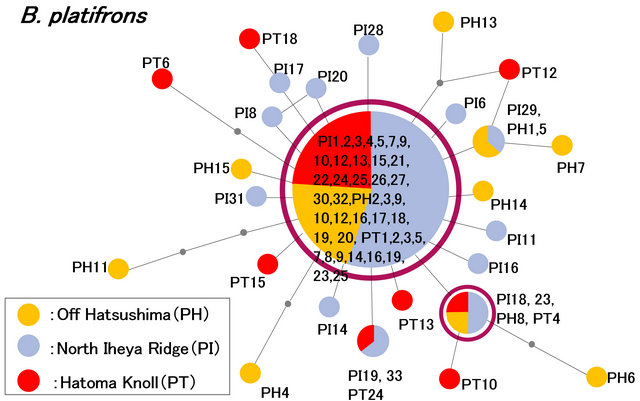
Figure 3. The genetic population structure of B. platifrons based on the 423-bp mitochondrial ND4 sequences. The minimum spanning tree was constructed using total 73 specimens from seeps of Off Hatsushima in the Sagami Bay (PH1-20, yellow) and vents of North Iheya Ridge (PI1-33, blue) and Hatoma Knoll (PT1-20, red) in the Okinawa Trough.
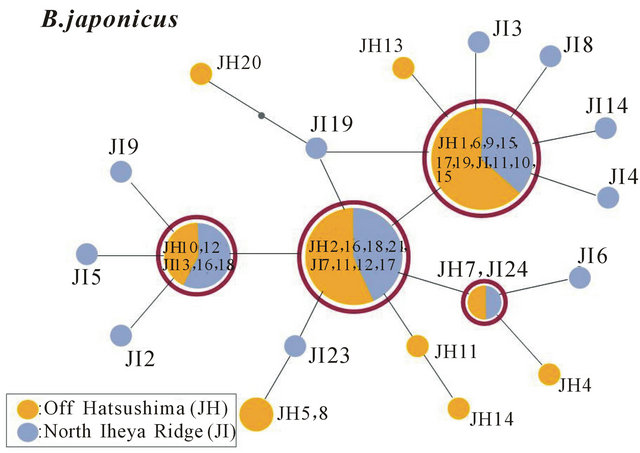
Figure 4. The genetic population structure of B. japonicus based on the 423-bp mitochondrial ND4 sequences. The minimum spanning tree was constructed using total 41 specimens from seeps of Off Hatsushima in the Sagami Bay (JH1-20, yellow) and vents of North Iheya Ridge (JI1-21, blue) in the Okinawa Trough.
Okinawa Trough and to be also nearly 0 (0.00463) between the seep and vent populations (Table 2). The values of Nm were estimated to be infinity between the two vent populations in the Okinawa Trough and between the Off Hatsushima seep and Hatoma Knoll vent populations, and to be very high (32.3) between the Off Hatsushima seep and North Iheya Ridge vent populations (Table 2). An Nm value of more than 1 is indicated to be sufficient to maintain genetic continuity among populations [37]. In B. japonicus, the values of Fst and Nm were estimated to be nearly 0 (0.00027) and infinity, respectively, between the seep and vent populations (Table 2). These results showed no significant genetic differentiation and high gene flow between the seep and vent populations, indicating that the two species has high dispersal ability and the environmental type (vent or seep) is not the primary factor responsible for habitat segregation.
Goodness-of-fit tests in the mismatch distributions showed no significant differences between the observed and model values (1.00 for the Off Hatsushima population, 0.10 for the North Iheya Ridge population, and 0.70 for the Hatoma Knoll population in B. platifrons; 0.55 for the Off Hatsushima population and 0.20 for the North Iheya Ridge population in B. japonicus). The τ values decreased in the order of the Off Hatsushima seep population, Hatoma Knoll vent population, and North Iheya Ridge vent population in B. platifrons (Figure 5), while the values decreased in the order of the North Iheya Ridge vent population and Off Hatsushima seep population in B. japonicus (Figure 6).
4. Discussion
Habitat segregation of deep-sea animals can be ascribed to depth (light strength, temperature, and hydraulic pressure), geographical distances, and environmental (chemical and physical) conditions etc., which are associated with physiological characteristics and evolutionary backgrounds of animal lineages. Habitat segregation in Calyptogena clams is attributable to depth, which is probably related to their differences in the physiological tolerance to hydraulic pressure [6,40]. Habitat segregation of some Bathymodiolus mussels is probably due to their preference to one (or some) specific ambient condi-
Table 2. Fst (below the diagonal) and Nm (above the diagonal) in two Bathymodiolus spp.
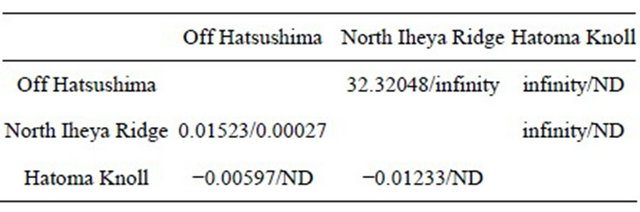
Values for B. platifrons are presented before slash and those for B. japonicus after slash. ND, no data.
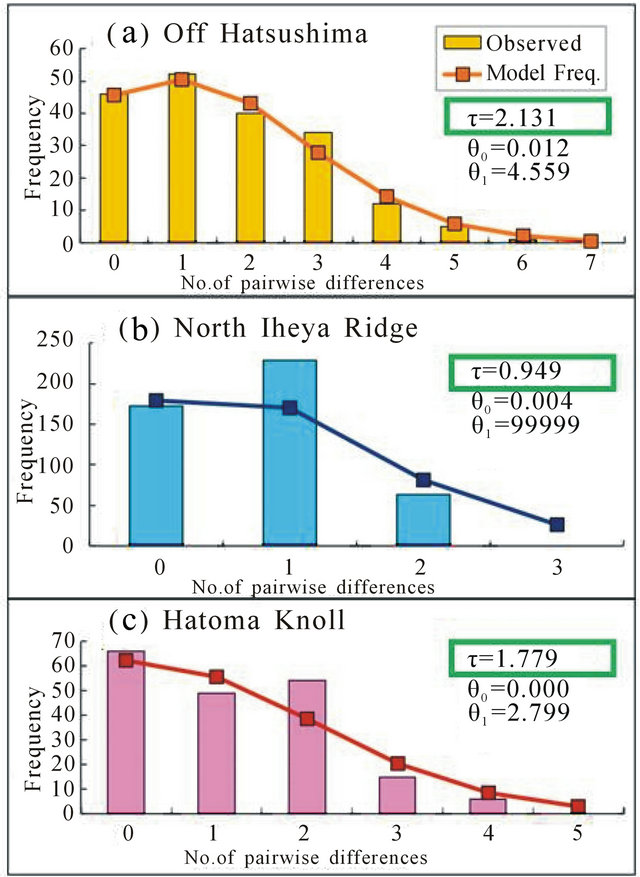
Figure 5. Mismatch distributions based on the 423-bp mitochondrial ND4 sequences in B. platifrons. (a) Off Hatsushima population in the Sagami Bay; (b) North Iheya Ridge population in the Okinawa Trough; (c) Hatoma Knoll population in the Okinawa Trough.
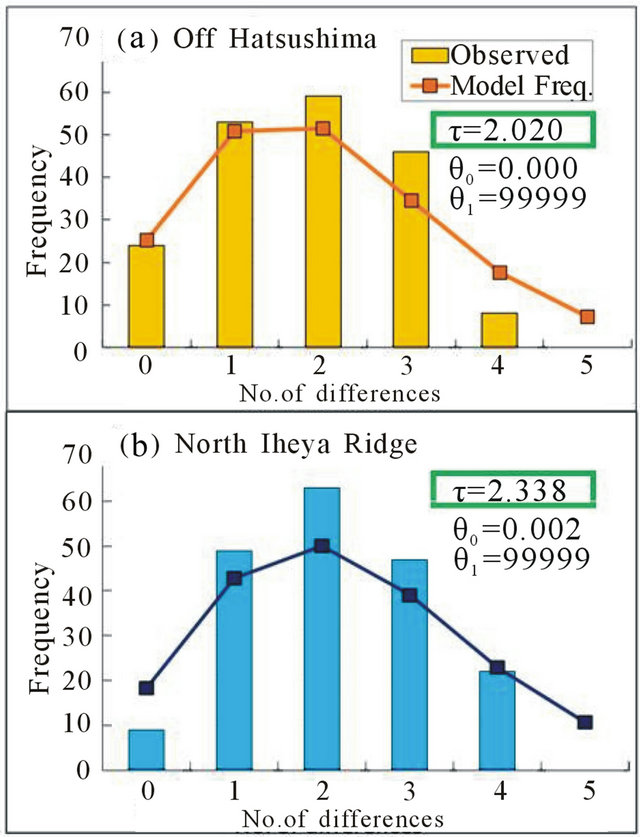
Figure 6. Mismatch distributions based on the 423-bp mitochondrial ND4 sequences in B. japonicus. (a) Off Hatsushima population in the Sagami Bay; (b) North Iheya Ridge population in the Okinawa Trough.
tion(s), but is not constrained by depth [41] or geographical distances [28].
Only three species, B. japonicus, B. platifrons, and B. aduloides, in the Japanese waters can inhabit both vents and seeps, while the other Bathymodiolus species live in either, indicating it difficult for deep-sea mussels to make different environments (vents and seeps) their habitats [11]. Therefore, we hypothesized that even though the vent and seep populations are settled by modern taxonomy in a single species, they must be genetically highly differentiated populations or possibly different species by adapting to their respective environments and accumulating genetic differences. However, the intermingled phylogenetic relationships (Figure 2), many shared haplotypes (Figures 3 and 4), and Fst of nearly 0 (Table 2) definitely indicated almost no genetic differentiation between the vent and seep populations both in B. japonicus and B. platifrons. The very large Nm clearly showed extensive gene flow between the vent and seep populations in the two species (Table 2). These results refuted our hypothesis. The two species have high adaptability to environments.
Environmental differences between vents and seeps have not been elucidated well probably due to large site-to-site variations, but we readily assume the differences. Vents seem more severe and ephemeral than seeps as environments for organisms. The temperature gradient from the maximum of emitting hot water (ca. 350˚C) to the minimum of ambient cold water (2˚C - 3˚C) is large in vents, but no such gradient in seeps. The positions of active emitting sites change frequently in vents. Such temperature regime is critical for deep-sea mussels, because they stick by byssi to substrates and cannot move promptly according to changes in the temperature regime. Since vents persist for only a few decades [3], vent animals should disperse propagules more effectively to other vent sites. On the other hand, active emitting sites in vents can supply more chemicals including methane and hydrogen sulfide than oozing sites in seeps. Methane and hydrogen sulfide are toxic to animals, but rear chemosynthetic symbionts that support life of deep-sea mussels. Bathymodiolus japonicus and B. platifrons have methanotrophs in their epithelial cells of gills [42]. Nevertheless, since the two species can inhabit both vents and seeps, it is possible that the environmental differences are smaller than we expect. However, it is not likely, because the other species except the three species in the Japanese waters can live only in either.
The Sagami Bay and the Okinawa Trough are approximately 1500 km away, but North Iheya Ridge and Hatoma Knoll in the Okinawa Trough is only 300 km away. It is surprising that in B. japonicus and B. platifrons, the same extents of genetic differentiation and gene flow were presented in this study between the Sagami Bay and the Okinawa Trough and between the two vent sites in the Okinawa Trough. These results indicate that the two species have high dispersal ability. The genetic similarity between the populations from the Sagami Bay and the Okinawa Trough was also shown in the deep-sea bresiliid shrimp Alvinocaris longirostris [43]. We assume that the intense stream by the Kuroshio Current, which runs in the direction from the Okinawa Trough to the Sagami Bay down to depth of 1000 m, has fundamental effects on transporting propagules. High dispersal ability of deep-sea animals has been suggested by previous studies [4-8,26-28]. Another factor that warrants long distance dispersal is developmental arrest at cold temperatures in deep sea [44]. The planktotrophic stage in deep-sea mussels can increase dispersal distances. Vestimentiferan Riftia pachyptila had a larval stage of approximately 38 d under conditions similar to the in situ environment, suggesting that larvae can disperse over 100 km [45]. The larvae of the verrucomorph barnacle Neoverruca sp. had a planktonic period of over 70 d at 4˚C under 1 atm [46].
Considering the present results, it is difficult to explain why B. japonicus is missing in Hatoma Knoll. No significant differences of dispersal ability and environmental adaptability were found between B. platifrons and B. japonicus. The only presence of B. platifrons in Hatoma Knoll may have been caused by its frontier effect. When the two species have similar ecological characteristics, it seems difficult that the second species colonize the same locality after the frontier established its ecological position. However, in North Iheya Ridge B. platifrons is more dominant than B. japonicus, suggesting that slight differences in environmental preference may exist between B. platifrons and B. japonicus and vents in the Okinawa Trough advantage B. platifrons over B. japonicus.
In mismatch distributions, our previous study failed to support by goodness-of-fit tests the match between the observed and model values for the Okinawa Trough population of B. platifrons, in which we used the combined samples from three different localities because of the lack of samples in a single locality [28]. Thereafter, we collected and accumulated samples and constructed mismatch distributions using more than 20 samples of B. platifrons each for two vent localities in the Okinawa Trough and one seep locality in the Sagami Bay. Consequently, we succeeded to support no significant difference between the observed and model values in the three localities for B. platifrons as well as the two localities for B. japonicus. The unimodal patterns in the mismatch distributions indicated population expansion in the two species.
Mismatch distributions in this study showed that the τ values decreased in the order of the Off Hatsushima seep population, Hatoma Knoll vent population, and North Iheya Ridge vent population in B. platifrons (Figure 5). The results support our previous conclusions [28], and suggest the immigration of ancestral B. platifrons from the Sagami Bay to the Okinawa Trough. The process is consistent with the history of the Japanese archipelago, because the Okinawa Trough has been habitable for animals in the chemosynthesis-based community since ca. 200 MYA, while the Sagami Bay since more than 500 MYA [47]. On the other hand, our new data for B. japonicus showed that the τ values decreased in the order of the North Iheya Ridge vent population and Off Hatsushima seep population (Figure 6). This suggests the opposite immigrational route from the Okinawa Trough to the Sagami Bay. However, it seems unlikely that these closely related species propagated in different ways. We think we cannot presume immigrational pathways under extensive gene flow between the relevant localities, because genetic compositions are rigorously mixed up. Therefore, our present data support extensive gene flow between the Okinawa Trough and the Sagami Bay rather than suggest immigrational pathways.
REFERENCES
- P. Lonsdale, “Clustering of Suspension-Feeding Macrobenthos near Abyssal Hydrothermal Vents at Oceanic Spreading Centers,” Deep-Sea Research, Vol. 24, No. 9, 1977, pp. 857-863. doi:10.1016/0146-6291(77)90478-7
- D. Jollivet, “Specific and Genetic Diversity at Deep-Sea Hydrothermal Vents: An Overview,” Biodiversity and Conservation, Vol. 5, No. 12, 1996, pp. 1619-1653. doi:10.1007/BF00052119
- V. Tunnicliffe and S. K. Juniper, “Cosmopolitan Underwater Fauna,” Nature, Vol. 344, No. 6424, 1990, p. 300. doi:10.1038/344300a0
- S. Kojima, S. Ohta, T. Yamamoto, T. Miura, Y. Fujiwara, K. Fujikura and J. Hashimoto, “Molecular Taxonomy of Vestimentiferans of the Western Pacific and Their Phylogenetic Relationship to Species of the Eastern Pacific. II. Families Escarpiidae and Arcovestiidae,” Marine Biology, Vol. 141, No. 1, 2002, pp. 57-64.
- S. Kojima, S. Ohta, T. Yamamoto, T. Miura, Y. Fujiwara and J. Hashimoto, “Molecular Taxonomy of Vestimentiferans of the Western Pacific and Their Phylogenetic Relationship to Species of the Eastern Pacific. I. Family Lamellibrachiidae,” Marine Biology, Vol. 139, No. 2, 2001, pp. 211-219. doi:10.1007/s002270100581
- K. Fujikura, S. Kojima, Y. Fujiwara, J. Hashimoto and T. Okutani, “New Distribution Records of Vesicomyid Bivalves from Deep-Sea Chemosynthesis-Based Communities in Japanese Waters,” Venus, Vol. 59, No. 2, 2000, pp. 103-121.
- R. C. Vrijenhoek, “Gene Flow and Genetic Diversity in Naturally Fragmented Metapopulations of Deep-Sea Hydrothermal Vent Animals,” Journal of Heredity, Vol. 88, No. 4, 1997, pp. 285-293. doi:10.1093/oxfordjournals.jhered.a023106
- S. Kojima, R. Segawa, Y. Fujiwara, K. Fujikura, S. Ohta and J. Hashimoto, “Phylogeny of Hydrothermal-VentEndemic Gastropods Alviniconcha spp. from the Western Pacific Revealed by Mitochondrial DNA Sequences,” Biological Bulletin, Vol. 200, No. 3, 2001, pp. 298-304. doi:10.2307/1543511
- V. C. Kenk and B. R. Wilson, “A New Mussel (Bivalvia: Mytilidae) from Hydrothermal Vents in the Galapagos Rift Zone,” Malacologia, Vol. 26, No. 1-2, 1985, pp. 253- 271.
- R. von Cosel, B. Métivier and J. Hashimoto, “Three New Species of Bathymodiolus (Bivalvia: Mytilidae) from Hydrothermal Vents in the Lau Basin and the North Fiji Basin, Western Pacific, and the Snake Pit Area, Mid-Atlantic Ridge,” Veliger, Vol. 37, 1994, pp. 374-392.
- J. Hashimoto and T. Okutani, “Four New Mytilid Mussels Associated with Deep-Sea Chemosynthetic Communities around Japan,” Venus, Vol. 53, 1994, pp. 61-83.
- R. von Cosel and K. Olu, “Gigantism in Mytilidae. A New Bathymodiolus from Cold Seep Areas on the Barbados Accretionary Prism,” ComptesRendus de l’Academie des Sciences Paris, Vol. 321, No. 8, 1998, pp. 655-663.
- R. G. Gustafson, R. D. Turner, R. A. Lutz and R. C. Vrijenhoek, “A New Genus and Five New Species of Mussels (Bivalvia: Mytilidae) from Deep-Sea Sulfide/Hydrocarbon Seeps in the Gulf of Mexico,” Malacologia, Vol. 40, No. 1-2, 1998, pp.63-112.
- R. von Cosel, T. Comtet and E. M. Krylova, “Bathymodiolus (Bivalvia: Mytilidae) from Hydrothermal Vents on the Azores Triple Junction and the Logatchev Hydrothermal Field, Mid-Atlantic Ridge,” Veliger, Vol. 42, 1999, pp. 218-248.
- J. Hashimoto, “A New Species of Bathymodiolus (Bivalvia: Mytilidae) from Hydrothermal Vent Communities in the Indian Ocean,” Venus, Vol. 60, No. 3, 2001, pp. 141- 149.
- R. von Cosel, “A New Species of Bathymodioline Mussel (Mollusca, Bivalvia, Mytilidae) from Mauritania (West Africa), with Comments on the Genus Bathymodiolus Kenk & Wilson, 1985,” Zoosystema, Vol. 24, 2002, pp. 259-271.
- R. von Cosel and B. A. Marshall, “Two New Species of Large Mussels (Bivalvia: Mytilidae) from Active Submarine Volcanoes and a Cold Seep off the Eastern North Island of New Zealand, with Description of a New Genus,” Nautilus, Vol. 117, No. 2, 2003, pp. 31-46.
- T. Okutani, K. Fujikura and T. Sasaki, “Two New Species of Bathymodiolus (Bivalvia: Mytilidae) from Methane Seeps on the Kuroshima Knoll off Yaeyama Islands, Southwestern Japan,” Venus, Vol. 63, 2004, pp. 97-110.
- J. Hashimoto and M. Furuta, “A New Species of Bathymodiolus (Bivalvia: Mytilidae) from Hydrothermal Vent Communities in the Manus Basin, Papua New Guinea,” Venus, Vol. 66, No. 1-2, 2007, pp. 57-68.
- R. von Cosel, “A New Bathymodioline Mussel (Bivalvia: Mytiloidea: Mytilidae: Bathymodiolinae) from Vent Sites near Kueishan Island, North East Taiwan,” The Raffles Bulletin of Zoololgy, Vol. 19, 2008, pp. 105-114.
- R. von Cosel and R. Janssen, “Bathymodioline Mussels of the Bathymodiolus (S. L.) childressi clade from Methane Seeps near Edison Seamount, New Ireland, Papua New Guinea (Bivalvia: Mytilidae),” ArchivfürMolluskenkunde, Vol. 137, No. 2, 2008, pp. 195-224.
- Y. Fujita, H. Matsumoto, Y. Fujiwara, J. Hashimoto, S. V. Galkin, R. Ueshima and J.-I. Miyazaki, “Phylogenetic Relationships of Deep-Sea Bathymodiolus Mussels with Their Mytilid Relatives from Sunken Whale Carcasses and Wood,” Venus, Vol. 67, No. 3-4, 2009, pp. 123-134.
- J.-I. Miyazaki, L. O. Martins, Y. Fujita, H. Matsumoto and Y. Fujiwara, “Evolutionary Process of Deep-Sea Bathymodiolus Mussels,” PLoS ONE, Vol. 5, No. 4, 2010, p. e10363. doi:10.1371/journal.pone.0010363
- J. Lorion, B. Buge, C. Cruaud and S. Samadi, “New Insights into Diversity and Evolution of Deep-Sea Mytilidae (Mollusca: Bivalvia),” Molecular Phylogenetics and Evolution, Vol. 57, No. 1, 2010, pp. 71-82. doi:10.1016/j.ympev.2010.05.027
- T. Koito, J. Hashimoto, S. Nemoto, M. Kitajima, M. Kitada and K. Inoue, “New Distribution Record of DeepSea Mussel, Bathymodiolus aduloides (Mollusca: Bivalvia: Mytilidae) from a Hydrothermal Vent, Myojinsho,” Marine Biodiversity Records, Vol. 5, 2012, pp. 1-5.
- R. A. Lutz, P. Bouchet, D. Jablonski, R. D. Turner and A. Warén, “Larval Ecology of Mollusks at Deep-Sea Hydrothermal Vents,” American Malacological Bulletin, Vol. 4, 1986, pp. 49-54.
- M. Le Pennec and P. G. Beninger, “Reproductive Characteristics and Strategies of Reducing-System Bivalves,” Comparative Biochemistry and Physiology A, Vol. 126, No. 1, 2000, pp. 1-16. doi:10.1016/S0742-8413(00)00100-6
- A. Kyuno, M. Shintaku, Y. Fujita, H. Matsumoto, M. Utsumi, H. Watanabe, Y. Fujiwara and J.-I. Miyazaki, “Dispersal and Differentiation of Deep-Sea Mussels of the Genus Bathymodiolus (Mytilidae, Bathymodiolinae),” Journal of Marine Biology, Vol. 2009, 2009, Article ID 625672.
- R. Varney, C. E. Galindo-Sánchez, P. Cruz, and P. M. Gaffney, “Population Genetics of the Eastern Oyster Crassostrea virginica (Gmelin, 1791) in the Gulf of Mexico,” Journal of Shellfish Research, Vol. 28, No. 4, 2009, pp. 855-864. doi:10.2983/035.028.0415
- E. A. Saillant, M. A. Renshaw, N. J. Cummings, and J. R. Gold, “Conservative Genetics and Management of Yellowtail Snapper, Ocyurus chrysurus, in the US Caribbean and South Florida,” Fisheries Management and Ecology, Vol. 19, No. 4, 2012, pp. 301-312. doi:10.1111/j.1365-2400.2011.00840.x
- E. McCartney-Melstad, T. Waller, P. A. Micucci, M. Barros, J. Draque, G. Amato and M. Mendez, “Population Structure and Gene Flow of the Yellow Anaconda (Eunectesnotaeus) in Northern Argentina,” PLoS ONE, Vol. 7, No. 5, 2012, p. e37473. doi:10.1371/journal.pone.0037473
- S. Kumar, K. Tamura and M. Nei, “MEGA3: Integrated Software for Molecular Evolutionary Genetics Analysis and Sequence Alignment,” Briefings in Bioinformatics, Vol. 5, No. 2, 2004, pp. 150-163. doi:10.1093/bib/5.2.150
- D. L. Swofford, “PAUP*: Phylogenetic Analysis Using Parsimony (and Other Methods) 4.0 Beta,” Sinauer Associates, Sunderland, 2002.
- M. Kimura, “A Simple Methods for Estimating Evolutionary Rate of Base Substitution through Comparative Studies of Nucleotide Sequences,” Journal of Molecular Evolution, Vol. 16, No. 2, 1980, pp. 111-120. doi:10.1007/BF01731581
- J. P. Huelsenbeck, F. Ronquist, R. Nielsen, and J. P. Bollback, “Bayesian Inference of Phylogeny and Its Impact on Evolutionary Biology,” Science, Vol. 294, No. 5550, 2003, pp. 2310-2314. doi:10.1126/science.1065889
- D. Posada and T. R. Buckley, “Model Selection and Model Averaging in Phylogenetics: Advantages of the AIC and Bayesian Approaches over Likelihood Ratio Tests,” Systematic Bioliology, Vol. 53, No. 5, 2004, pp. 793-808. doi:10.1080/10635150490522304
- L. Excoffier, G. Laval and S. Schneider, “Arlequin Ver. 3.0: An Integrated Software Package for Population Genetics Data Analysis,” Evolutionary Bioinformatics Online, Vol. 1, 2005, pp. 47-50.
- S. Schneider and L. Excoffier, “Estimation of Past Demographic Parameters from the Distribution of Pairwise Differences when the Mutation Rates Vary among Sites: Application to Human Mitochondrial DNA,” Genetics, Vol. 152, No. 3, 1999, pp. 1079-1089.
- L. Excoffier and P. E. Smouse, “Using Allele Frequencies and Geographic Subdivision to Reconstruct Gene Trees within a Species: Molecular Variance Parsimony,” Genetics, Vol. 136, No. 1, 1994, pp. 343-359.
- K. Olu, A. Duperret, M. Sibuet, J.-P. Foucher and A. Fiala-Médioni, “Structure and Distribution of Cold Seep Communities along the Peruvian Active Margin: Relationship to Geological and Fluid Patterns,” Marine Ecology Progress Series, Vol. 132, 1996, pp. 109-125. doi:10.3354/meps132109
- J.-I. Miyazaki, M. Shintaku, A. Kyuno, Y. Fujiwara, J. Hashimoto and H. Iwasaki, “Phylogenetic Relationships of Deep-Sea Mussels of the Genus Bathymodiolus (Bivalvia: Mytilidae),” Marine Biology, Vol. 144, No. 3, 2004, pp. 527-535. doi:10.1007/s00227-003-1208-3
- Y. Fujiwara, K. Takai, K. Uematsu, S. Tsuchida, J. C. Hunt and J. Hashimoto, “Phylogenetic Characterization of Endosymbionts in Three Hydrothermal Vent Mussels: Influence on Host Distributions,” Marine Ecology Progress Series, Vol. 208, 2000. pp. 147-155. doi:10.3354/meps208147
- G. Tokuda, A. Yamada, K. Nakano, N. Arita and H. Yamasaki, “Occurrence and Recent Long-Distance Dispersal of Deep-Sea Hydrothermal Vent Shrimps,” Biological Letters, Vol. 2, No. 2, 2006, pp. 257-260. doi:10.1098/rsbl.2005.0420
- F. Pradillon, B. Shillito, C. M. Young and F. Gaill, “Developmental Arrest in Vent Worm Embryos,” Nature, Vol. 413, No. 6857, 2001, pp. 698-699. doi:10.1038/35099674
- A. G. Marsh, L. S. Mullineaux, C. M. Young and D. T. Manahan, “Larval Dispersal Potential of the Tubeworm Riftia pachyptila at Deep-Sea Hydrothermal Vents,” Nature, Vol. 411, No. 6833, 2001, pp. 77-80. doi:10.1038/35075063
- H. Watanabe, “Dispersal and Evolution in Chemoautosynthesis-Based Communities in the Western Pacific-Verrucomorphs as Test Species for Evolutionary Studies on
- Hydrothermal Vent-Endemic Animals-,” Japanese Journal of Benthology, Vol. 58, 2003, pp. 44-49.
- A. Taira, “Nihon-Rettou No Tanjou,” Iwanami-Shoten, Tokyo, 2003.
NOTES
*Corresponding author.

