Open Journal of Immunology
Vol.3 No.2(2013), Article ID:32678,8 pages DOI:10.4236/oji.2013.32009
Evolutionary adaptations of human cancer for parasitic life

Department of Evolutionary Immunology, Andent, Inc., Jersey City, USA; rumyan1@yahoo.com
Copyright © 2013 Sergey N. Rumyantsev. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 5 May 2013; revised 21 May 2013; accepted 28 May 2013
Keywords: Cancer Genealogy; Cancer Physiology; Cancer Reproduction; Hereditary Immunity; Make-Up of Cancer; Ontogeny of Cancer
ABSTRACT
According to the xenogamous paradigm of cancer origin, pathogenesis and epidemic spread, human cancer is a disease caused by the appearance in the afflicted body of deviant multicellular structures whose cells are aggressive (gobble the afflicted body; grow and divide without respect to normal limits), invasive (invade and destroy adjacent tissues), metastatic (dispersed over embryogenesis at different locations in the body) and transmissible. The causative agent of the human disease has only just been identified as an ancient, unprecedentedly unique parasitic being that sustains itself at the expense of substances and energy derived from its victim’s body. Presented integrative discovery consists of a more systematic description of main adaptations of cancer causative agent to this specific way of life developed over its evolution. Focus is on the main stages of cancer existence including cancerous invasion of a human body, make-up of the parasite, its self-protection from the victim’s immune defense and regulatory management, disposition of cancer sub-units around afflicted body, the self-management of cancer and its nutrition, communication between dispersed cancer units, physiological synchronization between them, horizontal (reproductive) way of cancer transmission between humans.
1. INTRODUCTION
According to the xenogamous paradigm of cancer origin, pathogenesis and epidemic spread, human cancer is a disease caused by the appearance in the afflicted body of deviant multicellular structures whose cells are aggressive (gobble the affected body; grow and divide without respect to normal limits), invasive (invade and destroy adjacent tissues), metastatic (dispersed over embryogenesis at different locations in the body) and transmissible. The causative agent of the human disease has only just been identified as an ancient, unprecedentedly unique parasitic being that sustains itself at the expense of substances and energy derived from its victim’s body. Presented integrative discovery consists of a systematic description of main adaptations of cancer causative agent to this specific way of life developed over its evolution accounted for by hundreds of millennia of generations.
Focus is on the main stages of cancer existence which include cancerous invasion of a human, make-up of the parasite, its self-protection from the victim’s immune defense and regulatory management, disposition of cancer sub-units around the afflicted body, the self-management of cancer and its nutrition, physiological synchronization and communication between its dispersed sub-units, the reproduction of cancer and its transmission between humans. All these traits of human cancer are considered from the viewpoint of their involvement in the evolutionary adaptation of the parasite.
2. MATERIALS AND METHODS
The article presents new results from reconsidering and re-sensing various either direct or indirect data regarding cancer epidemiology, clinical manifestations, and molecular pathogenesis from the viewpoint of up-to-date, all-pathological, immunogenetic, genetic, and evolutionary discoveries up to cellular, subcellular and molecular levels. Various appropriate data regarding the theme from the literature has been summarized with the data of longterm investigations performed by the author together with the team he leads [1].
3. RESULTS AND DISCUSSION
3.1. Cancerous Invasion of Humans
Cancer is caused by the appearance in a human body of a deviant cell lineage uncontrolled by this body’s habitual system of regulation of cell division and tissue growth. The uncontrollability is predetermined by constitutional insusceptibility of cancerous cells to the mediators of habitual regulation of cell division and tissue growth [2,3]. This intrinsic trait of cancerous cells is their ultimate evolutionary adaptation for carcinogenesis. Such deviant cell lineages can appear in a human body as a result of genome transformation performed over the heterozygous crossbreeding between parental gametes with partially different (divergent) genotypes [4]. This is a kind of chimerism or cellular mosaicism, the occurrence in an individual of at least two or more cell clones of different constitutions of genome, derived from different parental individuals [5,6]. Such heterozygous mosaicism arises as a result of hybridization between organisms genetically different in some of the relevant traits [2,3]. For instance, one of the patents is constitutionally sensitive to appropriate physiological regulators whereas its mating partner is constitutionally immune to it [1].
The heterozygosity results in the coexistence in the offspring’s genome of at least two active allelomorphic genes. Both alleles function dominantly and create two allelic cell lineages. Heterozygous offspring express both alleles equally but in different sizes and in separate locations around the body. Over such xenogamous formation of the descendant’s zygote, its genome becomes admixed with a block of both habitual and aberrant genes, leading to the formation in the offspring’s body of coexisting cell clones with opposite relation to autochthonous regulators of cell division and tissue growth and with opposite predisposition to carcinogenesis [4,7]. Thus, genes of human cancer do not exist. The function of immediate genomic causative agent of carcinogenesis is performed by a deviant gamete inserted with deviant genes incongruent to the victim’s habitual system of cell regulation.
For most of its evolutionary history, humankind shared the Earth with multiple humanlike subspecies. Early Homo sapiens mated with these kindred species and produced fertile offspring, and people today carry DNA inherited from these hybrid ascendants. Such interbreeding enriched the offspring’s genomes and thus played a very important role in the explosive evolution of humankind by means of natural selection.
The set of constitutional adaptive traits could be thought to be a result of evolution over many hundreds of millennia. The date of its initiation could be referred, for instance, to regular hybridization and exchange of genes between mutual ancestors of chimps and humans that may have occurred over a few million years [8] as well as to the epoch of xenogamous intercourse of European Homo sapiens with Homo neandertalensis. The last gene flow from Neanderthals (or their relatives) into Europeans likely occurred 37,000 - 86,000 years ago, and most likely 47,000 - 65,000 years ago [9].
Xenogamous mating between members of such genetically different subspecies and ethnoses led to the intrusion of the descendant’s genome with components of deviant genetic information that induce intra-individual diversity of cell lineages [10]. Some of the cells appear to own the main trait of cancerous cells, the genetic resistance to habitual regulators of cell division. The descent and consequent subsistence of human cancer includes regular obligatory alternation of successive forms (Table 1).
The coexistence in xenogamous zygotes of relevant opposite genes leads to the appearance in the afflicted human body of cell lineages resistant to habitual regulation of cell division and tissue growth. The lineages and their extracellular associates initially formed the microlocations and then the clinically-detectable locations of cancerous tissue, the tumors.
3.2. Make-Up of Developed Human Cancer
The bankrupt paradigm initially allowed only one cancerous clone in an affected body. In contrast to its dogmas, the possibility of a number of such clones was recently documented. First doubts were revealed by integrative analyses of epidemiological observations [3], according to which multiple cancers comprise two or more primary cancers occurring in an individual that originate in a primary site or tissue and are neither an extension nor a recurrence or metastasis [12].
Cancer patients have a 20% higher risk of new primary cancer compared with the general population. Approximately one third of cancer survivors aged >60 years were diagnosed more than once with another cancer. As the number of cancer survivors and older people increases, occurrence of multiple primary cancers is also likely to increase [12-16].
Such observations induced the idea of the possible existence in cancerous tissue of a lot of appropriate clones. This means that like any other multicellular being, cancer may contain a variety of different cells and associated extracellular structures that are under different genetic regulation and may perform different functions at differrent stages of cancer development [3,7].
Later it was determined that cancer is sustained by the production of aberrant cells that vary in many morphological and physiological properties. Totally, the repopulation dynamics of 150 single lentivirus-marked lineages from ten human colorectal cancers were followed. Such functionally heterogeneous cell lineages varied with respect to their distinctive structural or physiological func-
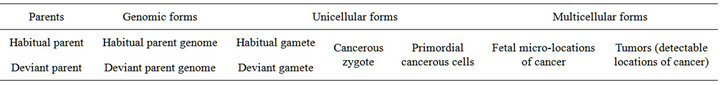
Table 1. Successive forms in cancer subsistence (according to [11], updated).
tions and potentials. Some clones were able to become dormant and undetectable, but became abundant in later generations [17].
The heterogeneity within the couple of tumor cell lineages may also determine the differences within the kinds of tumors and their locations. Cancer maintains its heterogeneous structural stability through many generations. The diversity of cancer composition remains stable over its sequential long-term propagation [17]. The presence of various slow-growing dormant clones was also evidenced by the re-emergence of previously minor clones after chemotherapy and their ability to initiate new tumors (although of a smaller size) over subsequent transplantations of the tumors in experiments [18].
Incipient micro-populations of cancerous cells are formed, distributed and disposed in the afflicted body before postnatal ontogenesis in a form of distantly separated micro-populations, and their initial sizes are differrent but very small. The cancerous sub-units are dispersed around the body either stochastically or in a manner not yet understood. Accordingly, the formation of subunits before postnatal ontogenesis allows them not to be eliminated by mechanisms of adaptive immunity performed by the lymphatic system [7].
Different locations of cancerous units begin to be clinically detectable at different times after initiation of malignant growth, which allows for the supposition of differences in their initially smallest sizes. The differences in initial cancer cell masses and their dislocation around the body predestine individual diversity in the course and severity of cancer when the disease develops [4].
At a relevant time of a victim’s life (mainly after 40 years of age), the uncontrollable growth of such microsub-populations becomes visible in the form of detectable extra cell masses of cancerous tissue, the malignant tumors. The largest of the sub-populations achieves the size of detectable tumors far earlier than the smaller ones, thus forming the first appeared cell mass usually called the “primary” tumor. The sub-populations of initially lesser sizes may become visible in the form of “secondary” detectable tumors, the “metastases”.
The set of separated cancer sub-units functions like the integral whole, the united organism consisting of many homologous sub-organisms. The make-up of cancer presents a kind of super organism. This undoubtedly adaptive trait enhances the possibility of the invading parasite to colonize in a victim’s body the maximal quantity of locations appropriate for further development.
3.3. Self-Protection of Cancerous Invader
3.3.1. Protection of the Victim Immune Defense
Human cancer invades its victim with no immune rejection. The malignant cells and tissues are inherently protected from destruction by cell and humoral mechanisms launching by the victim lymphatic system of responsive immunogenesis. Cancerous cell are not recognized by the victim immune system as non-self because their surface does not contain relevant molecules of the mayor histocompatibility complex that are essential to the antigen-processing pathway. Such traits allow cancer to evade the surveillance performed by the victim’s system of immunogenesis. This protection is predetermined by the germ line of the formation of cancerous cells directly from the zygote over the prenatal development of the afflicted organism [3]. This trait of cancer ontogeny is undoubtedly of evolutionary adaptation which provides the parasite with the long life ability to escape rejection by the victim’s immune response.
3.3.2. Protection of the Victim Management
Any living being is constitutionally provided with a physiological system that maintains normal body structure within its genetically predetermined shape, size and function. A special part of this very important and effective system is dedicated to managing the starting and revival of body structures and functions on molecular, subcellular, cellular, tissue and organ levels. Habitual cells of a normal organism grow and divide to form new cells as the body needs them. When cells grow old and die, new cells take their place. The regulation is realized on the level of cells and performed by means of molecular humoral agents.
In the case of cancer invasion, this orderly process goes wrong. The mighty system of body management and maintenance appears impotent in relation even to some its initially smallest parts, the sub-units of cancer. Cancerous cells grow and divide independent of habitual physiological management. That happens because cancer cells and tissues possess absolute constitutional immunity to the agents of habitual physiological management of cell division and tissue formation. Constitutional (hereditary) immunity of the cells against relevant physiological regulators can be created by structural incongruence between regulators and their receptors. The existence of such specific immunity is considered as the obligatory prerequisite to malignity [7].
Cancer cells continue dividing and forming masses of relevant tissue when the afflicted body does not need them. In addition, the cancerous cells of older generations do not die when their habitual peers do. Thus the appearance of extra cells which form the masses of tissue called malignant tumors. This innate (constitutional) trait of cancerous cells is of most adaptive, pathogenic importance. Innate immunity of cancerous cells enables them to perform their obligatory adaptation, which functions in all stages of cancer including initiation, development and subsequent progression.
3.4. Self-Management of Cancer
The uncontrollably growing populations of a cancerous unit could produce its own humoral regulators, some of which may mediate the own physiology of cancerous cells and tissue. The regulators may function within separated cancerous units of a cancer and between its distantly dispersed sub-units.
3.4.1. Communications between Dispersed Sub-Units
The existence of inter-tumor communications was hypothesized in [19] and confirmed in a host of other studies, many of which are reviewed in [20,21]. It was noted that large tumors inhibit the growth of smaller tumors and thwart the inception of new tumors [20,22-24]. Extirpation of larger tumors triggers accelerated proliferation of smaller, dormant or slow-growing cancerous units.
The removal of a primary tumor could accelerate the growth of sub-units that had been inhibited. Accelerated progression of cancerous units after foregoing resection was noted in experimental [25-27] and clinical [28,29] studies.
Acceleration in the rate of growth of secondary subunits was found after a 70% ectomy of cancerous liver [30]. Resection of other primary tumors was followed by a 32-fold increase in the rate of secondary tumor growth [24]. Maximally early extirpation of the first appeared cancer unit does not prevent subsequent appearance of “secondary” units [31,32]. This may mean that at the time of the resection, cancer already existed elsewhere in the body in the form of undetectable micro-populations.
The proposed mechanism was that tumors produce humoral factors able either to promote or inhibit tumor growth and angiogenesis. Removal of the primary tumor reduces the production of growth inhibitors and proapoptosis factors and signals, which accelerates the growth of smaller sub-units [24].
This important finding was directly confirmed in a number of well-documented clinical case studies involving various types of cancer. For instance, in eight cases of testicular cancer, resection of voluminous tumors caused a dramatic exacerbation of the disease [33]. Excision of primary melanomas precipitated the appearance of new sub-units in three skin cancer patients [34,35]. In one case of pancreatic cancer, excision of the primary adenocarcinoma caused surfacing in liver of numerous previously undetectable sub-units [36].
A woman diagnosed with breast cancer had a tumor of 10.3 cm3 in volume. The tumor was resected. However, eight years later, 37 previously undetectable cancerous sub-units were discovered in her bones, lung, lymph nodes and soft tissue. Volumes of 31 bone tumors ranged from 1.69 cm3 to 22.96 cm3. Three lung tumors had the volumes from 1.30 cm3 to 7.26 cm3, two lymph node tumors had the volumes of 2.85 cm3 and 9.66 cm3, and one tumor had the volume of 11.41 cm3. In two other breast cancer patients, 20 and 15 bone tumors became detectable 5.5 years and 9 months after primary resection, respectively [24].
Thus the life of all sub-populations of cancerous cells is controlled by their own united physiological mechanism which maintains the whole structure of cancer within a genetically predetermined size. The destruction of one or more sub-units of cancer boosts the growth of other sub-units. The set of separated cancer sub-units functions like the integral whole, a physiologically and ecologically-united organism consisting of many identical sub-organisms. This is a kind of multicellular super organism.
3.4.2. Physiological Synchronization between Cancer Sub-Units
Human cancer possesses its own schedule (program of ontogenesis) as well as physiological synchronization between its sub-units. The existence of these intrinsic traits has been initially estimated [2,3,21,37] with detailed presentation and discussion of the evidence. The proposed mechanism was that cancer’s genome contains a functional program of development over alternation of its successive forms in time. Later, the existence of these traits was supported by experiments showing the progress of experimental cancer (implanted melanoma) in the mouse model is synchronized with eventual progress of the disease in human patients, the source of mouse implantation. Conversely, melanomas that did not progress after surgical removal of the primary tumors from patients also developed slowly or inefficiently in implanted animals, even after repeated passages of tumor cells through several generations of mice. This finding also demonstrated that the key factors that regulate the rate of cancer and mode of development are intrinsic to the invading cancerous matter [38]. The existence of the functional in-time synchronization evidences the adaptive evolutionary origin of this characteristic of cancer.
3.4.3. Cancer’s Management of the Victim’s Body
The complex interactions among molecules by which cancer can influence its victim and how they affect victim structures and functions are now beginning to be elucidated. Solid cancers cannot grow beyond a certain size without an adequate blood supply [39]. The hypothesis that tumors produce a diffusible “angiogenic” substance was put forward in 1968 [40,41]. Cancer units produce humoral factors that are able both to induce and promote angiogenesis [42] addressed individually toward each of them and thus perform an “angiogenic switch” of their own unrestricted growth.
Angiogenesis is a critical, rate-limiting step of the multi-stage process leading to detectable cancerous subunits. The induction of angiogenesis is an important step in carcinogenesis. This angiogenic activity first appears in a subset of hyperplastic islets before the onset of tumor growth [43]. The angiogenic switch causes the tumor to advance in the progression pipeline [42]. One can hypothesize that it is a specific cancerous vascular endothelial growth factor, a signal protein produced by cells that stimulates vasculogenesis and angiogenesis and restores the oxygen and nutrient supply to cancerous units when the local blood circulation is inadequate. Many evolutionary adaptations of cancer belong to its ability to managing its own nutrition.
3.5. The Nutrition of Cancer
Like any other living beings, cancer sustains itself at the expense of substances and energy derived from its environments, i.e. from its victim’s body. What is more, the populations of cancerous cells subsist also on life supporting functions provided by the victim. Any individual cancer exists as a result of natural ecological relations between two living species in which the consuming one (the consumer) obtains the stuff and energy for its life at the expense of substances and physiologic functions composed of the consumed organism (the victim). Thus, cancer is a kind of ultimate parasitism.
The ultimate state of cancer disease is characterized by cancerous cachexia, catastrophically progressive weight loss provoked by intensive atrophy, mainly of skeletal muscle and adipose tissue that are used by cancer as main sources of lipids and proteins. Depending on the tumor type, weight loss occurs in 30% - 80% of cancer patients and is severe (with loss of >10% of the initial body weight) in 15% [44]. In pancreatic cancer, 85% of patients are cachectic even at diagnosis [45]. The forage of nutrients by cancerous cells functions as the leading cause of poor quality of life, poor physical function, and poor prognosis in cancer patients [44]. Human cancer is an exceptional man-eater.
The cancerous atrophy of skeletal muscle is characterized by the intense degradation of macromolecules of muscle proteins and the depression of their biosynthesis. The associated massive loss of adipose tissue is incited by extensive degradation of fat molecules. Cancer functions as a marauder which sucks the body of its victim dry. Beside, one can suppose some cyto-ecological regulators produced by cancerous cells inhibit the growth of normal cells, thus aggravating cancerous cachexy. Some of humoral agents of cancerous cells suppress the functions of the victim’s cells thus inducing the development of cachexia [45].
The development of this state is induced by the primordial existence in the afflicted organism of a symbiotic population of cancerous cells. The population exists inside the afflicted organism like a sponge. It develops intensively at the expense of both the structures (proteins, lipids, saccharides) and functions (the supply with oxygen, nutritive substances and means for reproduction) of the host organism. The cells are able to produce molecular agents specifically targeted on the enzymatic splitting of muscle proteins. Moreover, cancerous cells are able to secrete lipolytic enzymes, which make a substantial investment in the creation of cancerous cachexia.
When a cancer victim dies from cancer, it is mostly because its tumors have exhausted its life supporting stuffs and intoxicated its life supporting organs. Cancer has gobbled its victim. The development of either solitary or associated malignant tumors inevitably lead to death way before the victim’s genetically predetermined longevity. The marauding behavior exploited by cancerous tumors (the populations of cancerous cells and their subcellular structures) is performed mainly by their molecular enzymatic agents, targeted either on the splitting of the victim’s macromolecules or on producing functional inhibition of the victim’s cells. The possession by cancer of so specialized and undoubtedly wholesome toxins and nutritive factors evidences the adaptive evolutionary origin of cancerous marauding.
3.6. Genetic Program of Human Cancer
The earliest primordial cancerous cells are stochastically disposed in different areas of the embryo’s body before postnatal ontogeny in the manner used to create other embryonic tissues and organs. After the end of their disposition and initial multiplication, the cells exist at their stable places like the primordiums of future tumors, the sleeping cell masses of small but different sizes. After that, the cells continue to exist in the form of several distantly separated micro-populations, provided with life supporting nutrients and energy by the infected organism. The development of cancer is delayed for decades.
At an appropriate time of a breadwinner’s life (mainly after 40 years of age), probably according to a specific program of cancer ontogenesis and aging, the potentially cancerous micro-populations receive a specific impulse to awaken. This means that human cancer possesses its own schedule or intrinsic biological watch i.e., the genetic program of its development from zygote and primordial cancerous cells to the transmission between humans. This cancer subsistence program is different of those belonging to its victim. This is a specific cancerous germ line—the lineage of cells culminating in the germ cells.
The possession of these unique genomic traits provided cancer with many benefits of undoubtedly adaptive importance. The program favors those cancerous cell lineages whose schedule of life do not allow early restriction of reproductive, i.e., transmissive, functions of the afflicted person as well as the period of its effective care for offspring before 40 years of age.
3.7. Cancer Transmission between Humans
For many decades, human cancer was not generally considered a transmissible disease. Meanwhile, in the middle of the 20th century, cancer overtook many diseases as an important human killer. It became one of the biggest threats to global human health. It takes a terrible and growing human toll and its prevalence continues to grow. This could not be performed without a regularly functioning natural mechanism for the transmission of cancer between humans. This regular cancer transmission by means of human reproductive organs and functions has been guessed and deciphered quite recently [2, 3]. This long-expected result has been achieved by the multidisciplinary integrative reassessment of data about the main traits of cancer from the viewpoint of recent all-pathological, immunological, genetic and evolutionary discoveries. According to the new paradigm, cancer belongs to the group of invasive diseases whose subsistence depends on regular transmission of the causative agent from one victim’s body to another [46].
The saving and continuing of own life via self-reproduction and consequent transposition from the location of exploited resources toward unexploited ones is an extraordinarily important function of any form of living matter. Human cancer also performs these functions very regularly and effectively by means of human reproductive organs and functions. This peculiar form of life is characterized by a complex of evolutionary adaptive traits necessary for providing the victim’s ability to transmit deviant genomes into relevant gametes, execute multifold acts of fertilization and breed descendants to the stage of complete maturity. The absence of any of the abilities sharply diminishes the chances of the cancerous genome to prolong its life in the genomes of descendant generations.
4. CONCLUSIONS
The xenogamous paradigm of cancer origin, pathogenesis and epidemic spread has allowed the consideration of human cancer as a disease caused by the appearance in the afflicted body of deviant multicellular structures whose cells are aggressive (gobble the affected body; grow and divide without respect to normal limits), invasive (invade the body and destroy adjacent tissues), metastatic (dispersed over embryogenesis at different locations in the body) and transmissible. The causative agent of the human disease was identified just recently as an ancient unprecedentedly unique parasitic being that sustains itself at the expense of nutritive substances and energy derived from its victim’s body and is transmitted from xenogamous parent to its offspring by means of human reproductive organs and functions. Integrative analysis presented above performed first a systematic discovery of main adaptations of cancer causative agent to this specific way of life developed over its evolution.
Focus is on the adaptive traits revealed at the main stages of cancer existence, including cancerous invasion of a human body, make-up of the parasite, its self-protection from the victim’s immune defense and regulatory management, disposition of cancer sub-units around the afflicted body, the self-management of cancer and its nutrition, communication between dispersed cancer units, physiological synchronization between them and horizontal (reproductive) way of cancer transmission between humans. The above considered findings may be important in the improved understanding of cancer origin, epidemiology and pathogenesis as well as in the creation of non-traditional measures for its prevention and remedies for healing. Xenohamous paradigm of cancer origin did not confirm the usefulness of local (surgical and radiological) treatment of cancer disease such as prophylactic double mastectomy. In contrast, the paradigm accentuated the value of chemotherapy oriented on the ecology and physiology of causative agent as well as of the prophylaxis of the disease by means of the restriction of xenogamy and launching of cancer free genealogy.

REFERENCES
- Rumyantsev, S.N. (2008) Hereditary immunity: Fundamental principles and exploitation in life study and health care. Nova Publishers, New York.
- Rumyantsev, S.N. (2009) The discredit of cancer metastasis. Science Advisory Board. http://www.scienceboard.net/community/perspectives.227.html
- Rumyantsev, S.N. (2009) The uniqueness and ordinaryness of cancer origin and pathogenesis: New epidemiological, clinical and preventive perspectives. Journal of Clinical Medicine Research, 1, 32-36.
- Rumyantsev, S.N. (2011) Functions of hereditary immunity and xenogamy in cancer origin and pandemic spread. Open Journal of Immunology, 1, 27-40. doi:10.4236/oji.2011.12004
- Bonnicksen, A.L. (2009) Chimeras, hybrids and interspecies research: Politics and policymaking. Georgetown University Press, Washington.
- McLaren, A. (1976) Mammalian chimaeras. Cambridge University Press, Cambridge.
- Rumyantsev, S.N. (2010) Hypothesis: Towards the origin of cancer epidemics and pathogenesis. Journal of Carcinogenesis, 9, 1-7.
- Patterson, N., Richter, D.J., Gnerre, S., Lander, E.S. and Reich, D. (2006) Genetic evidence for complex speciation of humans and chimpanzees. Nature, 441, 1103- 1108. doi:10.1038/nature04789
- Sankararaman, S., Patterson, N., Li, H., Paabo, S. and Reich, D. (2012) The date of interbreeding between Neandertals and modern humans. PLoS Genet, 8, Article ID. e1002947.
- Rumyantsev, S.N. (2003) The intra-individual diversity in senescence. Biogerontology, 4, 171-178. doi:10.1023/A:1024137418419
- Rumyantsev, S.N. (2013) Human cancer is a parasite spread via intrusion in genome. Pure and Applied Biology, 2, 7-16.
- Soerjomataram, I. and Coebergh, J.W. (2009) Epidemiology of multiple primary cancers. Methods in Molecular Biology, 471, 85-105. doi:10.1007/978-1-59745-416-2_5
- Milan, T., Pukkala, E., Verkasalo, P.K., Kaprio, J., Jansen, C.T., Koskenvuo, M. and Teppo, L. (2000) Subsequent primary cancers after basal-cell carcinoma: A nationwide study in Finland from 1953 to 1995. International Journal of Cancer, 87, 283-288. doi:10.1002/1097-0215(20000715)87:2<283::AID-IJC21>3.0.CO;2-I
- Nugent, Z., Demers, A.A., Wiseman, M.C., Mihalcioiu, C. and Kliewer, E.V. (2005) Risk of second primary cancer and death following a diagnosis of nonmelanoma skin cancer. Cancer Epidemiology, Biomarkers & Prevention, 14, 2584-2590. doi:10.1158/1055-9965.EPI-05-0379
- Soerjomataram, I., Louwman, W.J., Lemmens, V.E., Coebergh, J.W. and De Vries, E. (2008) Are patients with skin cancer at lower risk of developing colorectal or breast cancer? American Journal of Epidemiology, 167, 1421- 1429. doi:10.1093/aje/kwn077
- Levi, F., Randimbison, L., Te, V.-C., Conconi, M.M. and Vecchia L.C. (2008) Risk of prostate, breast and colorectal cancer after skin cancer diagnosis. International Journal of Cancer, 123, 2899-3001. doi:10.1002/ijc.23816
- Kreso, A., O’Brien, C.A., Van Galen, P., Gan, O.I., Notta, F., Brown, A.M., Ng, K., Ma, J., Wienholds, E., Dunant, C., Pollett, A., Gallinger, S., McPherson, J., Mullighan, C.G., Shibata, D. and Dick, J.E. (2013) Variable clonal repopulation dynamics influence chemotherapy response in colorectal cancer. Science, 339, 543-548. doi:10.1126/science.1227670
- Marusyk, A. and Polyak, K. Cancer. (2013) Cancer cell phenotypes, in fifty shades of grey. Science, 339, 528-529. doi:10.1126/science.1234415
- Prehn, R.T. (1993) Two competing influences that may explain concomitant tumor resistance. Cancer Research, 53, 3266-3269.
- Retsky, M., Demicheli, R., Hrushesky, W., Baum, M. and Gukas, I. (2010) Surgery triggers outgrowth of latent distant disease in breast cancer: An inconvenient truth? Cancers, 2, 305-337. doi:10.3390/cancers2020305
- Rumyantsev, S.N. (2012) Toward the genomic roots of cancer. Journal of Medicine and Medical Sciences, 3, 638-659.
- Baum, M., Chaplain, M., Anderson, A., Douek, M. and Vaidya, J.S. (1999) Does breast cancer exist in a state of chaos? European Journal of Cancer, 35, 886-891. doi:10.1016/S0959-8049(99)00067-2
- Demicheli, R., Retsky, M., Hrushesky, W.J.M., Baum, M. and Gukas, I.D. (2008) The effects of surgery on tumor growth: A century of investigations. Annals of Oncology, 19, 1821-1828. doi:10.1093/annonc/mdn386
- Hanin, L. and Korosteleva, O. (2010) Does extirpation of the primary breast tumor give boost to growth of metastases? Evidence revealed by mathematical modeling. Mathematical Biosciences, 223, 133-141. doi:10.1016/j.mbs.2009.11.006
- De Jong, K.P., Lont, H.E., Bijma, A.M., Brouwers, M.A., De Vries, E.G., Van Veen, M.L., Marquet, R.L., Slooff, M.J. and Terpstra, O.T. (1995) The effect of partial heaptectomy on tumor growth in rats: In vivo and in vitro studies. Hepatology, 22, 1263-1272. doi:10.1002/hep.1840220436
- Garcia-Alonso, I., Palomares, T., Alonso, A., Portugal, V., Castro, B., Carames, J. and Mendez, J. (2003) Effect of hepatic resection on development of liver metastasis. Revista Española de Enfermedades Digestivas, 95, 765-767.
- Ikeda, Y., Matsumata, T., Takenaka, K., Sasaki, O., Soejima, K. and Sugimachi, K. (1995) Preliminary report of tumor metastasis during liver regeneration after hepatic resection in rats. European Journal of Surgical Oncology, 21, 188-190. doi:10.1016/S0748-7983(95)90468-9
- Elias, D., De Baere, T., Roche, A., Ducreux, M., Leclere, J. and Lasser, P. (1999) During liver regeneration following right portal embolization growth rate of liver metastases is more rapid than that of the liver parenchyma. British Journal of Surgery, 86, 784-788. doi:10.1046/j.1365-2168.1999.01154.x
- Von Schweinitz, D., Fuchs, J., Gluer, S. and Pietsch, T. (1998) The occurrence of liver growth factor in hepatoblastoma. European Journal of Pediatric Surgery, 8, 133- 136. doi:10.1055/s-2008-1071139
- Sorin, V., Mizrahi, A., Ohana, P., Ayesh, S., Birman, T., Hochberg, A. and Czerniak, A. (2009) Partial Hepatectomy in rats results in significant growth of liver metastases by increased expression of H19 gene. Cancer Therapy, 7, 240-244.
- Pockaj, B.A., Wasif, N., Dueck, A.C., Wigle, D.A., Boughey, J.C., Degnim, A.C., Gray, R.J., McLaughlin, S.A., Northfelt, D.W., Sticca, R.P., Jakub, J.W. and Perez, E.A. (2010) Metastasectomy and surgical resection of the primary tumor in patients with stage IV breast cancer: Time for a second look? Annals of Surgical Oncology, 17, 2419-2426. doi:10.1245/s10434-010-1016-1
- Giuliano, A.E., Hunt, K.K., Ballman, K.V., Beitsch, P.D., Whitworth, P.W., Blumencranz, P.W., Leitch, A.M., Saha, S., McCall, L.M. and Morrow, M. (2011) Axillary dissecttion vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: A randomized clinical trial. JAMA: Journal of the American Medical Association, 305, 569-575. doi:10.1001/jama.2011.90
- Lange, P.H., Hekmat, K., Bosl, G., Kennedy, B.J., Fraley, E.E. (1980) Accelerated growth of testicular cancer after cytoreductive surgery. Cancer, 45, 1498-1506. doi:10.1002/1097-0142(19800315)45:6<1498::AID-CNCR2820450633>3.0.CO;2-7
- De Giorgi, V., Massi, D., Gerlini, G., Mannone, F., Quercioli, E. and Carli. P. (2003) Immediate local and regional recurrence after the excision of a polypoid melanoma: Tumor dormancy or tumor activation? Dermatologic Surgery, 29, 664-667. doi:10.1046/j.1524-4725.2003.29163.x
- Tseng, W.W., Doyle, J.A., Maguiness, S., Horvai, A.E., Kashani-Sabet, M. and Leong, S.P.L. (2009) Giant cutaneous melanomas: Evidence for primary tumour induced dormancy in metastatic sites? British Medical Journal, Case Reports.
- Deylgat, B., Van Rooy, F., Vansteenkiste, F., Devriendt, D. and George, C. (2011) Postsurgery activation of dormant liver micrometastasis: A case report and review of literature. Journal of Gastrointestinal Cancer, 42, 1-4.
- Rumyantsev, S. (2010) Hypothesis: Toward the origin of cancer epidemics and pathogenesis. Journal of Carcinogenesis, 9, 1-7.
- Quintana, E., Piskounova, E., Shackleton, M., Weinberg, D., Eskiocak, U., Fullen, D.R., Johnson, T.M. and Morrison, S.J. (2012) Human melanoma metastasis in NSG mice correlates with clinical outcome in patients. Science Translational Medicine, 4, 159ra149. doi:10.1126/scitranslmed.3004599
- Carmeliet, P. and Rakesh, K.J. (2000) Angiogenesis in cancer and other diseases. Nature, 407, 249-257. doi:10.1038/35025220
- Ehrmann, R.L. and Knoth, M. (1968) Choriocarcinoma: Transfilter stimulation of vasoproliferation in the hamster cheek pouch studied by light and electron microscopy. Journal National Cancer Institute, 41, 1329-1341.
- Greenblatt, M. and Shubik, P. (1968) Tumor angiogenesis: Transfilter diffusion studies in the hamster by the transparant chamber technique. Journal National Cancer Institute, 41, 111-124.
- Hanin, L. (2013) Seeing the invisible: How mathematical models uncover tumor dormancy, reconstruct the natural history of cancer, and assess the effects of treatment. Advances in Experimental Medicine and Biology, 734, 261- 282. doi:10.1007/978-1-4614-1445-2_12
- Folkman, J., Watson, K., Ingber, D. and Hanahan, D. (1989) Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature, 339, 58-61. doi:10.1038/339058a0
- DeWys, W.D., Malone, W.F., Butrum, R.R., Sestili, M.A. (2006) Clinical trials in cancer prevention. Cancer, 58, 1954-1962. doi:10.1002/1097-0142(19861015)58:8+<1954::AID-CNCR2820581425>3.0.CO;2-A
- Tisdale, M.J. (2003) Pathogenesis of cancer cachexia. The Journal of Supportive Oncology, 1, 159-168.
- Rumyantsev, S.N. (2013) Human cancer is transmitted via genome. Open Journal of Genetics, 3, 6-11. doi:10.4236/ojgen.2013.31002

