Open Journal of Animal Sciences
Vol.3 No.1(2013), Article ID:27072,6 pages DOI:10.4236/ojas.2013.31003
Creatine supplementation in exercised rats: Effects on the aerobic capacity
![]()
1Biosciences Institute, Department of Physical Education, Universidade Estadual Paulista Júlio de Mesquita Filho, Rio Claro, Brazil; *Corresponding Author: mbujo@ig.com.br
2Faculty of Nutrition, Universidade Federal de Mato Grosso, Cuiabá, Brazil
Received 13 September 2012; revised 13 October 2012; accepted 27 October 2012
Keywords: Diet; Lactate; Maximal Lactate Steady State; Treadmill Running
ABSTRACT
This study aimed to analyze the possible metabolic disturbances caused by creatine supplementation on aerobic capacity of rats, inferred by the maximal lactate steady state. Forty male Wistar rats (90 days old) were distributed into two groups for eight weeks: trained group (T): rats that were submitted to a training protocol, and supplemented-trained group (TCr): rats that were submitted to a training protocol and received balanced diet supplemented with 2% creatine. The blood lactate concentrations equivalent to maximal lactate steady state during treadmill running were analyzed at the beginning and also at the end of the experiment. At the end of the experiment were done comparing the test results MLSS between the two groups. At the beginning of the experiment, prior to groups division, the majority of animals obtained MLSS at a speed of 26 m/min, blood lactate concentration of 3.79 ± 0.76 mmol/L. At the end of the experiment, most of trained rats in T presented MLSS at the speed of 28 m/min, blood lactate concentration of 3.37 ± 0.68 mmol/L. Most TCr had MLSS at the speed of 28 m/min, blood lactate concentration of 3.52 ± 0.69 mmol/L. We conclude that creatine supplementation was not the cause of the improvement in the aerobic capacity of rats in the treadmill exercise.
1. INTRODUCTION
It has been suggested that creatine supplementation may modify the substrate utilization and possibly improve performance during extended exercise (>150 seconds), submaximal, steady state [1,2], or interspersed segments of incorporated exercise in extended endurance events, such as multiple periodic sprints during cycling of a triathlon [2,3].
In recent years, creatine supplementation presented itself as important in physical performance within the global sporting outlook, and also practitioners of endurance exercise as well as aerobic nature [4-6].
Creatine is a physiologically active substance essential for muscle contraction. It is a methylguanidine acetic acid (amine nitrogen) found naturally in food and fully involved in human metabolism. In the body it is found in skeletal muscle, heart, brain, retina and other tissues [7].
The endogenous synthesis of creatine occurs in kidney, pancreas and mainly in the liver, made by three amino acids: glycine, arginine and methionine. The molecule of glycine is fully incorporated into creatine, while arginine provides only its amino group, and methionine, provides its methyl group [7]. This only occurs when the availability of creatine in the diet is insufficient to meet daily needs.
The process begins by transferring the amino group from arginine to glycine, by a process called transamination, forming guanidilacetate and ornithine, a reversible catalyzed reaction by the enzyme S-adenosylmethionine that requires methyltransferase enzyme to the irreversible reaction known as transmethylation [7,8].
The Maximal Lactate State Steady (MLSS) may be used to detect the highest exercise intensity tolerated without continuous increase of blood lactate concentrations [9,10] by representing the highest point of balance between production and removal of lactate [11,12]. This parameter is considered a good indicator of aerobic capacity [11-13] and the ratio of work associated with the MLSS may be used in the aerobic endurance training of athletes of high performance sports [12,14,15]. During exercise, there is a transition zone, in which a change occurs from, aerobic to anaerobic predominance, and this exercise zone is extremely important for physical conditioning, training and sports performance. For this reason, several investigations about this transition zone have been carried out in recent decades, resulting in different evaluation protocols. The Maximal Lactate Steady State (MLSS) test is widely used to determine this metabolic transition in rats. Gobatto et al. [16] have developed a study to determine the MLSS of rats during swimming exercise. Already Manchado et al. [9] have described a protocol for determining the MLSS of rats during exercise in treadmill running. More recently Araújo et al. [17] have used the MLSS test to identify the metabolic transition of rats supplemented with creatine.
In literature, there is a lack of studies evaluating the effects of creatine supplementation on exercise performance, performed at intensities equivalent to metabolic transition. Thus, the objective of this study was to analyze the possible metabolic disturbances caused by creatine supplementation on aerobic capacity of rats inferred by the MLSS.
2. MATERIALSAND METHODS
2.1. Study Site and Animals
Forty male Wistar rats (90 days old) were selected, receiving water and food ad libitum. The rats were housed in collective cages made of polyethylene (5 animals per cage), measuring 37.0 × 31.0 × 16.0 cm, under controlled temperature conditions (22˚C) with 12 h light/dark cycle. All experiments involving animals were approved by the Ethics Committee on Animal Experimentation at the Taubaté University—UNITAU, São Paulo State, Brazil (register CEEA/UNITAU No. 018/08).
2.2. Diets
The animals supplemented with creatine received balanced diet AIN-93M [18] added of 13 or 2% monohydrated creatine (All Chemistry, São Paulo, SP, Brazil) [19].
According to Hutman et al. [20] and Vandenbergue et al. [21] creatine supplementation must be offered in two phases, aiming to promote an overload of this substrate in the organism: firstly a loading phase and subsequently into a second phase named maintenance. In the present study the loading phase consisted in supplementing 13% of creatine for seven days and the maintenance phase 2% of creatine for the rest of the experiment. It is worth mentioning that creatine was administered to animals through the diet, seven days a week for eight weeks of the experiment. The non-supplemented animals received balanced diet AIN-93M [18], without addition of creatine. The detailed composition of the diet is described in the Table 1.
2.3. Selection of Running Rats and Adaptation to the Treadmill Exercise
The process of selection of running rats occurred prior to the training, and aimed to find the “naturally” running rats. For this, in the three weeks before the training period, the animals were adapted to the treadmill at speeds and progressive time according to Table 2, for later obtain the aerobic/anaerobic metabolic transition evaluated through the determination of Maximal Lactate Steady State (MLSS).
Table 1. Diet composition.

*According to the American Institute of Nutrition (AIN-93M) [18]; **Creatine maintenance diet according to Demenice [19]; ***Creatine loading diet adapted of Demenice [19] and according to Hultman et al. [20] and Vandenbergue et al. [21].
Table 2. Protocol of adaptation to the treadmill exercise.
2.4. Experimental Procedure and Blood Samples and Analysis
In the beginning of the experiment, to determine the MLSS, exercises series of 25 minutes were performed on a treadmill at different speeds fixed for each series, intervals of 48 hours between them and blood sampling (25 μL) every five minutes for lactate measurement. Blood samples were taken from a small cut at the tail end. A single incision made before the exercise was sufficient to collect all samples. The blood lactate concentration representative of the MLSS was considered at the higher speed where there was no variation of blood lactate greater than 1.0 mmol/L between 10 and 25 min of exercise [9,17]. The lactate concentration in blood was determined by enzymatic method [22]. Then, the animals were divided into two groups: 1) trained (T), rats that performed running training on a treadmill, and 2) supplemented-trained (TCr), rats that performed running training on a treadmill associated to creatine supplementation. The animals trained for 40 minutes/day, five days/week for eight weeks at a speed correspondent to the individual MLSS. At the end of the experimental period, all rats were submitted to a running session on a treadmill at the speed equivalent to the MLSS for 25 minutes. Blood samples were collected through an incision in the distal portion of tail, every five minutes/exercise to determine the lactate concentration. The intervention time of both, exercise and creatine supplementation was eight weeks.
3. STATICAL ANALYSIS
The analyses were performed with the aid of statistical packages, STATISTICA®, version 7.0. All experimental results were submitted to the normality test of ShapiroWilks, to establish the necessity for using parametric statistics. The data were determined to have a normal distribution. Results were expressed by mean ± standard deviation, and were statistically analyzed by Two-Way ANOVA followed by a post-hoc Tukey HSD, when necessary. For all analyses p < 0.05 was considered significant.
4. RESULTS
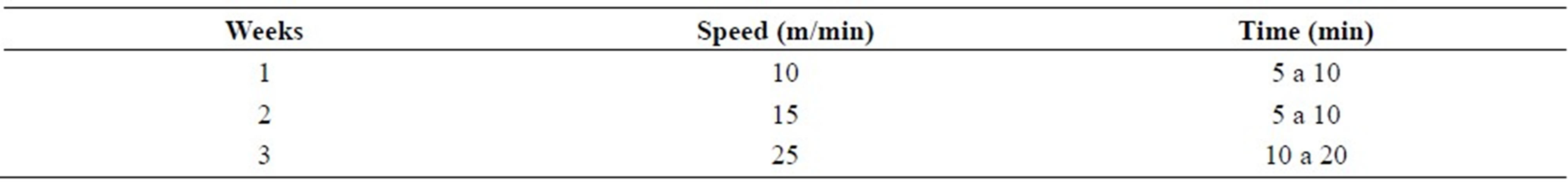
The blood lactate values during exercise test to determine the Maximal Lactate Steady State (MLSS) found at the beginning of the experiment, referring to a rat, as an example, are shown in Figure 1. For this animal, the MLSS occurred at the speed of 26 m/min, blood lactate concentration of 3.52 ± 0.49 mmol/L. Considering the whole batch of animals evaluated, 57% of them achieved the MLSS at the speed of 26 m/min, blood lactate concentration of 3.79 ± 0.76 mmol/L, and 42% achieved at the speed of 24 m/min, blood lactate concentration of 3.27 ± 0.68 mmol/L.
The blood lactate values during the stress test to determine the MLSS at the end of the experiment are shown in Figure 2. The animals of group T achieved MLSS at the speed of 28 m/min, blood lactate concentration of 3.37 ± 0.68 mmol/L. Regarding the rats of group
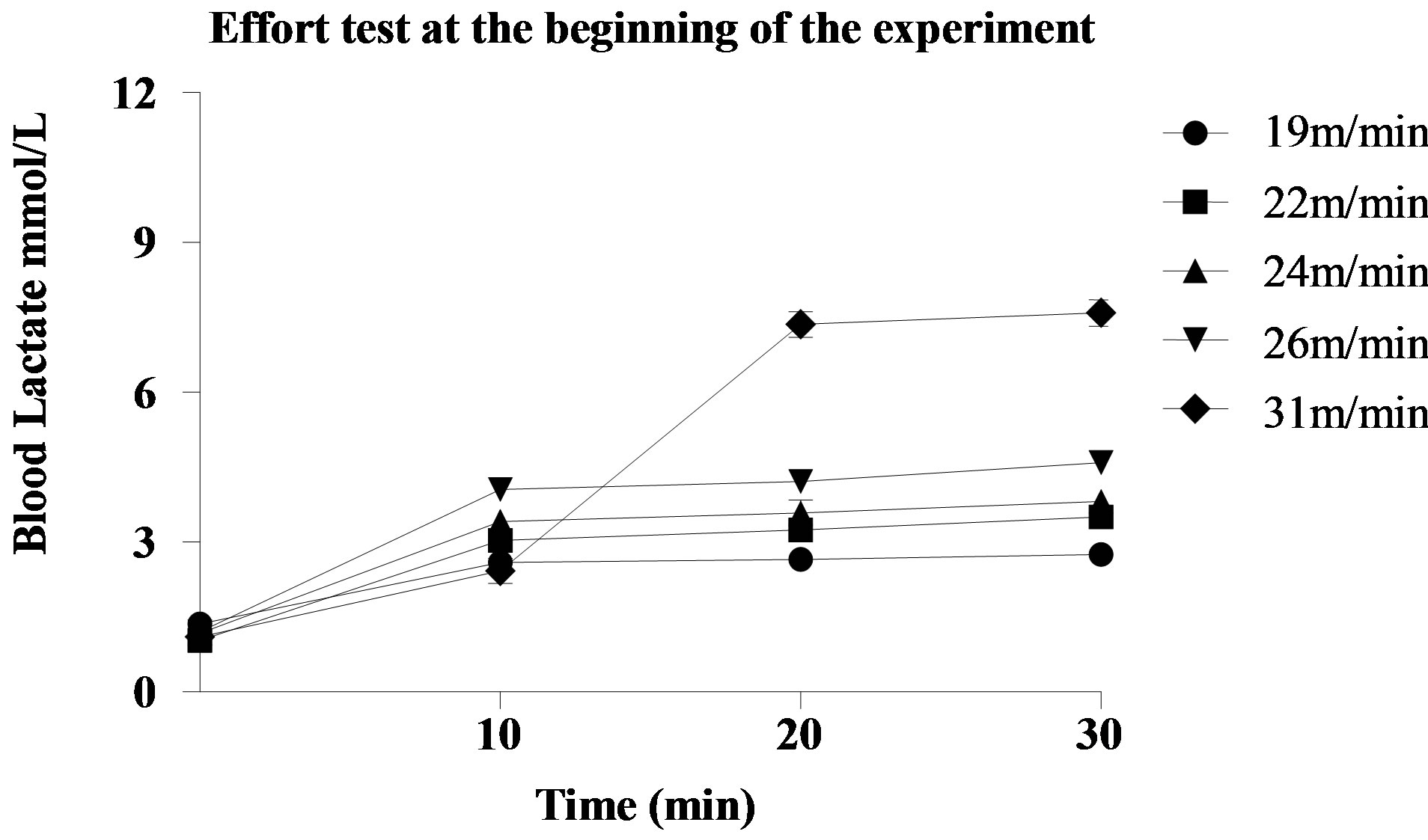
Figure 1. Blood lactate of an animal, as an example, during stress test to determine the Maximal Lactate Steady State in the beginning of the experiment. For this animal, the MLSS occurred at the speed of 26 m/min, blood lactate concentration of 3.52 ± 0.49 mmol/L.
 (a)
(a) (b)
(b)
Figure 2. Blood lactate of an animal, as an example, during stress test to determine the Maximal Lactate Steady State at the end of the experiment. (a) Group T; (b) Group TCr. For the rat belonging to group T, the MLSS occurred at the speed of 28 m/min, blood lactate concentration of 3.10 ± 0.41 mmol/L. For the rat belonging to the group TCr, the MLSS occurred at the speed of 28 m/min, blood lactate concentration of 2.98 ± 0.42 mmol/L. TCr = Trained Creatine; T = Trained.
TCr, the MLSS was also obtained at the speed of 28 m/min, blood lactate concentration of 3.52 ± 0.69 mmol/L.
5. DISCUSSION
Creatine supplementation has been widely adopted, especially by athletes, as a nutritional strategy that aims to enhance physical performance [23,24]. However, there is still some disagreement in the literature regarding the effectiveness of creatine and its possible interference in the exercise of aerobic nature. This study aimed at evaluating the possible effects of creatine supplementation on aerobic capacity of exercised rats at intensities equivalent to aerobic-anaerobic metabolic transition, through the Maximal Lactate Steady State (MLSS) test in rats.
The analyses results for MLSS tests, carried out at the beginning of the experiment with the purpose of defining the initial intensity of these animals training, showed that, in rats submitted to physical exercise on a treadmill, the blood lactate shows a pattern similar to that described in human beings [12]. Recently, similar results have been observed when using rats submitted to swimming exercise and treadmill running [9-16,25]. The average concentration of blood lactate equivalent to the MLSS for 57% of the animals evaluated at the beginning of our experiment was 3.79 ± 0.76 mmol/L and corroborates previous studies, which applied the MLSS test on sedentary and eutrophic animals [9,17,25]. In turn, the velocity associated to the MLSS in the animals was 26 m/min. At higher intensities, the blood lactate concentrations showed a sustained progressive increase and some rats did not tolerate the exercise maintenance. The value of maximal exercise intensity associated to aerobic energetic predominance, observed at the beginning of the present study was higher than that described by Araújo et al. [17] (20 m/min) in rats supplemented with creatine and Phillis et al. [26] (25 m/min), obtained by a different protocol. In this last study, the authors determined the anaerobic threshold in running rats using a multistage progressive test in a treadmill. The AT was estimated from individual’s plots of blood lactate vs. treadmill speed, and it was considered being the exercise intensity at which lactacidemia showed rapid and sudden increase. Langfort et al. [27] and Araújo et al. [28] have also reported similar intensity for AT to sedentary rats, calculated at the speed of running corresponding to the individual breaking point of the lactate curve using the two-segment linear regression (25 m/min).
To evaluate the effect of creatine supplementation and effectiveness of training protocol as a useful tool in improving aerobic fitness of animals, a MLSS test was performed at the end of the experiment. It is evident that the animals in both groups (T and TCr) improved the MLSS intensity. At the beginning of the experiment, in the MLSS test, the animals reached the speed of 26 m/min, though at the end of the experiment this speed was of 28 m/min in animals of both groups, this represents a 7% increase in values related to intensity corresponding to MLSS.
Several studies have suggested that creatine supplementation improves performance in exercises of anaerobic character [23,29]. Furthermore, in aerobic exercise, literature displays differences regarding the possible effects of using creatine supplementation [2,5]. However, there are studies that have showed improvements in the use of creatine on aerobic performance, this would be done by the role of anaerobic energetic buffer generated by creatine supplementation [24,30]. It has been proposed that creatine and creatine phosphate (CP) act as messenger molecules between mitochondria and subcellular sites of production and hydrolysis of adenosine triphosphate (ATP) and thereby may aid aerobic activities [8,29]. According to Souza et al. [24], another possible grounding for creatine supplementation on aerobic exercises would be related with the aid of creatine to buffer the elevations of adenosine diphosphate (ADP).
Supporting this notion and according to our results, some hypothesis may be raised, regarding to the reason why creatine supplementation did not promote the ergogenic effects expected. According to Casey and Greenhaff [31], in a study performed in human beings, the individuals sampled failed to retain an amount of muscle creatine capable of promoting the benefits expected of supplementation. According to the authors, seems to be no ergogenic effects after creatine supplementation, where the increase of muscle phosphocreatine concentration is less than 20 mmol/Kg dry weight. Based on this information, this could be a plausible hypothesis and so we could extrapolate to our animal model.
In addition, as viewed in this study, the results of stress tests, performed after exercise training showed that creatine supplementation did not affect the increase in aerobic capacity of rats, but the physical training itself led to a reduction in the accumulation of blood lactate during exercise. This indicates that the running protocol used without interference from creatine supplementation was effective in improving aerobic fitness of animals. Moreover, the results here obtained showed that aerobic training prevented the deterioration of aerobic fitness imposed by aging. Trained rats of both groups increased exercise intensity (velocity) equivalent to the metabolic transition during the experiment. This is evidence of evolution in aerobic fitness as a function of physical training.
We believe that the improvement in aerobic fitness observed in this study occurred, at least in part, due to the concentration of lactate stabilization, found at the end of the experiment in the animals of groups T and TCr, which were similar to those observed in running experimental protocols applied in rats training [7,9]. These findings identify that aerobic training intensities of MLSS may provide physiological changes such as increased capillary density, and mitochondrial, besides the enzymatic muscle oxidative capacity [12], thus promoting the improvement in aerobic fitness.
During exercise, blood lactate concentration is dependent of the ratio between the speed in which the substrate is produced by skeletal muscle, and the speed that the same substrate is removed from the bloodstream [32]. The mechanisms involved in the accumulation of lactate during exercise are diverse, and the increased intensity of exercise is one of its main causes [33]. In the exercise of low or moderate intensity, in human beings and rats, the blood lactate remains stable [33]. In this situation, the lactate production rate shows balance or even lower than its removal [34]. When individuals are submitted to highintensity exercise, the blood lactate concentration rises after three to four minutes of activity, thereby indicating that the rate of production exceeds the velocity of removal [32]. Differently from acute exercise, the training, mainly those of aerobic nature, generates considerable metabolic adaptations in relation to the turnover of lactate, and, as a consequence, results in the reduction of accumulation of lactate during exercise, thus causing an increase in the intensity of MLSS of animals [16,35]. This is an indicative of improvement in aerobic fitness [36].
6. CONCLUSION
In summary we can conclude that creatine supplementation was not the cause of the improvement in the exercise capacity of rats on a treadmill.
7. ACKNOWLEDGEMENTS
The authors are grateful for the technical support of Clarice Y. Sibuya and José Roberto R. da Silva who contributed greatly to this Project. This study was supported by “The State of São Paulo Foundation for Research Support” (FAPESP-Proc. 2009/52063-0).
REFERENCES
- Stroud, M.A., Holliman, D., Bell, D., Green, A.L., Macdonald, I. and Greenhaff, P.L. (1994) Effect of oral creatine supplementation on respiratory gas exchange and blood lactate accumulation during steady-state incremental treadmill exercise and recovery in man. Clinical Science, 87, 707-710.
- Williams, M.H., Kreider, R. and Branch, J.D. (2000) Creatina. Ed Manole, São Paulo.
- Engelhardt, M., Neumann, G., Berbalk, A. and Reuter, I. (1998) Creatine supplementation in endurance sports. Medicine Science Sports Exercise, 30, 1123-1129. doi:10.1097/00005768-199807000-00016
- Souza Junior, T.P., Dubas, J.P., Pereira, B. and Oliveira, P.R. (2007) Suplementação de creatina e treinamento de força: Alterações na resultante da força máxima dinâmica e variáveis antropométricas em universitários submetidos a oito semanas de treinamento de força (hipertrofia). Revista Brasileira de Medicina do Esporte, 13, 303-309.
- Gama, M.S. (2011) Efeitos da creatina sobre desempenho aeróbio: Uma revisão sistemática. Revista Brasileira de Nutrição Esportiva, 5, 182-190.
- Pereira Junior, M., Moraes, A.J.P., Ornellas, F.H., Gon- çalves, M.A., Liberali, R. and Navarro, F. (2012) Eficiência da suplementação de creatina no desempenho físico humano. Revista Brasileira de Prescrição e Fisiologia do Exercício, 6, 90-97.
- Araújo, M.B. and Mello, M.A.R. (2009) Exercício, estresse oxidativo e suplementação com creatina. Revista Brasileira de Nutrição Esportiva, 3, 264-272.
- Greenhaff, P.L. (1997) The nutritional biochemistry of creatine. Journal Nutrition Biochemistry, 11, 610-618. doi:10.1016/S0955-2863(97)00116-2
- Manchado, F.B., Gobatto, C.A., Contarteze, R.V.L., Papoti, M. and Mello, M.A.R. (2005) Maximal lactate steady in running rats. Journal Exercise Physiology, 8, 29-35.
- Pringle, J.S.M. and Jones, A.M. (2002) Maximal lactate steady state, critical power and EMG during cycling. European Journal Applied Physiology, 88, 214-226. doi:10.1007/s00421-002-0703-4
- Billat, V.L., Siverent, P., Py, G., Korallsztein, J.P. and Mercier, J. (2003) The concept of maximal lactate steady state: A bridge between biochemistry, physiology and sport science. Sports Medicine, 33, 407-426. doi:10.2165/00007256-200333060-00003
- Manchado-Gobatto, F.B. (2007) Protocolos invasivos e não invasivos para avaliação aeróbia e anaeróbia de ratos wistar. Tese de Doutorado, Pós-Graduação em Ciências da Motricidade Humana, UNESP, Rio Claro.
- Jones, A.M. and Carter, H. (2000) The effect of endurance training on parameters of aerobic fitness. Sports Medicine, 29, 373-376. doi:10.2165/00007256-200029060-00001
- Baldari, C. and Guidetti, L. (2000) A simple method for individual anaerobic threshold as a predictor of maximal lactate steady state. Medicine Science Sports Exercise, 32, 1798-1802. doi:10.1097/00005768-200010000-00022
- Almarwaey, O.A., Jones, A.M. and Tolfrey, K. (2004) Maximal lactate steady state in trained adolescent runners. Journal Sports Science, 22, 215-225. doi:10.1080/02640410310001641520
- Gobatto, C.A., Mello, M.A.R., Sibuya, C.Y., Azevedo, J.R.M., Santos, L.A. and Kokubun, E. (2001) Maximal lactate steady state in rats submitted to swimming exercise. Comparative Biochemistry Physiology, 130, 21-27. doi:10.1016/S1095-6433(01)00362-2
- Araújo, M.B., Moura, L.P., Ribeiro, C., Dalia, R.A., Voltarelli, F.A. and Mello, M.A.R. (2011) Oxidative stress in the liver of exercised rats supplemented with creatine. International Journal of Nutrition and Metabolism, 3, 58- 64.
- Reeves, P.G., Nielsen, F.H. and Fahey, G.C. (1993) AIN- 93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. Journal Nutrition, 123, 1939-1951.
- Deminice, R., Portari, G.V., Vannucchi, H. and Jordão, A.A. (2009) Effects of creatine supplementation on homocysteine levels and lipid peroxidation in rats. Brazilian Journal Nutrition, 102, 110-116. doi:10.1017/S0007114508162985
- Hultman, E., Soderlund, K., Timmons, J., Cederblad, G. and Greenhaff, P. (1996) Musclecreatine loading in man. Journal Applied Physiology, 81, 232-237.
- Vandenberghe, K., Goris, M., Van Hecke, P., Van Leemputte, M., Vangerven, L. and Hespel, P. (1997) Longterm creatine intake is beneficial to muscle performance during resistance training. Journal Applied Physiology, 83, 2055-2063.
- Engels, R.C. and Jones, J.B. (1978) Causes and elimination of erratic Blanc in enzymatic metabolic assays involving the use of NAD in alkaline hydrazine buffers: Improved conditions for assay of L-glutamate, L-lactate and other metabolites. Analytical Biochemistry, 88, 475- 484. doi:10.1016/0003-2697(78)90447-5
- Bemben, M.G. and Lamont, H.S. (2005) Creatine supplementation and exercise performance: Recent findings. Sports Medicine, 35, 107-125. doi:10.2165/00007256-200535020-00002
- Souza, R.A., Santos, R.M., Osório, R.A., Cogo, J.C., Prianti Júnior, A.C.G., Martins, R.A.B.L. and Ribeiro, W. (2006) Influência da suplementação aguda e crônica de creatina sobre as concentrações sanguíneas de glicose e lactato de ratos Wistar. Revista Brasileira de Medicina do Esporte, 12, 361-365. doi:10.1590/S1517-86922006000600012
- Contontarteze, R.V.L., Manchado, F.B., Gobatto, C.A. and Mello, M.A.R. (2007) Stress biomarkers in rats submitted to swimming and treadmil running exercises. Comparative Biochemistry Physiology, A, 1-8.
- Pillis, W., Zarzeczny, R., Langfort, J., Kaciuba-Uscilko, H., Nazar, K. and Wojtyna, J. (1993) Anaerobic threshold in rats. Comparative Biochemistry Physiology, 106, 285- 289. doi:10.1016/0300-9629(93)90513-4
- Langfort, J., Zarzeczny, R., Pillis, W., Kaciuba-Uscilko, H., Nazar, K. and Porta, S. (1996) Effect of sustained hyperadrenalinemia on exercise performance and lactate threshold in rats. Comparative Biochemistry Physiology, 14, 51-55. doi:10.1016/0300-9629(95)02087-X
- Araújo, M.B., Voltarelli, F.A., Manchado-Gobatto, F.B., Moura, L.P. and Mello, M.A.R. (2010) Treinamento em diferentes intensidades e biomarcadores de estresse oxidativo e do metabolismo glicídico musculoesquelético de ratos. Revistade Educação Física/UEM, 21, 695-707.
- Kreider, R.B. (2003) Species-specific responses to creatine supplementation. American Journal of Physiology—Regulatory, Integrative and Comparative Physiology, 285, R725-R726.
- Freire, T.O., Gulano, B., Leme, M.D., Polacow, V.O. and Lancha Júnior, A.H. (2008) Captação de glicose em ratos submetidos ao exercício físico. Revista Brasileira de Medicina do Esporte, 14, 431-435. doi:10.1590/S1517-86922008000500006
- Casey, A. and Greenhaff, P.L. (2000) Does dietary creatine supplementation play a role in skeletal muscle metabolism and performance? American Journal Clinical Nutrition, 72, 607S-617S.
- Wasserman, K. (1986) The anaerobic threshold: Definition, physiological significance and identification. Advances in Cardiology, 35, 1-23.
- Mcardle, W.D., Katch, F.I. and Katch, V.L. (2008) Fisiologia do exercício: Energia, nutrição e desempenho humano. Editora Guanabara Koogan S.A., Rio de Janeiro.
- Gobatto, C.A., Kokubun, E., Sibuya, C.Y. and Mello, M.A.R. (1991) Efeitos da desnutrição proteica-calórico e do treinamento físico na produção de acido lático em ratos machos adultos apos testes de cargas progressivas. Ciência e Cultura, 43, 725-736.
- Araújo, M.B., Manchado-Gobatto, F.B., Voltarelli, F.A., Ribeiro, C., Mota, C.S.A., Gobatto, C.A. and Mello, M.A.R. (2009) Efeitos do treinamento de corrida em diferentes intensidades sobre a capacidade aeróbia e produção de lactato pelo musculo de ratos wistar. Revista Brasileira de Medicinado Esporte, 15, 365-369. doi:10.1590/S1517-86922009000600009
- Voltarelli, F.A., Mello, M.A.R. and Gobatto, C.A. (2004) Limiar anaeróbio determinado pelo teste do lactato mínimo em ratos: Efeito dos estoques de glicogênio muscular e do treinamento Físico. Revista Portuguesa de Ciência Desportiva, 4, 16-25.

