Open Journal of Genetics
Vol.3 No.2(2013), Article ID:33613,9 pages DOI:10.4236/ojgen.2013.32012
Further evidence for the theory that crossover interference in Drosophila melanogaster is dependent on genetic rather than physical distance between adjacent crossover points*
![]()
Laboratory of Genetics, Department of Biology, University of Turku, Turku, Finland
Email: petter.portin@utu.fi
Copyright © 2013 Petter Portin. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 5 March 2013; revised 20 April 2013; accepted 16 May 2013
Keywords: Chiasma; Chromosome; Map Length; Meiosis
ABSTRACT
The effect of heat shock on certain meiotic parameters in Drosophila melanogaster was studied in the cv – v – f region of the X chromosome of females homozygous for the mus309 mutation, deficient in DNA double-strand break repair, or those of wild type. The heat shock in the wild females caused the frequencies of the single crossovers and double crossovers and all the map lengths to decrease while crossover interference remained unchanged. In the mus309 mutants all parameters, crossover interference included, remained unchanged despite the heat shock treatment. However, the mus309 mutation had a significant effect on all meiotic parameters both in the females not given the heat shock and in the heat shocked females with the exception that the recombination frequency of the v and f markers was the same in both genotypes in the females not given the heat shock. It seems that the heat shock treatment has an effect on crossing over which is independent on the mus309 gene and affecting the occurrence of crossing over itself. On the other hand, the mus309 gene has an effect on crossing over which is independent of the heat shock treatment and affects some precondition of crossing over. This precondition is probably the choice between two routes of the repair of double-strand DNA breaks known to be controlled by the mus309 gene. As explained in the discussion, the results are in accordance with the genetic models of interference in which interference depends on genetic distance between the crossover points, but in contradiction with physical models where interference is dependent on physical distance between the crossover points.
1. INTRODUCTION
1.1. General Introduction
Meiotic crossing over, the exchange of genetic material between homologous chromosomes during the generation of gametes in animals and sexual spores in plants and fungi, leads to recombination of genes and formation of chiasmata. A chiasma is a sufficient condition for the segregation of homologous chromosomes, which leads to the reduction of the chromosome number from diploid to haploid.
An important phenomenon, which has recently garnered considerable attention, associated with crossing over is crossover interference, i.e. the fact that multiple crossovers in each pair of homologous chromosomes are less frequent than would be expected on the basis of random coincidence of single crossovers [1-3]. The phenomenon of crossover interference is very likely responsible for the occurrence of obligate crossovers, and thus for the formation of obligate chiasmata.
The term “obligate crossover” refers to the fact that, in most species, it is rare to find chromosomes that do not undergo crossing over. For example, in Drosophila, there is usually one chiasma per chromosome arm. The feature of the obligate chiasma is biologically sensible because it ensures the disjunction of homologous chromosomes.
1.2. Models of Crossover Interference and the Purpose of the Present Study
In principle, there are two different categories of models of crossover interference. The first of these categories of models is called the genetic models and assumes that interference depends on the genetic (i.e. linkage map) distance, measured in Morgans, between adjacent crossovers [4]. To my knowledge, currently only one model, called the “counting model”, falls into this category [4,5].
The second category of models, “physical models”, hypothesizes that crossover interference is dependent on the physical distance (microns or base pairs) between the adjacent crossovers. In general, these models, which are many, suggest that some kind of physical signal travels along the bivalent and determines the distribution of crossovers.
Recently I presented evidence for the genetic models of crossover interference in Drosophila melanogaster [6]. The aim of the present study was to obtain further evidence for the theory that crossover interference is dependent on genetic rather than physical distances between adjacent crossover points. Crossing-over frequencies, crossover interference, recombination frequencies and map distances were compared in the cv – v – f region of the X chromosome of D. melanogaster in females bearing either wild type 3rd chromosomes (control) or having the DNA double-strand break repair deficient mus309D2/mus309D3 mutant constitution in the 3rd chromosomes (experiment) and either treated or untreated with a heat shock of 24 hours in 35˚C.
It was observed that the heat shock in the wild control females caused the frequencies of the single crossovers as well as double crossovers and all the map lengths to decrease while crossover interference remained unchanged. In contrast to this, in the experimental mus309 mutant females all meiotic parameters studied, crossover interference included, remained unchanged after the heat shock treatment. However, the mus309 mutation had a significant effect on all meiotic parameters both in females not given a heat shock and in the heat shocked females with the exception that the recombination frequency of the v and f markers was the same in both genotypes in the non-heat-shocked females. Thus, it appears that the heat shock has an effect on crossing over which is independent of the mus309 gene and affects the occurrence of crossing over itself. On the other hand, the mus309 gene has an effect on crossing over which is independent of the heat shock treatment and affects some precondition of crossing over. It is suggested that this precondition of crossing over is the repair of doublestrand DNA breaks known to be controlled by the mus309 gene. It should also be noted that the effect of the heat shock on the mutant females was generally speaking the opposite of its effect on the wild type females. As will be explained in the discussion chapter, these results are in accordance with the genetic models of interference, particularly the counting number model, in which interference depends on the genetic distance between the adjacent crossover points. However, the results are in contradiction with any physical model of interference where interference is dependent on physical distance between the adjacent crossover points.
1.3. The mus309 Gene and Molecular Models of Crossing over
Molecular models of meiotic crossing over suggest that crossing over is initiated by the formation of meiosis-specific double-strand breaks (DSBs) of DNA, catalyzed eventually in all eukaryotes by the topoisomeraselike Spo11 protein, encoded in Drosophila by the meiW68 gene [7], in co-operation with other enzymes. The birth of DSBs is followed by formation of heteroduplex DNA and rejoining of the ends created in the breakage involving a single-end-invasion intermediate. Following this, a physical structure called the displacement loop will be formed. Subsequent DNA synthesis and second end capture form a structure known as the double Holliday junction (dHJ), which is then resolved to form either crossovers or non-crossovers [8,9].
Two alternative pathways for the repair of the DSBs are known: the synthesis-dependent strand annealing (SDSA) pathway and the double-strand-break repair (DSBR) pathway. The former pathway leads exclusively to non-crossover products and the latter to both crossover and non-crossover products [10,11].
In D. melanogaster, the mus309 gene located on the right arm of chromosome three (86F4) encodes, in a manner similar to its orthologues in other organisms, a RecQ helicase [12-15] and, accordingly, is involved in DSB repair [10,11,16]. In particular, it is known that the product of the mus309 gene is involved in the SDSA pathway of the repair of the DSBs [17,18]. More specifically, in the mus309 mutants the SDSA pathway is blocked, while the DSBR pathway remains functional [19]. Thus, the mus309 gene seems to control the choice made by the oocyte between the two alternative pathways of DSB repair. The same is also true for the Sgs1 gene, the mus309 orthologue of yeast [20]. Consequently, if in mus309 mutants more DSBs are repaired as crossovers by the DSBR pathway, a change in the crossover/non-crossover ratio can be expected, since fewer non-crossovers are produced.
2. MATERIAL AND METHODS
2.1. Experimental Procedures
Crossing over frequency and interference in the X chromosome in the regions between the crossveinless (cv, 1 - 13.7), vermilion (v, 1 - 33.0) and forked (f, 1 - 56.7) markers in four different experimental procedures were studied. In each procedure, six daily broods of progeny were derived after a certain treatment of virgin females before they were mated with males. The progeny was collected as daily broods in order to obtain the best yield of progeny flies. In the analysis of the results, however, the materials of the broods were pooled. The females were isolated and the treatment started not later than twelve hours after their hatching from the pupa. In the control crosses, cv v f/+ + +; +/+ females were crossed with cv v f/Y males, and in the experimental crosses, cv v f/+ + +; mus309D2/mus309D3 females were crossed with cv v f/Y males. The experimental females were derived from the following preliminary cross: cv v f; mus309D3/ TM6, Tb females crossed with + + +/Y; mus309D2/TM6, Tb males (Tb; Tubby 3 - 90.6) and identified on the basis of their non-Tubby phenotype. The treatments in both the control crosses and in the experimental crosses were as follows: The virgin females were either given a heat shock of 24 hours in 35˚C ± 0.5˚C or they were kept in 25˚C ± 1˚C for 24 hours.
Both the mus309 alleles used carry mutational changes that could potentially impair or abolish at least the helicase function of the MUS309 protein. In mus309D2, there is a stop codon between the sequence motifs encoding the third and fourth helicase motif of the protein. mus309D3, for its part, has a glutamic acid to lysine substitution in the conserved helicase II motif, in addition to another amino acid substitution close to the C terminus [21]. It has been demonstrated that the genotype mus309D2/ mus309D3 is semi-sterile (Janos Szabad, personal communication; see also [21-23]).
Because of the semi-sterility of the females, the mutant female crosses were carried out in cultures in which three females were mated with 3 - 5 males, whereas the control crosses were single-female cultures. The same number (30) of crosses was made in both the control and the mutant female series. After the initial mating, the parental flies were transferred without etherization into fresh culture bottles every 24th hour for five consecutive days, and discarded after the sixth day of egg laying. The progeny, thus consisting of six daily broods in both the experimental and control procedure, were raised in 25˚C on a standard Drosophila medium consisting of semolina, syrup, agar-agar and both dried and fresh yeast.
2.2. Calculation of the Frequency of the True Single Crossovers
Some of the observed single crossovers in the cv-v and v-f intervals actually result from meioses that have two exchanges, one in each interval. Assuming no chromatid interference, the three classes of double-exchange tetrads, 2-, 3- and 4-strand doubles, occur in a 1:2:1 ratio [24]. Therefore, the true frequency of single crossovers, i.e. the number of single crossovers that resulted from meioses with only one exchange in the cv – v – f region, was calculated by subtracting the observed frequency of double crossovers from those of each of the single crossover classes.
2.3. Measurement of Interference
The coefficient of coincidence, C, was calculated according to the following formula of Stevens [25], which is a maximum likelihood equation
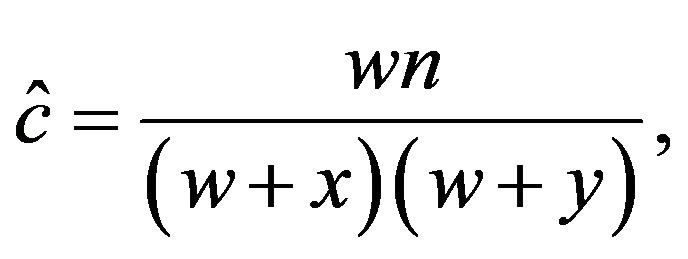
where w is the number of flies which were double crossovers, x and y are the numbers of flies which were single crossovers for cv and v, and v and f, respectively, and n is the total number of flies.
The variance of C was calculated according to the following formula, also given by Stevens [25]
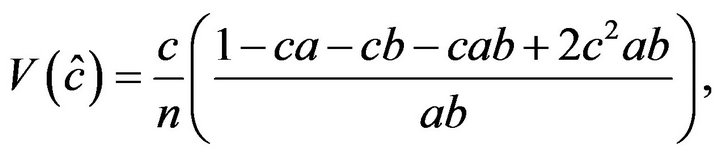
where a and b are the recombination frequencies of cv and v, and v and f, respectively. This is also a maximum likelihood equation.
2.4. Statistical Methods
In the calculations of the variance of the coefficient of coincidence, the formula of Stevens [25] given above was used. Otherwise, the variance of binomial frequencies, such as recombination frequencies, was calculated according to the usual formula: s2 = pq/n, where n is the total number of flies, p is the recombination frequency, and q is 1 – p. The standard deviation (S.D.) of all the binomial frequencies, the coefficient of coincidence included, is the square root of their variances.
In the analysis of the significance of difference of other parameters than the coefficients of coincidence a one-tailed Student’s t-test was employed. A one-tailed test in these cases is justified because non-crossing over is a priori more likely to occur than crossing over. This justification does not, however, apply to coefficients of coincidence since no value of C is a priori more probable than any other, and consequently the two-tailed Student’s t-test was employed.
3. RESULTS
The distribution of the progeny into different phenotypic classes in the control crosses is given in Table 1, and those in the experimental crosses in Table 2.
The effect of the heat shock on the phenomenon of crossing over including crossover interference in the control cross females is given in Table 3. It appears that all the parameters studied with the exception of the
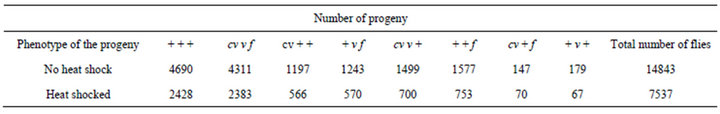
Table 1. Results of the control crosses. Distribution of progeny from the crosses in which cv v f/+ + +; +/+ females with or without a heat shock of 24 h in 35˚C were crossed with cv v f/Y; +/+ males.
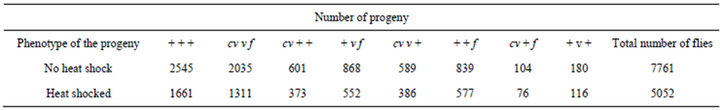
Table 2. Results of the experimental crosses. Distribution of progeny from the crosses in which cv v f/+ + +; mus309D2/mus309D3 females with or without a heat shock of 24 h in 35˚C were crossed with cv v f/Y; +/+ males.
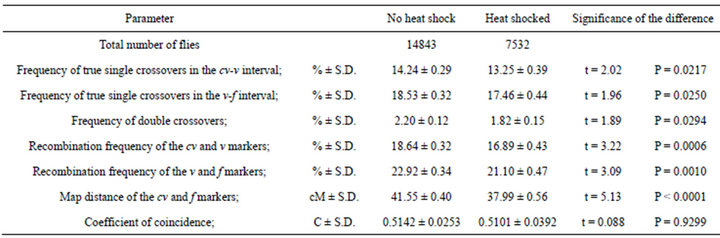
Table 3. Effect of heat shock on crossing over in females of wild type regarding the mus309 locus. Parameters measured from the results of the crosses in which cv v f/+ + +; +/+ females with or without a heat shock of 24 h in 35˚C were crossed with cv v f/Y; +/+ males.
coefficient of coincidence changed due to the heat shock treatment. The frequencies of true single crossovers decreased in both intervals studied, and as did the frequency of double crossovers. The recombination frequencies, directly giving the genetic map distances between the markers involved, firstly of cv and v markers and secondly of v and f markers decreased, and so did— of course—the map distance of the cv and f markers.
The respective figures derived from the experimental crosses are given in Table 4. The measurement of the parameters studied revealed results that were almost completely opposite to those of the control crosses: All the parameters, the coefficient of coincidence included, remained unaltered after the heat shock treatment.
Comparison of the meiotic parameters between the genotypes studied in non-heat-shocked and in heat shocked females are given in Tables 5 and 6 respectively. As can be seen from the tables, all parameters apart from the frequency of recombination of v and f markers in the non-heat-shocked females were different in both sets of data. It should specifically be observed that the frequency of double crossovers and the coefficient of coincidence were higher in the mus309 mutant females than in the wild type females. These data indicate that in both series the density of crossovers increased due to the effect of the mus309 mutation.
4. DISCUSSION
4.1. The mus309 Gene Controls the Choice Made by the Oocyte of the Route of Double Holliday Junction Repair
The first six broods after the initiation of egg laying by virgin females, i.e. the broods constituting the material of this study represent oocytes which, for the most part at least, were in the prophase stage of meiosis during the heat shock treatment, and had mainly passed the stage of DNA replication during the premeiotic interphase [26-
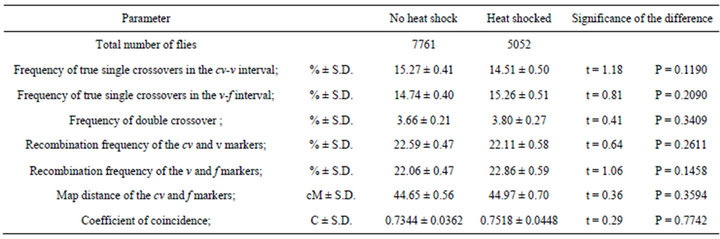
Table 4. Effect of heat shock on crossing over in mus309 mutant females. Parameters measured from the results of the crosses in which cv v f/+ + +; mus309D2/mus309D3 females with or without a heat shock of 24 h in 35˚C were crossed with cv v f/Y; +/+ males.
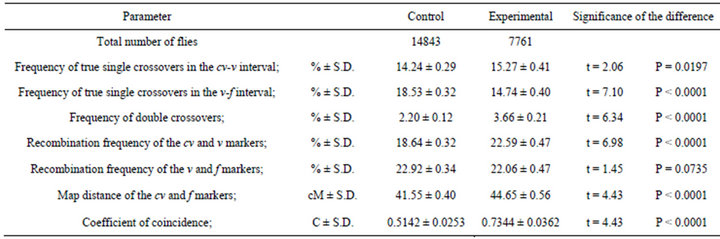
Table 5. Effect of the mus309 genotype on crossing over in females not given a heat shock. Comparison of parameters measured from the results of the crosses in which cv v f/+ + +; +/+ (control) and cv v f/+ + +; mus309D2/mus309D3 (experimental) females not given a heat shock were crossed with cv v f/Y; +/+ males.
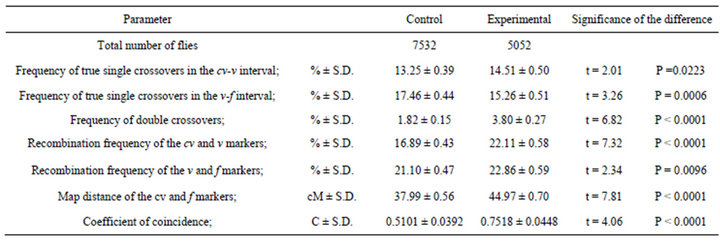
Table 6. Effect of the mus309 genotype on crossing over in heat shocked females. Comparison of parameters measured from the results of the crosses in which cv v f/+ + +; +/+ (control) and cv v f/+ + +; mus309D2/mus309D3 (experimental) females which had received a heat shock of 35˚C for 24 h were crossed with cv v f/Y; +/+ males.
29]. DSB formation occurs only during the earlier stages of meiotic prophase and initiates at a specific time after premeiotic DNA replication [29]. Crossing over in D. melanogaster for its part is known to occur during the pachytene stage of the meiotic prophase [29,30], and the progenies in the 3rd brood represent this stage of meiosis [28].
It is convincingly established that those meiotic mutants of D. melanogaster affecting crossing over which also affect interference involve preconditions of crossing over, whereas those mutants that affect crossing over without affecting interference involve the crossing over event itself [31]. Consequently, the genes involved are called precondition genes and exchange genes, respectively.
This was theoretically shown by Sandler et al. [32] as follows: Let a be the probability of the fulfillment of preconditions of crossing over in one region and only in that region in a three-point crossing-over experiment. Let b be the probability of fulfillment of the same in another region and only in that region. Let d be the probability of the fulfillment of the preconditions in both regions at the same time, and x the probability of exchange, given the preconditions. From this it follows that the coefficient of coincidence, C, is
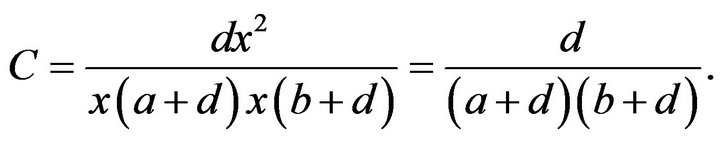
Since C is independent of x, if a mutant that acts on crossing over also affects interference, it must influence the preconditions of crossing over. If, however, interference remains unaltered, the target of the effect is the exchange itself.
What in this respect is true for meiotic mutants is, of course, also true for other factors that affect crossing over, such as the heat shock treatment in the present study.
The heat shock in the control females affected the crossing over frequencies but interference remained unaltered (Table 3). Thus, taking the foregoing into account, it can be concluded without any doubt that the heat shock in the control females affected the event of crossing over itself.
In contrast, heat shock in the experimental females affected none of the meiotic parameters studied crossover interference included (Table 4).
Interestingly, however, the mus309 mutation had a significant effect on all the meiotic parameters studied, crossover interference included, both in the non-heatshocked and heat-shocked females (Tables 5 and 6). The only exception was that the frequency of recombination of v and f markers in the females not given a heat shock was not significantly different in control and experimental females (Table 5).
As indicated by the fact that, while crossing over frequencies were also affected, interference decreased in the experimental mus309 mutant females as compared to the control females in both the non-heat-shock-treated and heat shocked females, it can be concluded that, independent of the heat shock, the mus309 mutation affected some precondition of crossing over. Therefore mus309 belongs to the class of mutations that Baker and Carpenter [33] referred to as “precondition mutants”, meaning that they act prior to the time when crossovers are actually generated.
Thus, it appears that the heat shock has a different effect on the phenomenon of crossing over than the mus309 gene. The heat shock affects the occurrence of crossing over itself, while the mus309 gene affects some precondition of crossing over. Moreover, these effects seem to be independent of each others.
As indicated in the introduction, the precondition of crossing over, which the mus309 gene product affects, is the repair of DSBs—a necessary condition for crossing over. In particular, it is known that the MUS309 protein is involved in the SDSA pathway of the repair of the DSBs. Specifically, it is also known that in the mus309 mutants the SDSA pathway is blocked, while the DSBR pathway remains functional [19].
As also indicated in the introduction, of these pathways the SDSA pathway leads exclusively to non-crossover end products of the repairing process, while the DSBR pathway leads to both non-crossover and crossover end products. Therefore, in the mus309 mutant females more DSBs are expected to be repaired as crossovers than in the wild type females. In other words, map lengths should be increased in the mus309 mutants as compared to the wild type females. This is precisely what was observed in the present study (Tables 5 and 6).
Moreover, it should also be noted that, as indicated in the results, the data show that in both the non-heatshock-treated and the heat shocked females the density of crossovers increased due to the effect of the mus309 mutation. This result shows that in the mus309 mutant females more DSBs are repaired as crossovers instead of non-crossovers than in the wild type females.
Consequently, it is suggested that the precondition of crossing over which the mus309 gene affects is the choice between the two routes of the DSB, or more precisely double Holliday junction, repair.
4.2. Testing the Models of Crossover Interference
This part of the discussion is mutatis mutandis similar to the respective discussion of an analogous series of experiments conducted by the present author where the effect of temperature on crossing over and crossover interference in mus309 mutants of D. melanogaster was investigated [6]. The results of these two studies reciprocally support each other.
As mentioned in the introduction, models of crossover interference can, in principle, be divided into two different categories. The first category of models, called genetic models [4], assumes that interference is dependent on genetic (i.e. linkage map) distance (Morgans) between adjacent crossovers. To my knowledge, currently only one model, called the “counting model” [4,5], falls into this category.
The central feature of the counting model is that recombinational intermediates (C’s) have two fates—they can be resolved with crossing over (Cx) or without (Co). The C’s are distributed at random with respect to each other, and interference results from constraints on the resolution of C’s. The basic constraint is that each pair of neighboring Cx’s must have a certain number, m, of Co’s between them, as if the meiocyte was able to “count” recombination events.
The second category of models, which may be called physical models, hypothesizes that crossover interference is dependent on physical distance (microns or base pairs) between the adjacent crossovers. In general, these models suggest that some kind of physical signal travels along the bivalent and determines the distribution of crossovers. One of the models belonging to this category, the reaction-diffusion model [34], is quantitative while the other models are qualitative.
According to the reaction-diffusion model, a “random walking” precursor becomes immobilized and matures into a crossover point. The interference is caused by a pair-annihilation of the random walkers, called the A particles, due to their collision together, or by annihilation of a random walker due to its collision with an immobilized point. This model has two parameters—the initial density of the random walkers, α, and the rate, h, of their processing into crossover points. It is logical to conclude that interference decreases if the α value increases and/or h decreases [34].
It is also quite logical to assume that if the mus309 mutations affect the proportion of the double Holliday junctions being resolved as crossovers instead of noncrossovers, the m value of the counting model should decrease, and consequently interference should diminish, in the mus309 mutants. The results of the present study are consistent with this idea. It is, therefore, very probable that the mus309 mutation affects the Drosophila counting number, thus being the first mutation of this kind identified. Consequently, the results of the present study support the view that crossover interference in Drosophila is tightly tied to genetic distance.
In contrast, however, the results of the present study are not compatible with the reaction-diffusion model. According to this model, interference depends on two factors only, viz. the initial density of crossover precursors, i.e. DSBs, and the rate of their processing into crossovers. Therefore, it is hard to conceive, in terms of the reaction-diffusion model, how the number of crossovers, i.e. the map distances, would change due to the effect of the temperature shock but their distances, i.e. interference, would not, as the initial density of DSBs does not change. This seems, however, to be the case in the results of the control crosses of the present study. Namely, because the coefficient of coincidence, C, did not change due to the heat shock treatment, it can be concluded that the initial density of the DSBs, i.e. the α value did not increase. Therefore, it cannot be assumed that the α value in the experimental crosses would change either.
Thus, if the reaction-diffusion model is correct, h in the experimental crosses should decrease due to the heat shock treatment. This means that the coefficient of coincidence, C, should decrease. In fact, however, C slightly increased.
The results are also in contradiction with any model of crossover interference based on physical distance on the following grounds: The map distances in the experimental and control females are different, and react differently to heat shock, the map distances in the control crosses being heat shock sensitive while those in the experimental crosses are not. However, the crossover interference is independent of the temperature shock treatment in both series of crosses. These results are in contradiction with the models based on physical distance. In fact, if interference was dependent on physical distance, how could it remain unchanged when the genetic map distances change but the physical distances do not? In other words, if interference, the distance between the crossover points, was dependent on physical distance, how could it react in a similar way to the heat shock treatment in the two series of crosses when the map distances, the number of the crossover points, react in a different way?
4.3. A Closer Look at the “Counting Model” of Crossover Interference in Drosophila
The “counting model” of crossover interference assumes that in the wild type of Drosophila melanogaster the value of m is equal to four, i.e. that there are four noncrossover outcomes (Co’s) of the recombinational intermediates between each pair of adjacent crossovers (Cx’s) [4].
The conclusion of the present study is that mus309 has a phenotype similar to that expected for a mutant that reduces the value of m. There are two ways in which m might be reduced: 1) Most interesting, but less likely, would be a mutant that changed m but retained the same rigor. E.g. m dropped from 4 to 2 in a manner that adjacent crossovers were now always separated by 2 noncrossover outcomes. 2) A more likely way would be a mutant that has reduced ability to ensure a non-crossover outcome for each of the four non-crossovers that fall between adjacent crossovers. The loss would be more or less random, so that as the mean number of non-crossovers between adjacent crossovers fell, the actual numbers became variable. Distinguishing between these possibilities is in principle possible but would require immense amounts of data. The test would involve comparing the data with the so called S3 curves in the Figure 2 of Foss et al. [4], and equations presented by them.
A further, fundamentally different but at the same time improbable, possibility which, however, cannot be ruled out is that, in individual mus309 cells, interference did not change. The mus309 mutant may have variable penetrance, such that some meiocytes or flies have better RecQ helicase activity than do others. Meiocytes with the least activity would have the most crossovers, but crossovers would now be non-uniformly distributed in the gamete population so that the crossover interference, as measured, would be reduced.
5. ACKNOWLEDGEMENTS
Thanks are given to Professor Janos Szabad (Szeged, Hungary) for introducing me to the mus309 gene, and the generous donation of the mutant stocks which, however, are also available from different stock centers. Skilful technical assistance by Mirja Rantanen, M.Sc. is gratefully acknowledged. I am very grateful to docent Samuli Helle, PhD, Department of Biology, University of Turku for his expert advice in statistical affairs. My sincere thanks go to Professor Franklin W. Stahl, University of Oregon, USA for critical reading of the manuscript and many valuable suggestions. Special thanks are given to Maaria Tringham, M.Sc., and Damon Tringham, M.Phil., for checking the language.
REFERENCES
- Sturtevant, A.H. (1915) The behavior of the chromosomes as studied through linkage. Zeitschrift für Induktive Abstammungs und Vererbungslehre, 13, 234-287
- Muller, H.J. (1916) The mechanism of crossing over. American Naturalist, 50, 193-221. doi:10.1086/279534
- Hillers, K.J. (2004) Crossover interference. Current Biology, 14, R1036-R1037. doi:10.1016/j.cub.2004.11.038
- Foss, E., Lande, R., Stahl, F.W. and Steinberg, C.M. (1993) Chiasma interference as a function of genetic distance. Genetics, 133, 681-691.
- Mortimer, R.K. and Fogel, S. (1974) Genetical interfereence and gene conversion. In: Grell, R.F., Ed., Mechanisms in Recombination, Plenum Press, New York, 263- 275. doi:10.1007/978-1-4684-2133-0_23
- Portin, P. (2011) Effect of temperature on crossing over in the mus309 mutant, deficient in DNA double-strand break repair, of Drosophila melanogaster suggests a mechanism for crossover interference. Open Journal of Genetics, 1, 38-47. doi:10.4236/ojgen.2011.13008
- McKim, K.S. and Hayashi-Hagihara, A. (1998) mei-W68 in Drosophila melanogaster encodes a Spo11 homolog: Evidence that the mechanism for initiating meiotic recombination is conserved. Genes and Development, 12, 2932-2942. doi:10.1101/gad.12.18.2932
- Olsen-Krogh, B. and Symington, L.S. (2004) Recombination proteins in yeast. Annual Reviews of Genetics, 38, 233-271. doi:10.1146/annurev.genet.38.072902.091500
- Lorenz, A. and Whitby, M.C. (2006) Crossover promotion and prevention. Biochemical Society Transactions, 34, 537-541. doi:10.1042/BST0340537
- Heyer W-D., Ehmsen, K.T. and Solinger, J.A. (2003) Holliday junctions in eukaryotic nucleus: resolution in sight? Trends in Biochemical Sciences, 28, 548-557. doi:10.1016/j.tibs.2003.08.011
- Heyer, W.-D. (2004) Recombination: Holliday junction resolution and crossover formation. Current Biology, 14, R56-R58. doi:10.1016/j.cub.2003.12.043
- Ellis, N.A., Groden, J., Ye, T-Z., Staughen, J., Lennon, D.J., Ciocci, S., Proytcheva, M. and German, J. (1995) The Bloom’s syndrome gene-product is homologous to RecQ helicases. Cell, 83, 655-666. doi:10.1016/0092-8674(95)90105-1
- Karow, J.K., Chakraverty, R.K. and Hickson, J.D. (1997) The Bloom’s syndrome gene product is a 3’-5’ DNA helicase. Journal of Biological Chemistry, 272, 30611-30614. doi:10.1074/jbc.272.49.30611
- Mohaghegh, P., Karow, J.K., Brosh Jr., R.M., Bohr, V.A. and Hickson, I.D. (2001) The Bloom’s and Werner’s syndrome proteins are DNA structure-specific homologues. Nucleic Acids Research, 29, 2843-2849. doi:10.1093/nar/29.13.2843
- Wu, L., Davies, S.L., Levitt, N.C. and Hickson, I.D. (2001) Potential role for the BLM helicase in recombinetional repair via a conserved interaction with RAD51. Journal of Biological Chemistry, 276, 19375-19381. doi:10.1074/jbc.M009471200
- Brabant, A.J., van Stan, R. and Ellis, N.A. (2000) DNA helicases, genome instability, and human genetic disease. Annual Reviews of Genomics and Human Genetics, 1, 409-459. doi:10.1146/annurev.genom.1.1.409
- Adams, M.D., McVey, M. and Sekelsky, J.J. (2003) Drosophila BLM in double-strand break repair by synthesis-dependent strand annealing. Science, 299, 265-267. doi:10.1126/science.1077198
- Laurencon, A., Orme, C.M., Peters, H.K., Boulton, C.L., Vladar, E.K., Langley, S.A., Bakis, E.P., Harris, D.T., Harris, N.J., Wayson, S.M., Hawley, R.S. and Burtis, K.C. (2004) A large-scale screen for mutagen sensitive loci in Drosophila. Genetics, 167, 217-231. doi:10.1534/genetics.167.1.217
- Portin, P. (2005) mus309 mutation, defective in DNA double-strand break repair, affects intergenic but not intragenic meiotic recombination in Drosophila melanogaster. Genetical Research, 86, 185-191. doi:10.1017/S0016672305007883
- Rockmill, B., Fung, J.C., Branda, S.S. and Roeder, G.S. (2003) The Sgs1 helicase regulates chromosome synapsis and meiotic crossing over. Current Biology, 13, 1954- 1962. doi:10.1016/j.cub.2003.10.059
- Kusano, K., Johnson-Schlitz, D.M. and Engels, W.R. (2001) Sterility of Drosophila with mutations in the Bloom syndrome gene—Complementation by Ku70. Science, 291, 2600-2602. doi:10.1126/science.291.5513.2600
- Boyd, J.B., Golino, M.D., Shaw, K.E.S., Osgood, C.J. and Green, M.M. (1981) Third-chromosome mutagensensitive mutants of Drosophila melanogaster. Genetics, 97, 607-623.
- Beal, E.L. and Rio, D.C. (1996) Drosophila IRBP/Ku p70 corresponds to the mutagen-sensitive mus309 gene and is involved in P-element excision in vivo. Genes and Development, 10, 921-933. doi:10.1101/gad.10.8.921
- Weinstein, A. (1936) The theory of multiple-strand crossing over. Genetics, 21, 155-199.
- Stevens, W.L. (1936) The analysis of interference. Journal of Genetics, 32, 51-64. doi:10.1007/BF02982501
- Grell, R.F. and Chandley, A.C. (1965) Evidence bearing on the coincidence of exchange and DNA replication in the oocyte of Drosophila melanogaster. Proceedings of the National Academy of Sciences of the USA, 53, 1340- 1346. doi:10.1073/pnas.53.6.1340
- Grell, R.F. (1972) Recombination and DNA replication in the Drosophila melanogaster oocyte. Genetics, 73, 87- 108.
- Portin, P. and Suormala, T. (1973) Timing of meiotic crossing-over in Drosophila melanogaster. Hereditas, 75, 267-272. doi:10.1111/j.1601-5223.1973.tb01168.x
- Mehrotra, S. and McKim, K.S. (2006) Temporal analysis of meiotic DNA double-strand break formation and repair in Drosophila females. Public Library of Sciences Genetics, 2, 1883-1897.
- Joyce, E.F. and McKim, K.S. (2009) Drosophila PCH2 is required for a pachytene checkpoint that monitors double-strand-break-independent events leading to meiotic crossover formation. Genetics, 181, 39-51. doi:10.1534/genetics.108.093112
- Baker, B.S. and Hall, J.C. (1976) Meiotic mutants: Genetic control of meiotic recombination and chromosome segregation. In: Ashburner, M. and Novitski, E., Eds., The Genetics and Biology of Drosophila, Academic Press, London, New York, San Francisco, 351-434.
- Sandler, L., Lindsley, D.L., Nicoletti, B. and Trippa, G. (1968) Mutants affecting meiosis in natural populations of Drosophila melanogaster. Genetics, 60, 525-558.
- Baker, B.S. and Carpenter, A.T.C. (1972) Genetic analysis of sex chromosomal meiotic mutants in Drosophila melanogaster. Genetics, 71, 255-286.
- Fujitani, Y., Mori, S. and Kobayashi, I. (2002) A reaction-diffusion model for interference in meiotic crossing over. Genetics, 161, 365-372.
NOTES
*Unfortunately the first printing of this paper, published in Open Journal of Genetics 2, 155-162 (2012) contained several mistakes in the statistical analysis of the data and errors in some tables. The mistakes in statistics resulted in changes in the content of the paper. Therefore the whole paper will here be published anew as a corrected version. The main conclusions of the paper concerning the mechanism of crossing over, however, remain unaltered.

