Advances in Biological Chemistry
Vol.2 No.4(2012), Article ID:24537,12 pages DOI:10.4236/abc.2012.24042
LCA studies for alkaline and enzyme catalyzed biodiesel production from palm oil
![]()
School of Engineering and Information Technology Universiti Malaysia Sabah, Sabah, Malaysia
Email: *dr_ravindra@hotmail.com, gunnerswizard_89@yahoo.com
Received 6 August 2012; revised 9 September 2012; accepted 20 September 2012
Keywords: Biodiesel; Life Cycle Assessment (LCA); Enzyme Catalyzed Process
ABSTRACT
The life cycle analysis of biodiesel production from palm oil (PO) involves 3 stages: 1) Cultivation; 2) Oil Extraction and 3) Transesterification of PO. There are several different approaches to the production process. The enzyme catalyzed process, was investigated and its environmental performance was compared with the conventional alkali-catalyzed process by using life cycle analysis (LCA). The enzyme catalyzed process is found to be technically a simpler production process and offers the production of less environmentally damaging wastes.
1. INTRODUCTION
Across the globe, the production of conventional petroleum will soon reach saturation. Therefore there is a need in introducing renewable energy and the production of biofuels such as biodiesel. Biodiesel significantly reduces emissions from the transportation sector and also helps in increased energy security. Biodiesel reduces net carbon dioxide emissions by 78% and it has also been shown to have dramatic improvements on engine exhaust emissions. Combustion of neat biodiesel decreases carbon monoxide (CO) emissions by 46.7%, particulate matter emissions by 66.7% and unburned hydrocarbons by 45.2%. In order to meet the global fuel demand, there is a need for investment in large scale biodiesel production plant. Therefore, selecting an appropriate process for the biodiesel production is a key for a cleaner environment.
The reaction can be carried out by various forms of catalysts, but so far industrially only chemical homogenous catalysts are used in large scale and no significant studies on enzymatic catalysts for large scale application. In order to evaluate the potential and obstacles in introducing enzyme-catalyzed process, analysis and comparison with the conventional process must be conducted. Sustainability is the key principle in natural resource management as it involves operational efficiency, socioeconomic and minimization of environmental impact considerations [1,2]. The assessment of environmental impacts is quite subjective. Therefore, one of the tools that can be employed to help answer this issue is life cycle assessment (LCA).
LCA is an environmental assessment tool to evaluate the impact that a product (or service) has on the environment over the entire period of its life. It involves from the extraction of the raw materials from which it is made, through the manufacturing, packaging and marketing processes, and the use, re-use and maintenance of the product, on to its eventual recycling or disposal as waste at the end of its useful life [3,4]. The purpose of this study is to compare the potential environmental impact for both alkali-catalyzed and enzymatic catalyzed biodiesel production, using palm oil as raw material.
2. MATERIAL AND METHODS
In this study, LCA method is used as a tool for comparative evaluation of alkali-catalyzed and enzymatic catalyzed biodiesel production process with palm oil as the oil source. The environmental load produced from each process was estimated using information obtained from the process design using process simulator, Aspen HYSYS Version 2006. The materials and energy used during biodiesel production are obtained and used as inputs for the LCA analyses. Simapro 7, developed by PRé Consultants, Netherlands is used for LCA analysis.
Life Cycle Impact Assessment
SimaPro v7 software program developed by PRé Consultants from Netherlands is used to create the model of Life Cycle Impact Assessment for both alkali and enzyme catalyzed biodiesel production. Eco-indicator 99(E) is chosen in this study taking into consideration eleven types of environmental impacts such as carcinogens, respiratory organics, respiratory inorganics, climate change, radiation, ozone layer, eco-toxicity, acidification/eutrophication, land use, minerals and fossil fuel. The unit used for carcinogens, respiratory organics, climate change, radiation, ozone layer is DALY (Disability Adjusted Life Years); for ecotoxicity, acidification/eutrophication, land use is PDF (Potentially Disappeared Fraction of Plant Species); for minerals and fossil fuel is MJ (Mega Joule, surplus energy requirement to compensate lower future ore grade). For Eco-indicator 99 method, “E” refers to the weighting set belonging to the egalitarian perspective. Egalitarian is preferred due to the long term perspective of the assessment and the valuation of all substances and impact categories are equal.
The overall functional unit is the product biodiesel. However, the sub-functional unit for agriculture stage is per hectare of cropland. For oil extraction, the sub-functional unit is 1000 kg of oil production. The assumptions were however carefully made based on the reliable data to make the assessment possible and comparable. In this study, we assume that both the processes are being set up in the same location to standardize transportation costs. Other than that, it is assumed that the biodiesel plant is set up nearby to the oil mill. Therefore, transportation between the places are assumed to be negligible. Of all the steps defined by the impact assessment phase in the LCA methodology, only the classification and characterization stages are considered. Normalization and evaluation are excluded, since they are optional elements and according to the objective of the study, they would not provide any extra useful information [5,6].
In this study, the system boundary is shown as Figure 1.
• Cultivation Palm tree cultivation is the cradle for palm oil production. Therefore, that is the first step to be considered. It is important to know the procedures to cultivate seeds of palm oil. If there is no care shown to the seeds they will only germinate after a few years. In order to cultivate the seeds, they must be placed in research stations. In the research stations, seeds tend to germinate faster by 90 - 100 days. It had to be noticed that seeds must be planted in small plastic containers. The young seedling stays in the container for 4 to 5 months. When the bifid leafs are
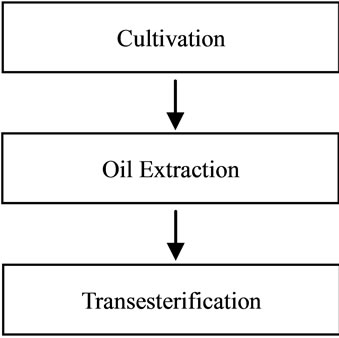
Figure 1. System boundary for biodiesel production.
coming up, the seedlings are transferred into the nursery. The seedling stays in the nursery for 1 year. When seedling is 16 - 18 month old it is ready to be planted in the palm grove. Young palm oil that had been planted will first produce male flowers. The flowers form at the base of each leaves. Female flowers are only produced after a few months. The male flowers are grouped in spikes while the female flowers form other spikes. The male flowers fertilize the female flowers. After the female flower gets fertilized it turns into a cluster of fruit. A hectare of palm oil can yield up to 7250 liter oil per year. It is important to include information’s such as type of fertilizers used and the amount of water used for the growth of the palm oil trees for the LCA studies.
• Oil Extraction Oil extraction is usually done by separating oil from the palm fruit. This process is usually done in the oil mill. For every 100 kilogram fruit bunches, 1.6 kilogram of kernel oil and 22 kilogram of palm oil can be extracted. In order to extract oil, there will be energy used and emissions from extraction of oil. In order to run LCA studies, sufficient data of the process is needed such as energy used in sterilization process, amount of palm fruit fed to the sterilizer, amount and type of gaseous emitted during this process and so on.
There are a few steps of handling the palm oil fruit before the oil could be extracted. Steps such as bunch reception, threshing, sterilization, digestion, pressing, clarification and drying of oil, oil storage and kernel recovery are vital in the oil extraction from palm oil fruit.
• Bunch reception Fresh fruits from the field are filled into empty wagons to be weighed and at times weighing bridge is used to weigh the amount of fruit in the trucks. The mill can’t change the quality of the oil that extracted from the fruit. The quality of the oil that produced depends on genetic, age of tree, growth environment, harvesting technique, handling and transport. Some control need to be implied on the harvesting techniques, transport, handling and growth of the palm oil tree.
• Threshing Threshing is a process where the fruits are removed from the bunch. In the mill, this is done by using a mechanized system. A rotating drum or fixed drum equipped with rotary beater bars detach the fruit from the brunch and leaves the spikelets on the stem. The empty fruit bunches are incinerated and the ash is returned to the plantation as fertilizers. Besides that, empty fruit bunches are then used to fuel the boiler in the mill.
• Sterilization Sterilization is an action of treating the fruit bunches with pressurized steam. Treatment of the fruit bunches been done at high temperature. The fruit bunches need to be treated due to few reasons. Heat treatment destroys oil splitting enzymes and avoids hydrolysis. Besides that, fruit bunches that are heat treated weakens the fruit stem and makes it easy to remove the fruit from bunches. Removal of fruits can be done by shaking or tumbling in the threshing machine. Heat also helps to solidify proteins in which oil-bearing cells are microscopically dispersed. Protein coagulation allows the oil-bearing cells to come together and flow more easily on application of pressure. During the sterilization process, it is important to ensure evacuation of air from the sterilizer. This is done because air acts as the barrier to heat transfer and leads to poor bleach ability of resultant oil.
• Digestion of fruit This process is done by releasing the palm oil in the fruit through the rupture or breaking down of the oilbearing cells. The digester consists of a steam heated cylindrical vessel fitted with a central rotating shaft carrying a number of stirring arms. Fruit will be pounded by the action of the rotating stirring arms. Pounding of fruit at high temperature helps to reduce viscosity of the oil, destroys the fruit outer covering and completes the disruption of the oil cells already begun in the sterilization process.
• Pressing There are two types of method of extracting oil from the digested material. One method uses mechanical presses which is the dry method. The wet method uses hot water to leach out the oil.
• Clarification and drying The main reason behind this step is to separate the oil from its impurities. A mixture of palm oil, water, debris, fibrous material and non-oily solids comes out of the pressing step. Hot water is added to the mixture to thin the mixture. The dilution provides a barrier causing the heavy solids to fall to the bottom of the container while lighter oil droplets flow through the watery mixture to the top. The water is added at the ratio of 3:1 in order to break the emulsion. Then, the mixture is passed through a screen in order to remove the fibers. The mixture is boiled for one to two hours and allowed to settle by gravity in a large tank. The clear oil will be decanted into a reception tank. The moisture content of oil needs to be reduced to 0.15 to 0.25 percent. The mixture of oil will be reheated and skimmed off to remove residual moisture.
• Oil storage Oil will be stored in storage tank and purified to meet the specifications.
• Kernel processing Mills usually recovers fiber and nutshells to fire steam boilers. The steam is used to drive turbines to generate electricity for the mill. In large scale kernel recovery process, the nuts contained in the press cake are separated from the fiber in a depericarper. Nuts are dried and cracked in centrifugal cracker to release the kernels. The kernels are separated from the shells by using combinations of winnowing column and hydrocyclones. The kernels are then dried in silos to a moisture content of 7 percent.
• Transesterification In order to produce biodiesel, transesterification process is a reaction of raw palm oil and methanol with the help of enzyme or chemical catalyst. Catalyst is usually used to increase the reaction rate and yield. Transesterification reaction is an equilibrium reaction. In order to shift the equilibrium towards the product side excess alcohol is used in the reaction. One of most popular process for producing biodiesel from oils/fats is transesterification of triglyceride by methanol to make methyl esters of straight chain fatty acids. Oil and fats needs to undergo transesterification process to lower their viscosity. There are two types of catalyst used in transesterification process, homogenous catalyst and heterogeneous catalyst. The types of homogenous catalyst for transesterification are sodium hydroxide, potassium hydroxide and sulphuric acid. Heterogeneous catalysts are carbonates and metal oxides. Transesterification is a process of exchanging the alkoxy group of an ester compound by another alcohol (Demirbas, 2008). These reactions are often catalyzed by the addition of base or catalyst. Bases can catalyze the reaction by removing proton from the alcohol thus making it more reactive, while acids can catalyze the reaction by donating a proton to carbonyl group, thus making it more reactive [8]. There are many factors such as amount of oil, methanol, enzyme and yield considerations to conduct LCA studies. Biodiesel plant is quite specific, because equipments are designed to suit the type of transesterification. Series of data would be essential from biodiesel plant or stimulation software in order to evaluate LCA studies of this particular section.
3. THEORY
In order to determine the environmental impacts of biodiesel production through life cycle assessment (LCA), material and energy data of the process are required. Simulation models for each process option have been developed using Aspen HYSYS. Flow sheets are developed for each case, using sodium hydroxide (NaOH) for chemical catalyzed process and the enzyme lipase from Burkholderia cepacia species for enzyme catalyzed process. The process flow sheets developed are continuous models converting palm oil, via transesterification reaction, to biodiesel. The reaction is represented as Figure 2. Since the reaction is reversible, alcohol will be used in excess to shift the equilibrium to the product side [2]. The alkali catalyzed process is based on the approach of [7].
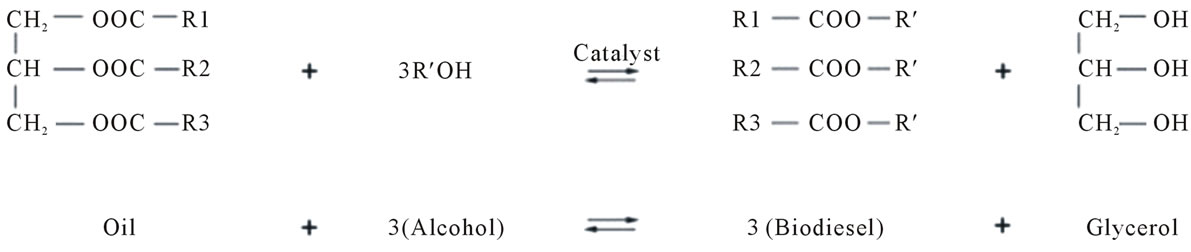
Figure 2. Transesterification of oil with alcohol (Source: [4]).
3.1. Process Design
3.1.1. Alkali Catalyzed Process
The catalyst used in the transesterification process will determine the process routes for the biodiesel production. In this study, the raw material, refined palm oil does not contain free fatty acids in its composition [6]. Therefore, alkali catalyzed process is used. Alcohol and catalyst are mixed initially before entering the transesterification reactor together with oil. Methanol enters at a 6:1 molar ratio with respect to the oil (twice the stoichiometric requirement) allowing the equilibrium to be shifted towards biodiesel production. After that, the product mixture is sent to a distillation tower to recycle excess methanol. The bottom product is then washed with water in a liquid-liquid extraction tower to separate polar and non-polar substances. Zhang et al. [10] recommended 11 kg of wash water per 1050 kg fresh oil feed and the use of a second unspecified purification unit. However, in this study, the amount of water used is increased to 154 kg to achieve separation in a single step. The polar substances such as glycerol, salts and residual substances are then neutralized with acid. The product from this neutralization process, sodium phosphate can be used as artificial fertilizer or to be dispose off and the non-polar product phase is purified by distillation.
3.1.2. Enzyme Catalyzed Process
Using the flow sheet model for biodiesel production with an alkali catalyst as outlined above, relevant changes are made to accommodate an enzyme catalyst. Enzymes and oil are mixed in the reactor after which alcohol is added for transesterification reaction. The enzyme loading is maintained at 4 wt% (biomass and support). Methanol is fed such that the concentration is kept low as the concentration higher than half the stoichiometric amount will denature the catalyst. Then, the product mixture is sent to a decanter to separate the non-polar and polar phases. The polar glycerol as the bottom product has a very high purity, since it is not mixed with water. The non-polar phase is distilled due to the lower yield of the enzymatic process compared to the alkali catalyzed processes. In this process, no excess methanol is recycled. In addition, since no alkali is present in the system, there is no need for neutralization process.
3.2. Life Cycle Impact Assessment (LCA)
Comparison of the different routes of biodiesel production is conducted with the LCA software, SimaPro v7 developed by PRé Consultants from Netherlands, using Eco-indicator 99 evaluation assessment method. The study included all raw materials, agricultural inputs and biodiesel processing required for the final biodiesel product. The impacts of process plant and equipment construction are not included in the LCA results. The results do not include impacts of the biological catalyst since the immobilized enzyme is re-used.
3.3. Method for Manual Calculation and Aspen Hysys Calculation
1) Aspen Hysys stimulation needs to be done after the manual calculation been done. First, a chemical reaction needs to be listed.
2) Then, the feed for the reaction such as flowrate of methanol, triolein and catalyst need to be listed. By using the molecular weight of methanol and triolein, amount of mole of two of the components can be found.
3) Then, the limiting reactant of the reaction can be found. The limiting reactant can be used to find the amount of glycerol, methyl oleate and excess of reactants. This must be done by using the stoichiometry of reaction and molecular weight of components.
4) Data obtained by using Aspen Hysys also need to be listed and then deviation can be found by comparing both of the manual calculation and Aspen Hysys values.
5) A few important lines are selected of the Aspen Hysys and manual calculation and listed in Table 1. Only important lines are selected to show the amount of excess methanol, catalyst, methyl oleate and glycerol present in those lines.
3.4. Method for Alkali Catalyzed Transesterification (Aspen Hysys)
1) First the components of the reactions such as triolein, methanol, glycerol, sodium hydroxide, methyl oleate are selected.
2) Then, the fluid package NRTL is selected in the stimulation due to the presence of polar components such as glycerol and methanol.
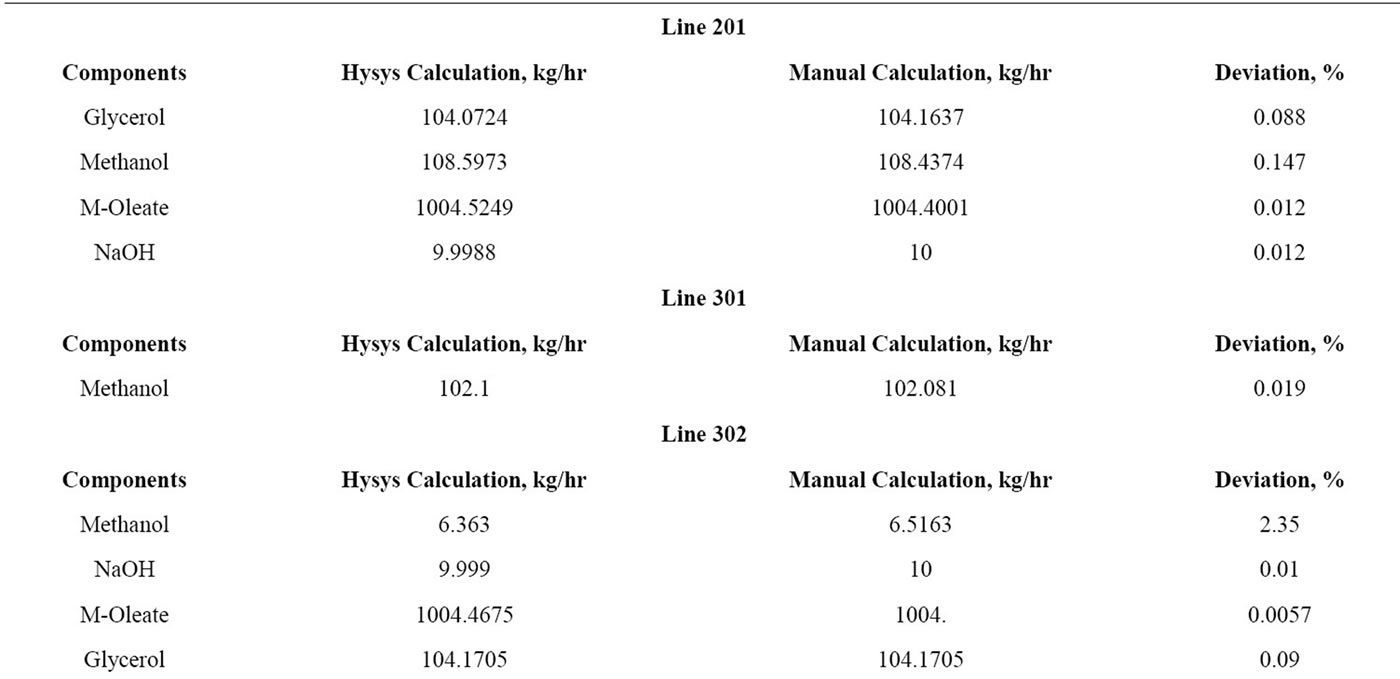
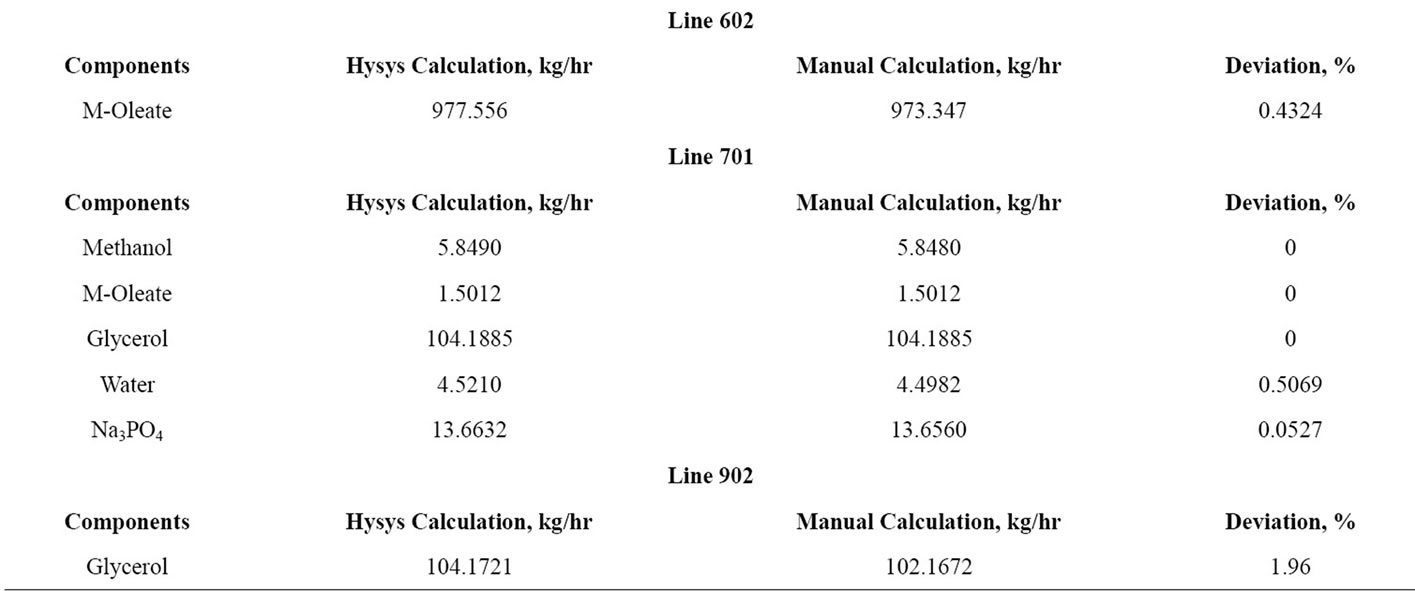
Table 1. Verification of Hysys and manual calculation of alkali catalyzed process.
3) Next, the stimulation can be preceded to the Stimulation Environment.
4) A line is selected as the feed of methanol for the alkali catalyzed transesterification. The properties that required such as temperature, pressure, flowrate, mole fraction need to be inserted.
5) Then, the line is connected with the mixer and Step 4 is repeated for the feed of sodium hydroxide because both needs to be mixed before fed to the reactor.
6) Next, another line is inserted at the end of the mixer and it is connected to the mixer. Step 4, 5 and 6 need to be repeated for each of the equipment that assembled.
7) Adding reactor to the Aspen Hysys is quite an important step. Lines are added at the outlet of the reactor and connected to the reactor. Then, the reaction and its stoichiometry are added to the fluid package of the reactor. The conversion of the reaction must be at 99%. The phase of the reaction takes place at the liquid phase.
8) The similar steps need to be taken for the rest of the equipments and the software indicates that it works when those lines turns into dark complexion.
3.5. Method for Enzyme Catalyzed Transesterification (Aspen Hysys)
1) First the components of the reactions such as triolein, methanol, glycerol, enzyme, methyl oleate are selected.
2) Then, the fluid package NRTL is selected in the stimulation due to the presence of polar components such as glycerol and methanol.
3) Next, the stimulation can be preceded to the Stimulation Environment.
4) A line is selected as the feed of methanol for the enzyme catalyzed transesterification. The properties that required such as temperature, pressure, flowrate, mole fraction need to be inserted.
5) Then, the line is connected with the reactor and Step 4 is repeated for the feed triolein because both need to be fed to the reactor.
6) Next, another line is inserted at the end of the reactor and it is connected to the reactor. Step 4, 5 and 6 need to be repeated for each of the equipment that assembled.
7) Adding reactor to the Aspen Hysys is quite an important step. Lines are added at the outlet of the reactor and connected to the reactor. Then, the reaction and its stoichiometry are added to the fluid package of the reactor. The conversion of the reaction must be at 99%. The phase of the reaction takes place at the liquid phase.
8) The similar steps need to be taken for the rest of the equipments and the software indicates that it works when those lines turns into dark complexion.
3.6. Matching and Verification of Manual and Hysys Calculation
3.6.1. Alkali Catalyzed Process
Referring to the Table 1, data of manual calculation and Hysys calculation of the important lines has been provided in order to compare its deviation. Line 201 explains of feed of the distillation column which is methanol, palm oil, sodium hydroxide and yield of biodiesel. Line 301 refers to the amount of methanol recycled of the first reactor since it’s the excess reactant of the transesterification process. After the recycle of methanol, bottom of the distillation column gives out a small amount of methanol, biodiesel, sodium hydroxide and glycerol through Line 302. The yield of biodiesel after going through separation process had been showed in Line 602. Line 701 evaluates the yield of second reactor where Na3PO4 had been used as catalyst. After the separation process, Line 902 shows the amount of glycerol separated from biodiesel.
3.6.2. Enzyme Catalyzed Process
Referring to Table 2, a few important lines of the Hysys calculation and manual calculation had been evaluated to identify its deviation of enzyme catalyzed transesterification. Line 101 is the yield of the reactor which contains biodiesel, methanol, palm oil and glycerol. After going through separation process, Line 201 (contains biodiesel and palm oil) and Line 202 (contains glycerol and methanol) are separated. Distillation column had been used to separate and obtain biodiesel which had been done in Line 301. Glycerol had been obtained through Line 402 by using distillation column to separate it from methanol. For both alkali and enzymatic processes, the deviations observed to be within 5%.
4. RESULTS AND DISCUSSIONS
4.1. Comparison of Transesterification Process
4.1.1. Process Simulation
Aspen HYSYS version 2006 from AspenTech is used as the process simulator in this study. The development of process model for simulations involves defining the chemical components, selecting appropriate thermodynamic models, proper unit operations and operating conditions such as temperature, pressure, and flow rate. For chemical components that are not readily available in the simulator library, such as palm oil, phosphoric acid and sodium phosphate, they are defined using “Hypothetical Manager” function by entering the required molecular weight, normal boiling point and density. In this case, the properties used to define palm oil are of 884 g/mol, 450˚C and 899 kg/m3 at 25˚C. Biodiesel is simulated as methyl oleate, which is available in the simulator library.
Due to the presence of polar compounds such as methanol and glycerol in the process, the non-random two liquid (NRTL) thermodynamic models are selected for use as the property package for calculation of activity coefficient of the liquid phase in the simulation. Since some binary interaction parameters were not available in the simulation databank, they were estimated using the UNIFAC vapor-liquid equilibrium and UNIFAC liquidliquid equilibrium methods.
The process flow sheets are constructed by selecting and connecting proper unit operations such as reactors, liquid-liquid extractor, distillation columns, heat exchangers. Then, conditions for each unit operation such as temperature, pressure, flow rates and composition of the input and output streams in each stream are specified. The process is simulated. Mass and energy values are obtained from flow sheets for each case used in life cycle analysis. Further, manual calculation for the process is calculated.
Comparing the process conditions (Table 3) in alkali catalyzed process, the methanol to oil ratio used is 6:1 and for enzyme catalyzed process, 3:1. The lower ratio in enzymatic process is selected, since higher ratio of alcohol will denature the enzyme. Therefore, lower ratio of methanol is preferred in the process and there is no need of distillation column for methanol recovery. Another advantage is the optimum temperature and pressure for enzyme catalyzed is lower than alkali catalyzed process is preferably 25˚C under atmospheric pressure. Other than that, due to the reusability of enzyme, the catalyst disposal issue is less concern as compared with alkali
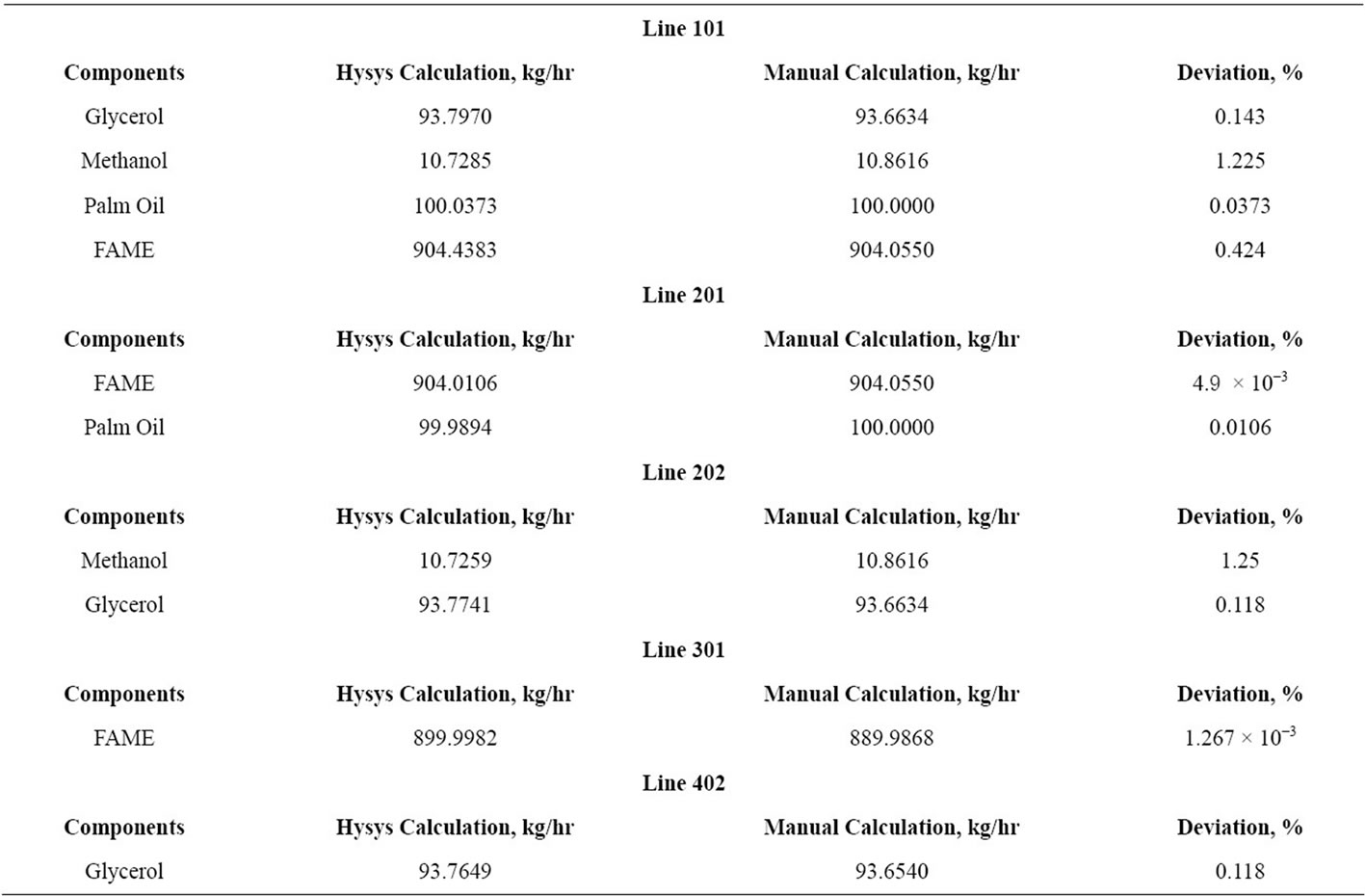
Table 2. Verification of Hysys and manual calculation of enzyme catalyzed process.
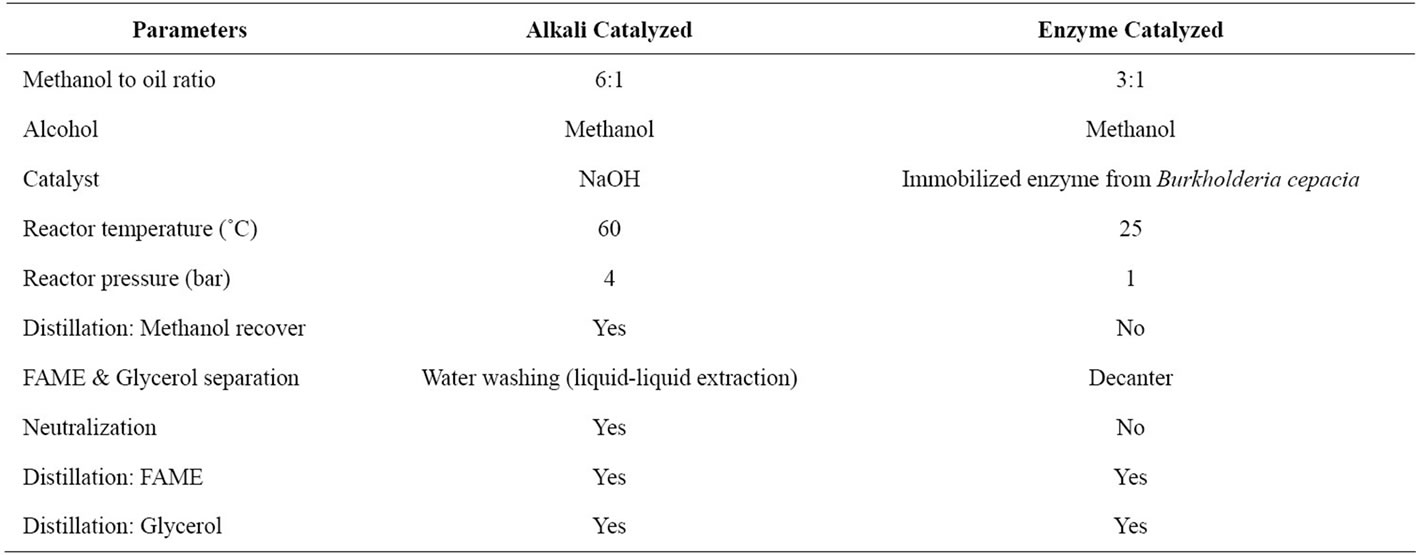
Table 3. Comparison of process conditions of biodiesel production.
catalyzed process. In alkali catalyzed process, direct disposal of catalyst sodium hydroxide in not favorable as it causes environmental damage. Therefore, alkali neutralization is needed for a safer way of waste disposal.
4.1.2. Mass and Energy Values
Data presented in Table 4 shows the amount of feed, product and waste obtained out of enzymatic catalyzed and alkali catalyzed Transesterification. By observing at the amount of biodiesel obtained it through both processes, the yield of biodiesel is right about the same. But it’s obviously noted that the amount of glycerol produced at the alkali catalyzed process is much higher which makes the separation process of biodiesel and glycerol
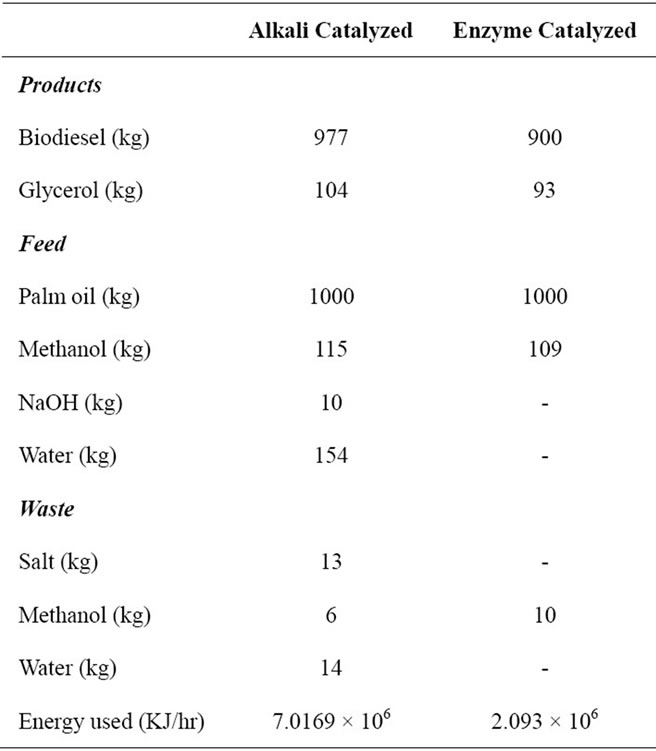
Table 4. Mass and energy values obtained from flow sheets (4.1.2).
difficult. The information regarding the feed shows that alkali catalyzed process uses more methanol compared to enzymatic catalyzed process. There has been usage of sodium hydroxide and water in the alkali catalyzed process but these are not needed in the enzymatic catalyzed process. The waste section shows that enzymatic process gives out more methanol waste compared to the alkali catalyzed waste. There has been formation of salt and water at the waste end of alkali catalyzed process, where that is not the case in enzymatic catalyzed process. It is observed that the energy requirement for the alkali catalyzed process is much higher compared to enzymatic catalyzed process due to tedious separation process stages.
Both Figures 3 and 4 are the processes, simulated using Aspen HYSYS software. From Figure 4, it is obviously seen that enzymatic catalyzed process is much simpler than alkali-catalyzed process. Since no excess of methanol is added in enzymatic method, there is littleneed for methanol recovery by distillation. Hence distillation column for methanol recovery is omitted. Also, since no alkali present in the process, there is no need for neutralization by acid and the neutralization reactor is omitted. Water washing column is not used in enzymatic process due to the absence of chemical ions in the process, instead decanter is used to separate the biodiesel and glycerol. Therefore, the energy used in enzymatic catalyzed process will be lesser when compared to alkali catalyzed process.
4.2. Life Cycle Impact Assessment
4.2.1. Characterization
Eco-indicator 99(E) which is used in the analysis characterized the impact to 11 impact categories: carcinogens, respiratory organic, respiratory inorganic, climate change, radiation, ozone layer, eco-toxicity, acidification/eutrophication, land use, minerals and fossil fuel. The characterizations of the parameter are shown in Figure 5 and the results are tabulated as shown in Table 5.
The result of the comparison shows that enzyme catalyzed biodiesel production is considered as having lower environmental impacts (Figure 5). All impact categories excepting respiratory organic justify the comparison result. The reason behind the higher impact on respiratory organic for enzyme catalyzed process is due the presence of methanol in higher amount within the process. The ratio of amount of methanol at the feed is (3:1) for enzymatic catalyst and (6:1) in alkali catalysed process. This shows that the feed of methanol at the alkali catalyzed transesterification process is much higher compared to the enzymatic catalyzed transesterification. But it is important to consider the amount of waste methanol obtained at the end of both processes when conducting the LCA study. The enzymatic catalyst transesterification gives out methanol waste of 10 kg and the alkali catalyzed transesterification process gives out methanol waste of 6 kg. That may be the reason for impact on the respiratory organics is much higher for enzyme catalyzed process compared to the alkali catalyzed process. The methanol released to the air if inhaled, will cause headache, dizziness and nausea. Methanol is the agent that causes respiratory organics.
4.2.2. Single Score
The processes stage in Figure 6 which contributed to the single score result, compiled all 11 impacts categories from characterization into single score for both processes. Each value from each category was calculated into a score and added to form the single score.
The single score result points out that land use contributes the most for the environmental impact for both processes (70% for alkali and 75% for enzyme). And, the final total score indicates obviously that the enzyme catalyzed biodiesel production process contributes less than the alkali catalyzed process to the environmental impact.
5. CONCLUSION
From the assessment of these two processes, the contribution of enzyme catalyzed biodiesel production to the 10 environmental impacts: carcinogens, respiratory inorganics, climate change, radiation, ozone layer, ecotoxicity, acidification, land use, minerals and fossil fuel are
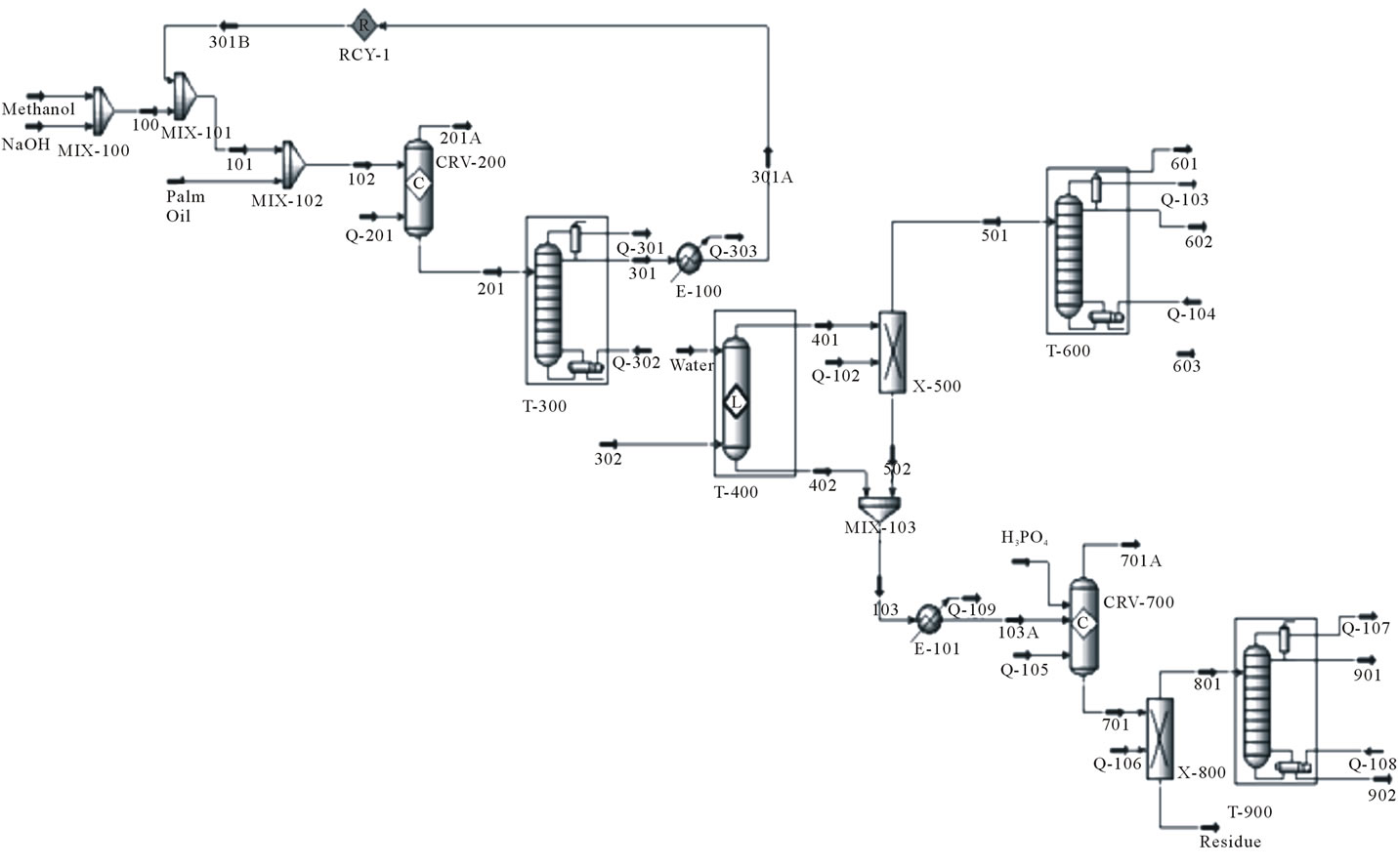
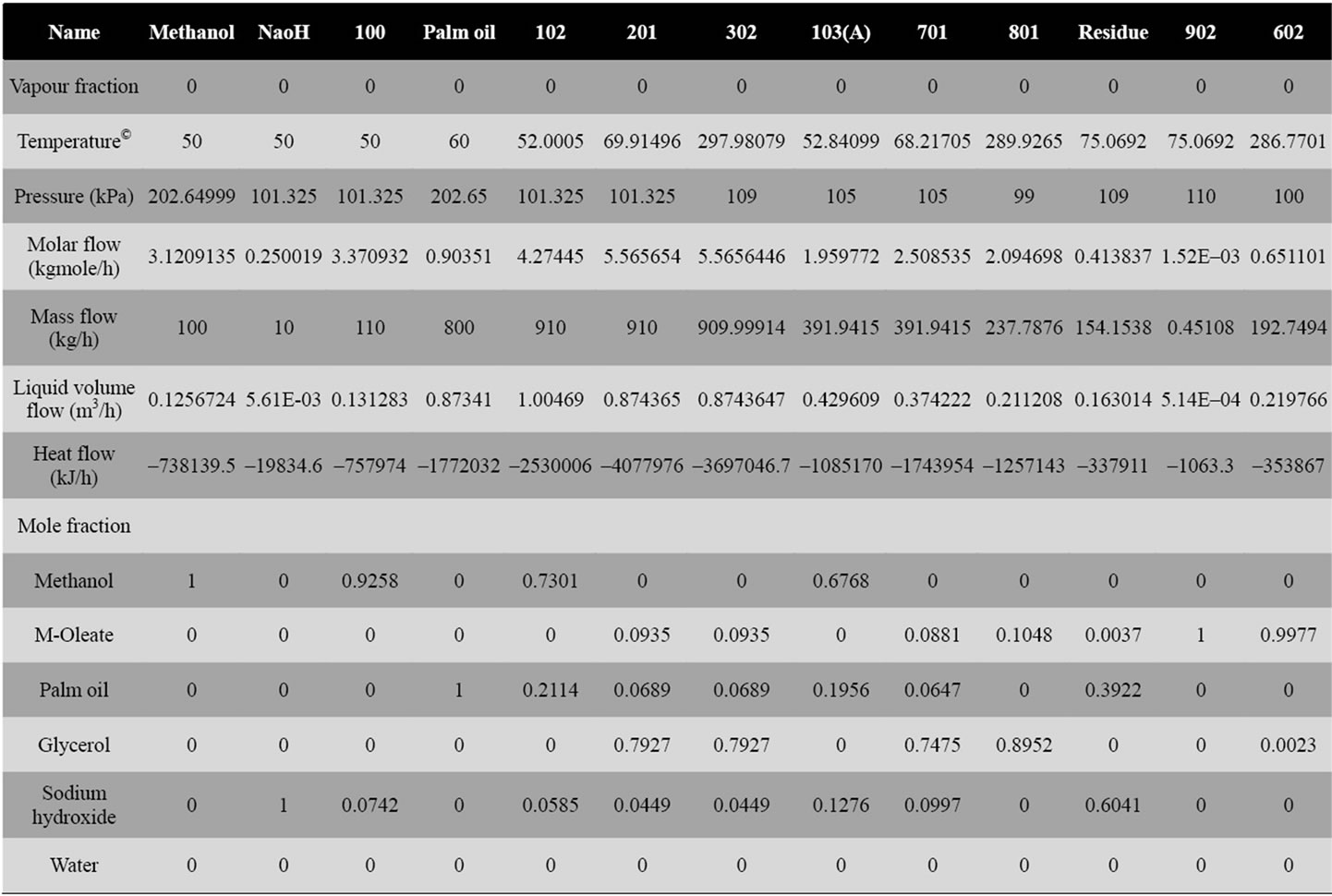
Figure 3. The alkali catalyzed process to produce biodiesel from palm oil.
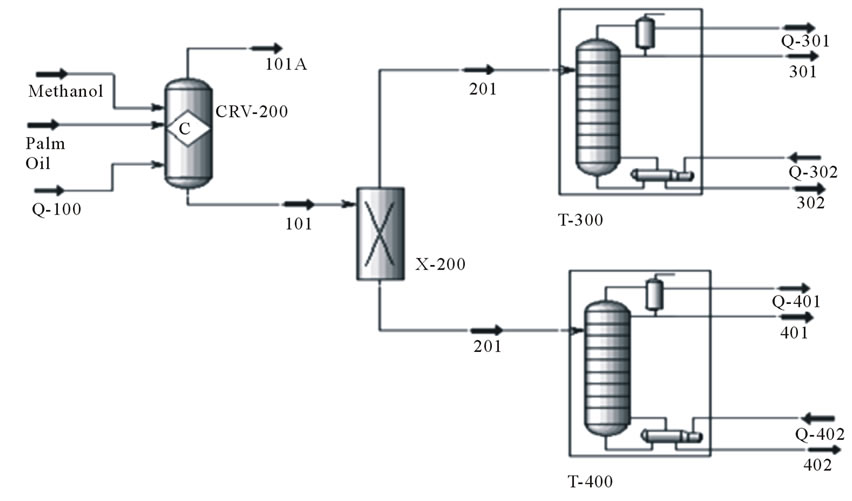

Figure 4. The enzymatic catalyzed process to produce biodiesel from palm oil.
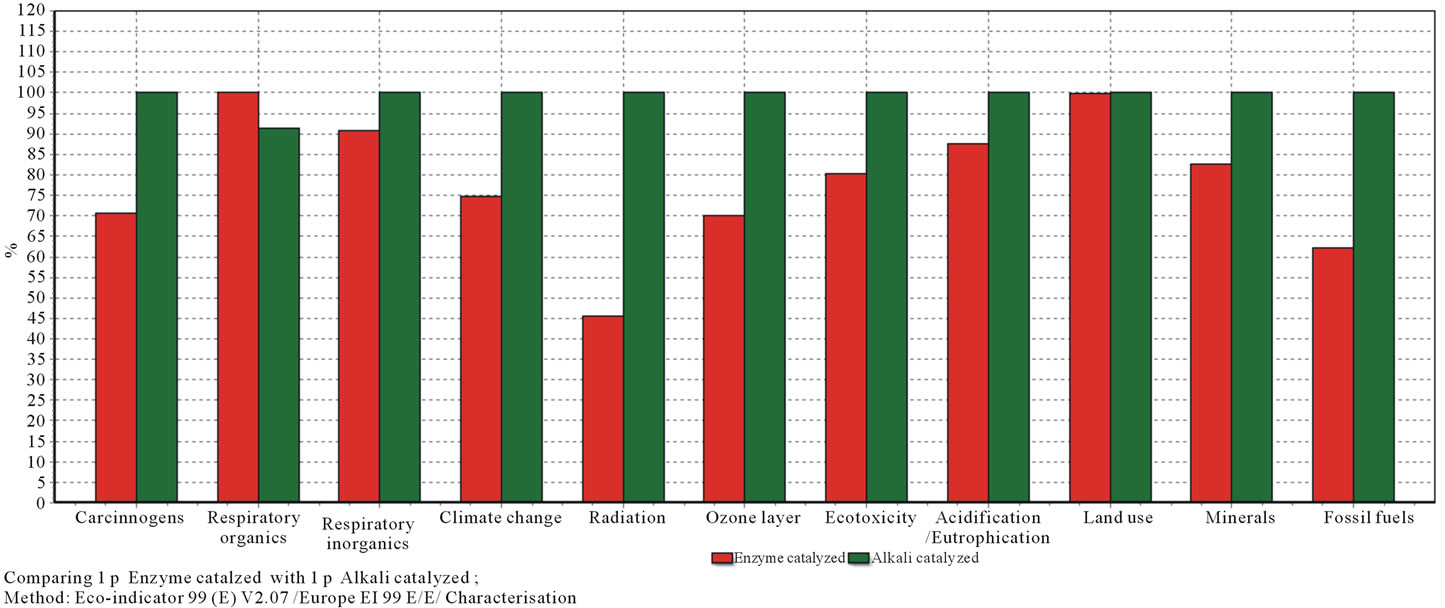
Figure 5. The characterization result in bar chart.
Figure 6. Single score in bar.
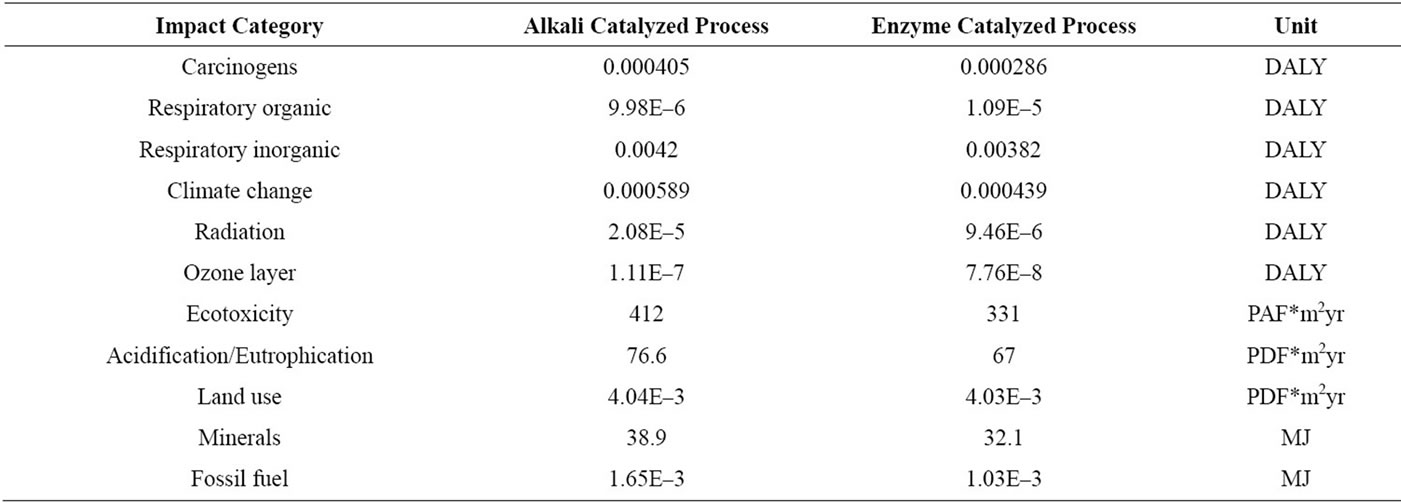
Table 5. Characterization result of impact categories on alkali and enzymatic catalyzed process.
proven as less to the alkali catalyzed biodiesel production. The exception applies only in respiratory organic due to the higher quantity of methanol presence in the emission of air for enzyme catalyzed biodiesel production. As known broadly, the reusability of enzyme will decrease the need for new catalyst and it is much cleaner in the emission perspective. This LCA study gives additional supportive information that enzyme catalyzed biodiesel production contributes less impacts to the environment due to chemical catalyst and neutralizing acid is not required in the process. The lower pressures and temperatures obtained help to give lower energy usage in the operation.
![]()
![]()
REFERENCES
- Brennan, L. and Owende, P. (2010) Biofuels from microalgae: A review of technologies for production, processing, and extractions of biofuels and co-products. Renewable & Sustainable Energy Reviews, 14, 557-577. doi:10.1016/j.rser.2009.10.009
- Choosak, K., Chanporn, T. and Pornpote, P. (2009) LCA studies comparing biodiesel synthesized by conventional and supercritical methanol methods. Journal of Cleaner Production, 17, 143-153. doi:10.1016/j.jclepro.2008.03.011
- Demirbas, A. (2008) Biodiesel a realistic fuel alternative for diesel engines. Springer-Verlag London Limited, London.
- Demirbas, A. (2009) Production of biodiesel from algae oils. Energy Sources, 31, 163-168.
- Harding, K.G., Dennis, J.S., Blottnitz, H.V. and Harrison, S.T.L.J. (2007) A life cycle comparison between inorganic and biological catalysis for the production of biodiesel. Clean Production, 16, 1368-1378. doi:10.1016/j.jclepro.2007.07.003
- Jegannathan, K.R., Leong, J.Y., Chan, E.S. and Ravindra, P. (2010) Production of biodiesel from palm oil using liquid core lipase encapsulated in κ-carrageenan. Fuel, 89, 2272-2277. doi:10.1016/j.fuel.2010.03.016
- Morais, S., Mata, T.M., Martins, A.A., Pinto, G.A. and Costa, C.A.V. (2010). Simulation and life cycle assessment of process design alternatives for biodiesel production from waste vegetable oils. Journal of Cleaner Production, 18, 1251-1259. doi:10.1016/j.jclepro.2010.04.014
- Sanz Requena, J.F., Guimaraes, A.C., Quiros Alpera, S., Relea Gangas, E., Hernandez-Navarro, S., Navas Gracia, L.M., Martin-Gil, J. and Fresneda Cuesta, H. (2010) Life Cycle Assessment (LCA) of the biofuel production process from sunflower oil, rapeseed oil and soybean oil. Fuel Processing Technology, 92, 190-199. doi:10.1016/j.fuproc.2010.03.004
- Schuchardt, U., Ricardo Sercheli, R. and Vargas, R.M. (1998). Transesterification of vegetable oils: A review. Journal of the Brazilian Chemical Society, 9, 199-210. doi:10.1590/S0103-50531998000300002
- Theocharis, T., Victor, K., Thodoris, Z. and Spyros, F. (2010) Life Cycle Assessment for biodiesel production under Greek climate conditions. Journal of Cleaner Production, 18, 328-335. doi:10.1016/j.jclepro.2009.11.002
- Zhang, Y., Dube, M.A., McLean, D.D. and Kates, M. (2003) Biodiesel production from waste cooking oil: 1. Process design and technological assessment. Bioresource Technology, 89, 1-16. doi:10.1016/S0960-8524(03)00040-3
NOTES
*Corresponding author.

