Open Journal of Pediatrics
Vol. 2 No. 4 (2012) , Article ID: 25249 , 4 pages DOI:10.4236/ojped.2012.24044
Case of purine nucleoside phosphorylase deficiency presented with hematuria*
![]()
1Department of Pediatric Allergy and Pulmonology, Behcet Uz Pediatric Diseases and Surgery Educational and Research Hospital, Izmir, Turkey
2Department of Pediatric Immunolgy, Behcet Uz Pediatric Diseases and Surgery Educational and Research Hospital, Izmir, Turkey
3Department of Pediatrics, Behcet Uz Pediatric Diseases and Surgery Educational and Research Hospital, Izmir, Turkey
4Department of Biochemistry, Division of Rheumatolgy & Immunology, Duke Univesity Medical Center, Durham, USA
Email: saniyegirit@gmail.com
Received 31 July 2012; revised 4 September 2012; accepted 18 September 2012
Keywords: Purine Nucleoside Phosphorylase Deficiency; Infant; Hematuria
ABSTRACT
Purine Nucleoside Phosphorylase (PNP) deficiency is a rare autosomal recessive metabolic disorder. In PNP-deficiency disorder, the deficient enzyme leads to accumulation of toxic metabolites, especially in lymphocytes and the metabolites exert toxic effect on T-cell generation. Purine nucleoside phosphorylase deficiency causes decreased numbers of T cells and lymphopenia. The patients suffering from PNP-deficiency may be admitted with recurrent infections, as well as neurological and autoimmune findings. We hereby presented a case admitted with the symptom of hematuria in which we established the diagnosis of PNP-deficiency early on the basis of detection of lymphopenia and low level of uric acid.
1. INTRODUCTION
Purine Nucleoside Phosphorylase (PNP) deficiency is a rare autosomal recessive metabolic disorder. Although the exact incidence has yet to be determined, 67 cases of PNPase deficiency from 49 families have been reported [1].
In PNP-deficiency disorder, the deficient enzyme leads to accumulation of toxic metabolites, especially in lymphocytes and the metabolites exert toxic effect on T-cell generation. Purine nucleoside phosphorylase deficiency causes decreased numbers of T cells and lymphopenia [2].
We hereby presented a case admitted with the symptom of hematuria in which the diagnosis of PNP-deficiency early on the basis of detection of lymphopenia and low level of uric acid was established.
2. CASE REPORT
A 7-month-old girl who was born at term without any complication admitted with the complaint of hematuria. She has been treated for urinary tract infection for one month. However, one week before admitting to the hospital she had intermittent hematuria again. Her neuromotor development has been reported to be slight retardation. The family history revealed no close kinship and the patient was the first child.
In the physical examination, body weight, the length, the head circumference were 7860 grams (25 - 50 percentile), 72 cm (10 - 25 percentile), 43 cm (25 - 50 percentile), respectively. The tonsillar tissues appeared smaller than normal. The neurological examination was normal, but she was unable to sit without any support. No pathology was identified in the other system examinations.
She was found to have mild anemia with a level of Hb: 9.9 gr/dl, Htc: 29% and PLT: 648,000/mm3. Her WBC: 3810/mm3 and the absolute lymphocyte count was 520/mm3. The results from urinalysis were as follows: density: 1020; pH: 6.5; protein (+); blood (+); 5 - 6 leucocytes and 9 - 10 erythrocytes under microscope at the magnification setting 40 × 10. No bacterial growth was evident in urine culture. On ultrasound examination of the urinary tract, bladder wall appeared thickening slightly and all other urinary system organs were normal. The uric acid level was detected to be <0.5 mg/dl, which is lower than normally expected. Serum xanthine and hypoxanthine levels were found to be <0.01 mmol/L and 0.10 mmol/L, in respective order. Uric acid, Ca/Cr ratio and urine osmolality were identified to be 5.2 mg/dl (low), 0.08, 603 mOsm/kg/water, respectively, with negative cystein capacity. Arterial blood gas analysis was normal. Lymphocytic subgroup analysis revealed low T and B cell counts along with normal NK count (Table 1).
The immunoglobulin levels measured in the serum were within normal range, (IgG: 718 mg/dl, IgM: 49 mg/dl, and IgA: 38 mg/dl).
The diagnosis of PNP-deficiency was established upon detection of no PNP activity in the lymphocytes in peripheral blood. However, adenosine deaminase level was measured to be within normal range (Table 2).
The patient was homozygous for a C > T mutation of nt 593 which changes codon 198 from CCC > CTC resulting in the substitution of Leu for Pro (Pro 198 > Leu). Both parents were heterozygous for this mutation. The patient was placed on monthly intravenous immunoglobulin (500 mg/kg) and TMP-SMX prophylaxis for Pneumocystis carinii.
Bone marrow transplantation could not be performed due to the lack of any donor with matching HLA.
Accordingly, the patient was placed on a search program for domestic and international donors. While patient was in the waiting list, she died due to clinical sepsis with fatal course at 17 months of age. No bacterial growth was evident in blood culture.
3. DISCUSSION
The discovery in the 1970s of deficiencies of adenosine deaminase and purine nucleoside phosphorylase in children with lymphocytopenia and combined immune deficiency, and subsequent investigation of pathogenesis, has established the critical importance of purine nucleoside metabolism to lymphocyte development and immune function [3]. In both disorders, the nucleoside
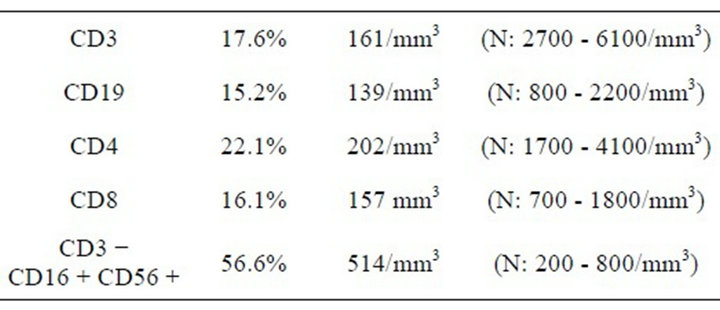
Table 1. The lymphocyte subgroups in the peripheral blood of our patient.
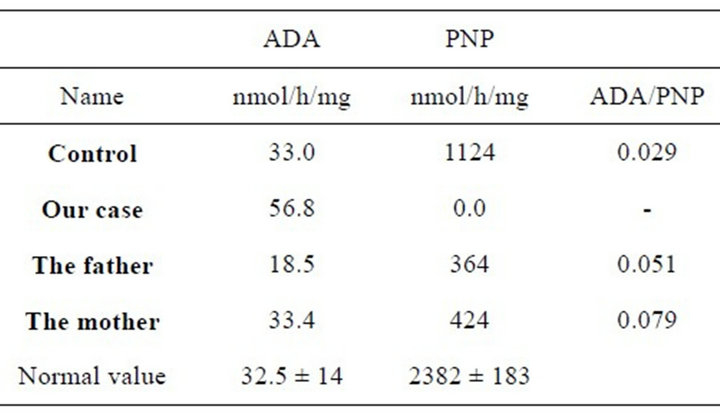
Table 2. ADA and PNP levels in our case and her family.
substrates of the missing enzyme are generated at high levels in thymus and bone marrow from the breakdown of nucleic acids associated with active cell turnover. In the absence of ADA or PNP, these purine nucleosides are converted to metabolites that accumulate in, and are toxic to, immature lymphocytes [2,3]. In ADA deficiency, dATP derived from deoxyadenosine blocks DNA synthesis and repair, resulting in depletion of T-cells, B-cells, and Natural Killer (NK)-cells; in PNP deficiency, dGTP derived from deoxyguanosine has a similar action, but predominantly affects Tand B-cell development [3,4]. dGTP can also accumulate in mitochondria and interfere with their function; this has been proposed as the cause of neuronal dysfunction, which is a more prominent feature of PNP than ADA deficiency [5]. Since the products of PNP, hypoxanthine and guanine are precursors of uric acid, PNP deficiency results in very low levels of uric acid in plasma and urine [6]. The total lymphocyte count in patients with PNP deficiency is often below 500/mm3, as in our patient. Her T and B cell counts were low, but NK cells were in the normal range, as were serum immunoglobulin levels. Even when Ig levels are normal, patients with PNP deficiency usually cannot mount a normal antigen-specific antibody response following immunization, and this capacity declines over time to cause a true combined immune deficiency [7]. Patients with PNP deficiency are also at increased risk of developing autoimmunity, which may manifest as hemolytic anemia, immune thrombocytopenic purpura and other cytopenias, as well as systemic lupus erythematosus [8,9].
Patients with PNP deficiency can become symptomatic over a broad period between the ages of 4 months and 6 years. The most frequent findings are recurrent otitis, pharyngitis, sinus infections, pneumonia, disseminated varicella infections, diarrhea and urinary tract infections [10]. Except for a presumed urinary tract infection accompanied by hematuria, our patient had no history of infection. Physical examination showed small tonsils, often found in patients with PNP deficiency.
Sometimes, neurological symptoms present first, before infections or autoimmune problems [9]. PNP deficiency has been associated with a broad spectrum of neurological abnormalities in about half of affected patients. Delay in motor development, tremor, hypertonia or hypotonia, behavioral changes, and mental-motor retardation have been reported, and may suggest the diagnosis of cerebral palsy [4,11]. In a series of 5 children with a disequilibrium syndrome characterized by hypotonia, spastic diplegia, and difficulty in sustaining posture, PNP deficiency was detected in 3 cases [12]. Our patient was unable to sit without support when she when she was diagnosed at 7 months of age; this disability persisted during her follow-up until 1 year of age.
Bone marrow or stem cell transplantation is the only curative therapy for PNP deficiency [13]. The treatment options discussed included allogeneic hematopoietic stem cell transplantation from a matched family donor, a mismatched haploidentical donor, and unrelated bone marrow donor. The survival following matched sibling donor or matched family donor transplants was extremely good in 27 of 30 patients (90% survival). Matched unrelated donor transplants were performed in 8 patients at 4 centers. These patients received pretransplant preparative regimens. Five survivors were reported (63% survival). The poorest outcome was in patients who received haploidentical family donor transplants. Overall, 21 of 42 patients (50%) survived; of the survivors, T-cell engraftment was present in 14 patients (66%) [14].
Among 11 patients transplanted for PNP deficiency, the procedure was successful in 4, although a second transplant was required in 1 patient; the causes of death in the other 7 patients were infection, graft failure and GvHD [15]. In one patient stem cell transplantation from cord blood was performed and, the level of PNP activity in lymphocytes and in vitro lymphocyte function were normal after 12 months from transplantation [16].
When no eligible stem cell donors were found among family members, our patient was treated with monthly IVIG and prophylactic TMP-SMX. A search was undertaken to identify HLA-matched domestic and international stem cell donors. However, the patient died of clinical sepsis with fatal course at 17 months of age. Because PNP deficiency is a rare cause of combined immune deficiency, the diagnosis may not be considered in a timely manner, particularly when the clinical presentation is atypical.
Our patient did not have a history of recurrent or opportunistic infections, autoimmunity, or obvious neurologic dysfunction. Her chief complaint of hematuria and a maternal history of nephrolithiasis, combined with a very low serum urate concentration, might suggest so-called renal hypouricemia, an inherited defect in renal uric acid excretion due to mutations in the gene for the URAT 1 uric acid transporter [17]. However, in that condition, nephrolithiasis results from markedly increased urinary uric acid excretion, contrary to findings in our patient. The other key finding in our patient was lymphopenia, which always raises a concern for immunodeficiency. Our patient demonstrates that, even prior to the emergence of clinical immunodeficiency, or the related findings of autoimmunity and neurologic dysfunction, the combination of lymphopenia and marked hypouricemia.
Such supportive managements as diagnosing the disease prior to emergence of the related symptoms, IVIG replacement and prophylactic administration of antibiotics may prove valuable in prolonging the survival and increasing the chance for BMT to be undertaken in the sufferers.
REFERENCES
- Walker, P.L., Corrigan, A., Arenas, M., Escuredo, E., Fairbanks, L. and Marinaki, A. (2011) Purine nucleoside phosphorylase deficiency: A mutation update. Nucleosides Nucleotides Nucleic Acids, 30, 1243-1250. doi:10.1080/15257770.2011.630852
- Hershfield, M.S. and Mitchell, B.S. (2001) Immunodeficiency diseases caused by adenosine deaminase deficiency and purine nucleoside phosphorylase deficiency. In: Scriver, C.R., Beaudet, A.L., Sly, W.S. and Valle, D., Eds., The Metabolic and Molecular Basis of Inherited Disease, McGraw-Hill, New York, 2585-2625.
- Papinazath, T., Min, W., Sujiththa, S., Cohen, A., Ackerley, C., Roifman, C.M. and Grunebaum, E. (2011) Effects of purine nucleoside phosphorylase deficiency on thymocyte development. Journal of Allergy and Clinical Immunology, 128, 854-863. doi:10.1016/j.jaci.2011.07.039
- Micheli, V., Camici, M., Tozzi, M.G., Ipata, P.L., Sestini, S., Bertelli, M. and Pompucci, G. (2011) Neurological disorders of purine and pyrimidine metabolism. Current Topics in Medicinal Chemistry, 11, 923-947. doi:10.2174/156802611795347645
- Cohen, A., Gudas, L.J., Ammann, A.J., Staal, G.E.J. and Martin, D.W. (1978) Deoxyguanosine triphosphate as a possible toxic metabolite in the immunodeficiency associated with purine nucleoside phosphorylase deficiency. The Journal of Clinical Investigation, 61, 1405-1409. doi:10.1172/JCI109058
- Alangari, A., Al-Harbi, A., Al-Ghonaium, A., Santisteban, I. and Hershfield, M. (2009) Purine nucleoside phosphorylase deficiency in two unrelated Saudi patients. Annals of Saudi Medicine, 29, 309-321. doi:10.4103/0256-4947.55320
- Gelfand, E.W., Dosch, H.M., Biggar, W.D. and Fox, I.H. (1978) Partial purine nucleoside phosphorylase deficiency: Studies of Lymphocyte function. The Journal of Clinical Investigation, 61, 1405-1409.
- Notarangelo, L.D. (2009) Primary immuno deficiencies (PIDs) presenting with cytopenias. Hematology: American Society of Hematology Education Program Book, 2009, 139-182. doi:10.1182/asheducation-2009.1.139
- Tabarki, B., Yacoub, M., Tlili, K., Trabelsi, A., Dogui, M. and Essoussi, A.S. (2003) Familial spastic paraplegia as the presenting manifestation in patients with purine nucleoside phosphorylase deficiency. Journal of Child Neurology, 18, 140-141. doi:10.1177/08830738030180021001
- Ozkinay, F., Pehlivan, S., Onay, H., van den Berg, P., Vardar, F., Koturoglu, G., Aksu, G., Unal, D., Tekgul, H., Can, S. and Ozkinay, C. (2007) Purine nucleoside phosphorylase deficiency in a patient with spastic paraplegia and recurrent infections. Journal of Child Neurology, 22, 741-743. doi:10.1177/0883073807302617
- Parvaneh, N., Ashrafi, M.R., Yeganeh, M., Pouladi, N., Sayarifar, F. and Parvaneh, L. (2007) Progressive multifocal leukoencephalopathy in purine nucleoside phosphorylase deficiency. Brain & Development, 29, 124-130. doi:10.1016/j.braindev.2006.07.008
- Soutar, R.L. and Day, R.E. (1991) Dysequilibrium/ataxic diplegia with immunodeficiency. Archives of Disease in Childhood, 66, 982-983. doi:10.1136/adc.66.8.982
- Classen, C.F., Schulz, A.S., Sigl-Kraetzig, M., Hoffmann, G.F., Simmonds, H.A., Fairbanks, L., Debatin, K.M. and Friedrich, W. (2001) Successful HLA-identical bone marrow transplantation in a patient with PNP deficiency using busulfan and fludarabine for conditioning. Bone Marrow Transplantation, 28, 93-96. doi:10.1038/sj.bmt.1703100
- Fischer, A., Landais, P., Friedrich, W., Gerritsen, B., Fasth, A., Porta, F., Vellodi, A., Benkerrou, M., Jais, J.P., Cavazzana-Calvo, M., Souillet, G., Bordigoni, P., Morgan, G., Van Dijken, P., Vossen, J., Locatelli. F. and di Bartolomeo, P. (1994) Bone marrow transplantation (BMT) in Europore primary immunodeficiencies other than severe combined immunodeficiency: A report from the European group for BMT and the European group for immunodeficiency. Blood, 183, 149-154.
- Carpenter, P.A., Ziegler, J.B. and Vowels, M.R. (1996) Late diagnosis and correction of purine nucleoside phosphorylase deficiency with allogeneic bone marrow transplantation. Bone Marrow Transplantation, 17, 121-125.
- Delicou, S., Kitra-Roussou, V., Peristeri, J., Goussetis, E., Vessalas, G., Rigatou, E., Psychou, F., Salavoura, K. and Grafakos, S. (2007) Successful HLA-identical hematopoietic stem cell transplantation in a patient with purine nucleoside phosphorylase deficiency. Pediatric Transplantation, 11, 799-803. doi:10.1111/j.1399-3046.2007.00772.x
- Hamada, T., Ichida, K., Hosoyamada, M., Mizuta, E., Yanagihara, K., Sonoyama, K., Sugihara, S., Igawa, O., Hosoya, T., Ohtahara, A., Shigamasa, C., Yamamoto, Y., Ninomiya, H. and Hisatome, I. (2008) Uricosuric action of losartan via the inhibition of urate transporter 1 (URAT1) in hypertensive patients. American Journal of Hypertension, 21, 1157-1219. doi:10.1038/ajh.2008.245
NOTES
*Conflict of interest: Authors have not a financial relationship with the organization that sponsored the research.

