Open Journal of Internal Medicine
Vol.3 No.4(2013), Article ID:40659,5 pages DOI:10.4236/ojim.2013.34027
Chronic eosinophilic pneumonia: A case report and review of the literature
![]()
Department of Respirology, Qingdao Municipal Hospital, Qingdao, China
Email: geyunjie66@126.com, hanxiudi@163.com, xuedongliu@263.net
Copyright © 2013 Yunjie Ge et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 1 September 2013; revised 1 October 2013; accepted 8 October 2013
Keywords: Chronic Eosinophilic Pneumonia; Case Report
ABSTRACT
Objective: We introduce one case of chronic eosinophilic pneumonia (CEP) and review the literature nearly 10 years in order to improve the understanding of this rare disease. Methods: A case of CEP diagnosed by transbronchial lung biopsy with clinical and follow-up data was analyzed and its clinical features, diagnosis and treatment combined with the literature were discussed. Result: CEP is a chronic pulmonary eosinophilic inflammation with unknown etiology, characterized by history of allergic disease, cough, sputum, but often breathlessness and wheezing; eosinophil in peripheral blood and/or sputum and/or bronchoalveolar lavage fluid significantly increased; chest X-ray showed progressively peripheral non-segment distribution of high-density infiltrates, often called “reversed pulmonary edema sign”; Pathology showed eosinophil infiltration in lung interstitial, bronchial submucosal and excessive eosinophil exudation in alveolar. Oral corticosteroids had a good response, but easy to relapse. Conclusions: Eosinophil count of bronchoalveolar lavage or lung biopsy can confirm the diagnosis of CEP timely in suspected patients. Bronchoscope can play an important role in assisting diagnosis and improving symptoms.
1. INTRODUCTION
Chronic eosinophilic pneumonia (CEP), a rare disorder of unknown etiology, is associated with intense and abnormal eosinophilic lung infiltration [1-4]. Most cases occur in patients with prior history of atopy and asthma, and are characterized by subacute or chronic presentation, alveolar and blood eosinophilia, peripheral pulmonary infiltrates on chest imaging, and rapid, dramatic response to systemic corticosteroid therapy and favorable prognosis, but tend to relapse. We report a case of CEP which was misdiagnosed as bronchial asthma with infections and bronchiectasis on admission.
2. EASE
A 62-year-old male patient was admitted with a 50-year history of asthma treated with fluticasone propionate/ salmeterol xinafoate inhalation, and shortness of breath with worsening symptoms for three months. Patient details were as follows: Occupation: retired worker; Ethnicity: Chinese; Weight: 79.6 Kg; Height: 172.5 cm; Medical history: a history of asthma since age 11, who was allergic to mites, multivalent mold and pollen, and had been given desensitization therapy. Otherwise unremarkable; Family history: unremarkable; Patient habits: nonsmoker, no alcohol consumption. Three months ago, the patient appeared with an exacerbation of wheezing due to a bad mood, accompanied with sticky sputum difficult to expectorate. He complained of coughing up pale green airway-shape sputum, hard as rubber, and obviously reduced exercise tolerance, but no fever, night sweats, weight loss, hemoptysis, chest pain, palpitation, arthralgia, arthritis, or skin rash. There were no neuronlogical or gastrointestinal symptoms and no travel history. A persistent inhalation of fluticasone propionate/salmeterol xinafoate (250 ug/50 ug, twice a day), other bronchodilators and multiple courses of antibiotics (clarithromycin, levofloxacin and cefixime) were administered over a 2-month period, but no improvement of symptoms, in contrast, gradual deterioration.
On examination his vital signs were stable, he was afebrile and oxygen saturation was 96% on ambient air. Auscultation of the lungs was decreased and revealed wheezing and scattered coarse crackles bilaterally. The remainder of the physical examination was unremarkable.
Results of laboratory investigations were as follows: His white blood cell count was 10.81 × 106/L with 33.6% eosinophils on differential count, hemoglobin, 131 g/L; Erythrocyte sedimentation rate(ESR) was 40 mm/h; Procalcitonin (PCT) was 0.10 ng/ml; C-reactive protein (CRP) was 30.91 mg/l; CEA was 19.32 ng/ml; Serum IgE level was 1180 IU/ml. Antinuclear antibody, antimitochondrial antibody, anti-smooth muscle antibody, antidouble-strand DNA ,ANCA and rheumatoid factor were negative. Tuberculosis antibody and tuberculin test were negative. Sputum etiologic test did not show aspergillus’s mycelium, aspergillus’s spores or other fungi. Bone marrow examination was unremarkable.
Arterial blood gas analysis was pH 7.44, PCO2 39 mmHg, PO2 76 mmHg, and SO2 96%. Lung function revealed moderate obstructive ventilatory dysfunction, and FEV1/FVC (%) was 59.45%, FEV1/predictive value was 53.33%, VC/predictive value was 81.71%. Six-minute walk test (6MWT) was 360 m. Chest computed tomography (CT) scan revealed bilateral peripheral patchy shadows and multiple cystic columnar shadows, predominant on right lung (Figure 1). Repeated electronic bronchoscope showed significantly congestive bronchial mucosa and the lumen impacted by massive thick yellow-white mucous plugs (Figure 2). The percentage of eosinophils in the bronchial alveolar lavage fluid (BALF) was 68%, BALF bacterial culture was Enterobacter cloacae; the histological examination of transbronchial lung biopsy (TBLB) showed remarkable accumulation of eosinophils and lymphocytes in the alveoli and interstitium, with mild interstitial fibrosis (Figure 3). Based on these observations, the diagnosis of idiopathic chronic eosinophilic pneumonia was confirmed.
Intravenous methylprednisolone 40 mg daily for five days followed by 40 mg of oral prednisolone was started. Patient showed dramatic response to the treatment within 5 days. Seven days later, peripheral eosinophilia and
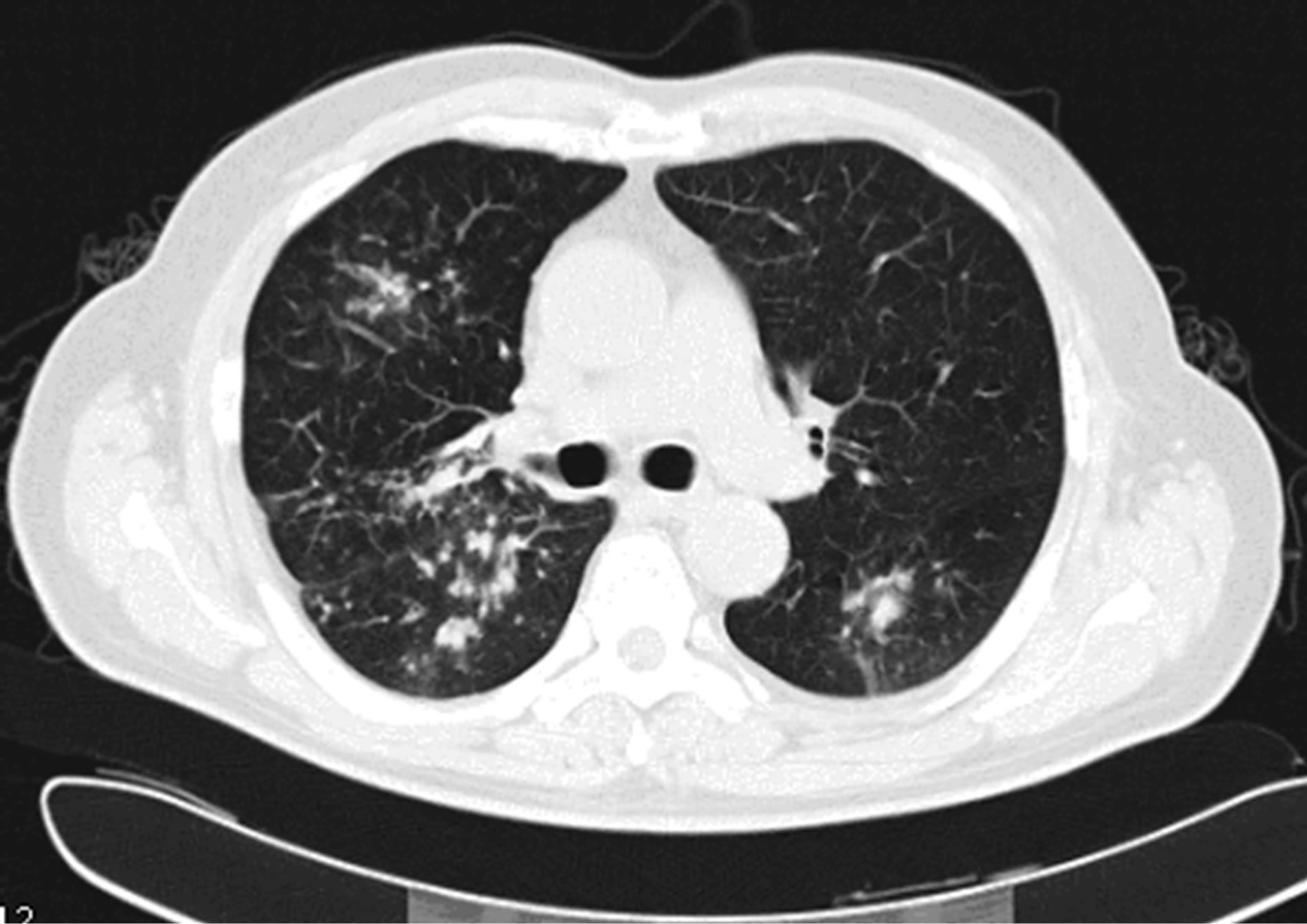
Figure 1. Computed tomography of the chest revealed bilateral peripheral patchy shadows and multiple cystic columnar shadows, predominant on right lung.
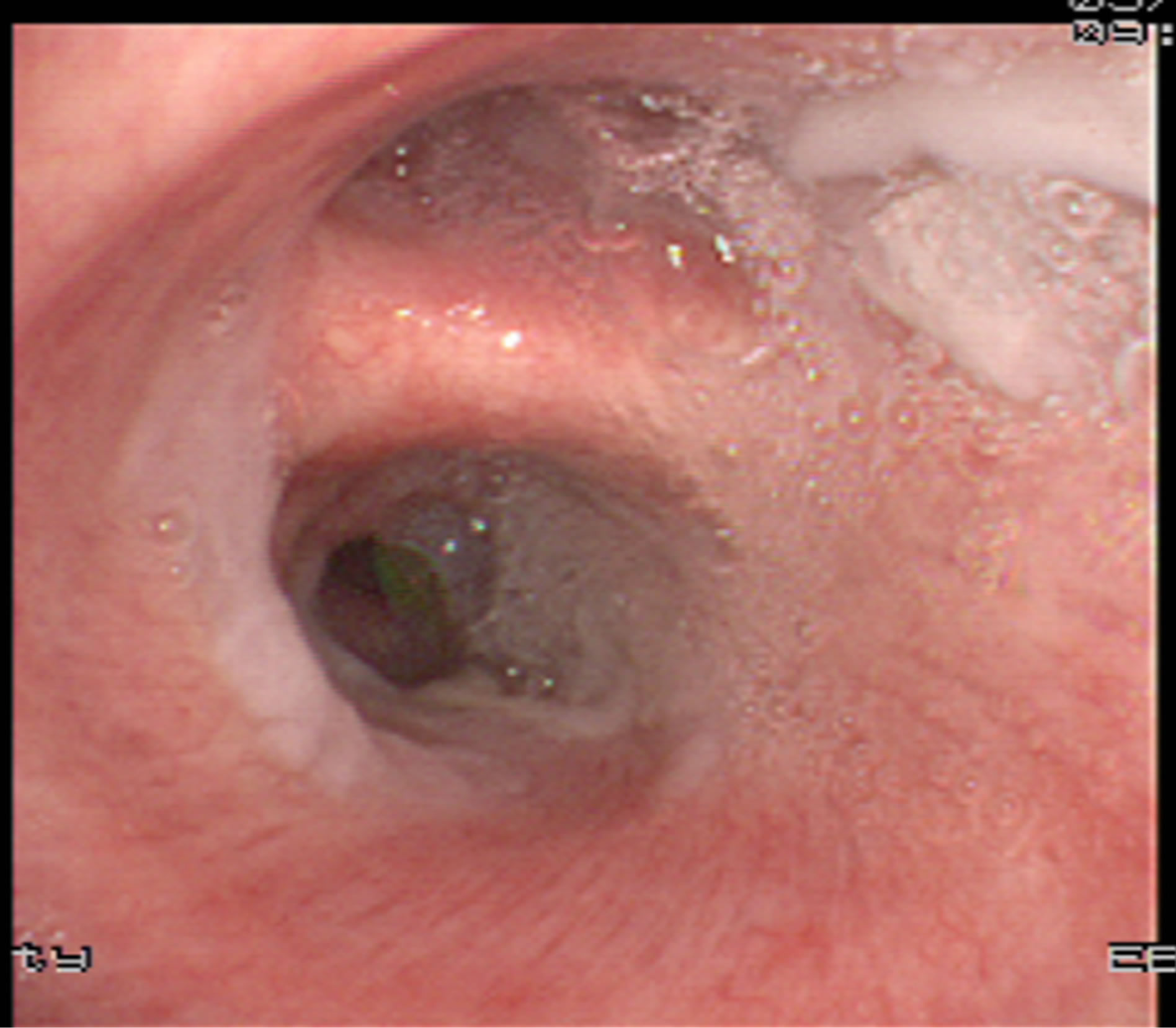
Figure 2. Electronic bronchoscope showed significantly congestive bronchial mucosa and thick yellow-white mucous plugs in the right main airway.
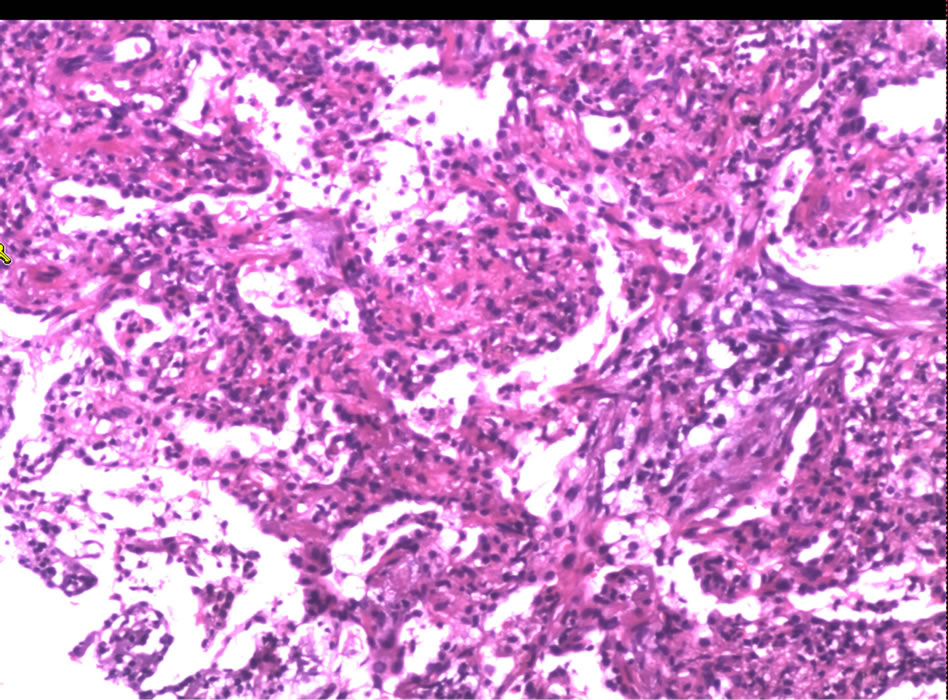
Figure 3. A transbronchial lung biopsy of the right lower lobe revealed remarkable accumulation of eosinophils and lymphocytes in the alveoli and interstitium, with mild interstitial fibrosis (HE stain, ×100).
ESR, CRP and IgE decreased to a normal level. The patient was discharged to receive 40 mg prednisolone per day. The dosage of predonisolone was gradually tapered. Following the steroid therapy, symptoms of cough, expectoration and shortness of breathing were obviously getting well. Two months later, the patient came back to our hospital for reviewing of laboratory tests, CT and bronchoscope examination were made in order to evaluate the effect of glucocorticoid therapy. The results showed that 6MWT was 588 m; lung function and chest CT scans were normalized (Figure 4). Bronchoscope reexamination was found no mucoid impaction and a marked decrease in the eosinophils in BALF. To reduce systemic steroid side effect and avoid relapse of CEP, ICS/ LABA inhaler was used continually.
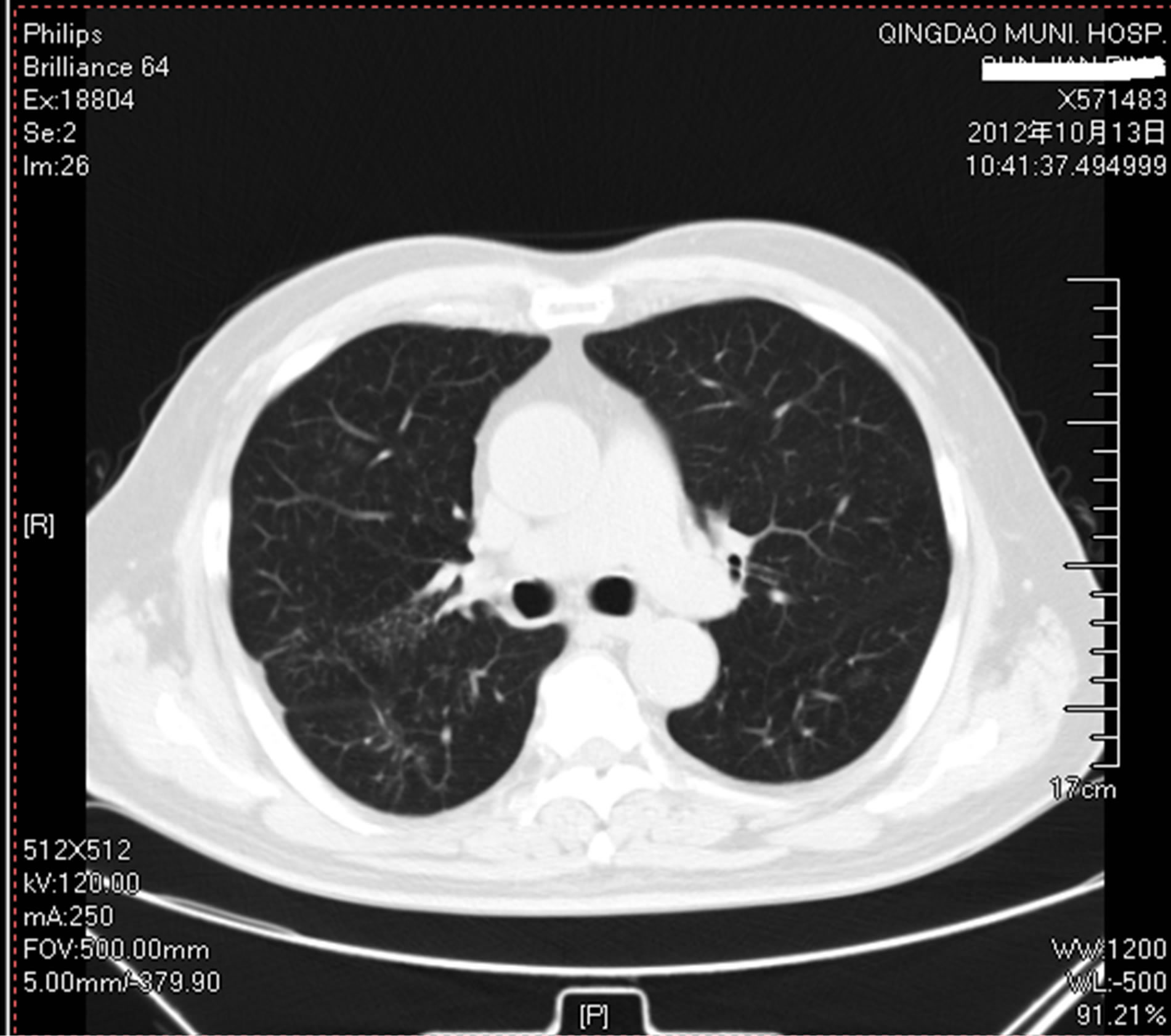
Figure 4. CT scan of the chest showed absolute resolution of bilateral lesion after receiving glucocorticoid therapy.
3. DISCUSSION
CEP was first described by Carrington CB in 1969 as chronic variants of Loffler’s syndrome [1]. The disease may affect every age group [5], with a mean age of 45 years [6]. For unexplained reasons, women are more frequently affected than men [5-8]. One-third to one-half of affected patients has a past history of allergies such as asthma and rhinitis [9,10]. Asthma may precede CEP ranging from a few weeks to more than 25 years [5,11- 13]. In contrast with acute eosinophilic pneumonia (AEP), the onset of CEP is usually insidious, either subacute or chronic. Accordingly, the symptoms appear most often at least several months before diagnosis is made. The clinical feature of AEP is progressive and characterized by dyspnea, cough, fever, asthenia, weight loss (sometimes marked), and nocturnal sweats.
The diagnosis of CEP is usually based on the association of: 1. respiratory symptoms of usually more than 2 weeks’ duration; 2. alveolar or blood eosinophilia (alveolar eosinophilia >25%, and especially ≥40% on BALF differential cell count; blood eosinophilia >1000/ mm3, and especially >1500/mm3); 3. a usually nonsegmental pulmonary infiltration with peripheral predominance (so called “photographic negative of pulmonary edema”) involving mainly the upper lobe on chest imaging; 4. exclusion of the following eosinophilic lung diseases: 1) idiopathic eosinophilic lung diseases: ① AEP: it is of acute onset, with severe fever, cough, dyspnea, chest pain and acute respiratory failure [14,15]; some overlap with CEP such as eosinophilia on BALF ≥ 25% or edema and eosinophilic infiltrate in lung biopsy; but peripheral eosinophilia, an atopic background, or a history of asthma are usually absent; dramatic response to steroids, no relapses. ② Churg-Strauss syndrome (CSS): it is characterized by bronchial asthma, eosinophilia, and systemic necrotizing vasculitis, and extravascular granulomas; single or multiple neuropathy; sinus disease [16- 18]. 2) eosinophilic lung diseases of known cause: ① drug-induced eosinophilic pneumonias: it is of acute onset, associated with a detailed drug consumption (including nonsteroidal antiinflammatory agents and antibiotics). The severity of clinical manifestations varies, and the first treatment is to stop the suspicious drugs. Corticosteroids may help to shorten the course. ② Allergic bronchopulmonary aspergillosis (ABPA): it also presents with asthma, migratory pulmonary infiltrates, and high total IgE levels [19,20]. However, the presence of central bronchiectasis, a type I skin test, and circulating precipitins directed against Aspergillus fumigatus points to the diagnosis of ABPA. 3) cryptogenic organizing pneumonia (COP): it has a subacute clinical presentation comprising both general and respiratory symptoms and presents with alveolar infiltrates that often have a migratory behavior on chest imaging [21,22]. However, it is not associated with blood eosinophilia, and lymphocytes outnumber eosinophils in BALF.
In the present case, the diagnosis of the patient met all the above criteria. But, it was difficult for us initially to distinguish CEP from asthma given to our under-recognition to this disease. Meanwhile we found significantly congestive bronchial mucosa and mucoid impaction; this feature happened to coincide with the issue by XIE et al. [23]. Due to the presence of mucous plugs, the airways obstruction may not be responsive to initial simple bronchodilators. The response to corticosteroid treatment was characterized by rapid resolution of symptoms, radiologic opacities, and peripheral eosinophilia; ESR, CRP and IgE also gradually reduced to normal levels; at the same time, the patient’s pulmonary function and quality of life improved extremely. However, we believed that the examination of bronchoscope has also played an important role in sputum drainage; so that the airway obstruction obtained relief faster. So far, there was no relapse in the patient for two years.
4. CONCLUSION
CEP is characterized by chronic and progressive clinical features and specific pathological findings. We review the epidemiology, clinical, diagnosis, and therapy of the CEP. In patients who are difficult in expectoration, accompanied with severe airway obstruction, bronchoscope can play an important role in assisting diagnosis and improving symptoms.
REFERENCES
- Carrington, C.B., Addington, W.W., Goff, A.M., Madoff, I.M., Marks, A., Schwaber, J.R. and Gaensler, E.A. (1969) Chronic eosinophilic pneumonia. The New England Journal of Medicine, 280, 787-798. http://dx.doi.org/10.1056/NEJM196904102801501
- Allen, J.N. and Davis, W.B. (1994) Eosinophilic lung diseases. American Journal of Respiratory and Critical Care Medicine, 150, 1423-1438. http://dx.doi.org/10.1164/ajrccm.150.5.7952571
- Marchand, E. and Cordier, J.F. (2006) Idiopathic chronic eosinophilic pneumonia. Seminars in Respiratory and Critical Care Medicine, 27, 134-141. http://dx.doi.org/10.1055/s-2006-939516
- Cottin, V. and Cordier, J.F. (2005) Eosinophilic pneumonias. Allergy, 60, 841-857. http://dx.doi.org/10.1111/j.1398-9995.2005.00812.x
- Jederlinic, P.J., Sicilian, L. and Gaensler, E.A. (1988) Chronic eosinophilic pneumonia. A report of 19 cases and a review of the literature. Medicine (Baltimore), 67, 154- 162. http://dx.doi.org/10.1097/00005792-198805000-00002
- Marchand, E., Reynaud-Gaubert, M., Lauque, D., Durieu, J., Tonnel, A.B. and Cordier, J.F. (1998) Idiopathic chronic eosinophilic pneumonia. A clinical and follow-up study of 62 cases. The Groupe d’Etudes et de Recherche sur les Maladies “Orphelines” Pulmonaires (GERM”O”P). Medicine (Baltimore), 77, 299-312. http://dx.doi.org/10.1097/00005792-199809000-00001
- Fox, B. and Seed, W.A. (1980) Chronic eosinophilic pneumonia. Thorax, 35, 570-580. http://dx.doi.org/10.1136/thx.35.8.570
- Naughton, M., Fahy, J. and Fitzgerald, M.X. (1993) Chronic eosinophilic pneumonia. A long-term follow-up of 12 patients. Chest, 103, 162-165. http://dx.doi.org/10.1378/chest.103.1.162
- Baldini, C., Talarico, R., Della Rossa, A. and Bombardieri, S. (2010) Clinical manifestations and treatment of Churg-Strauss syndrome. Rheumatic Disease Clinics of North America, 36, 527-543. http://dx.doi.org/10.1016/j.rdc.2010.05.003
- Sibrack, L.A., Mazur, E.M., Hoffman, R. and Bollet, A.J. (1982) Eosinophilic fasciitis. Clinics of Rheumatic Disease, 8, 443-454.
- Pearson, D.L. and Rosenow, E.C.III. (1978) Chronic eosinophilic pneumonia (Carrington’s): A follow-up study. Mayo Clinic Proceedings, 53, 73-78.
- Gaensler, E.A. and Carrington, C.B. (1977) Peripheral opacities in chronic eosinophilic pneumonia: The photographic negative of pulmonary edema. AJR American Journal of Roentgenology, 128, 1-13. http://dx.doi.org/10.2214/ajr.128.1.1
- Marchand, E., Etienne-Mastroianni, B., Chanez, P., Lauque, D., Leclerc, P. and Cordier, J.F. (2003) Idiopathic chronic eosinophilic pneumonia and asthma: How do they influence each other? European Respiratory Journal, 22, 8-13. http://dx.doi.org/10.1183/09031936.03.00085603
- Philit, F., Etienne-Mastroianni, B., Parrot, A., Guerin, C., Robert, D. and Cordier, J.F. (2002) Idiopathic acute eosinophilic pneumonia: A study of 22 patients. American Journal of Respiratory and Critical Care Medicine, 166, 1235-1239. http://dx.doi.org/10.1164/rccm.2112056
- Shorr, A.F., Scoville, S.L., Cersovsky, S.B., Shanks, G.D., Ockenhouse, C.F., Smoak, B.L., Carr, W.W. and Petruccelli, B.P. (2004) Acute eosinophilic pneumonia among US Military personnel deployed in or near Iraq. JAMA, 292, 2997-3005. http://dx.doi.org/10.1001/jama.292.24.2997
- Clumbley, L.C., Harrison Jr., E.G. and DeRemee, R.A. (1977) Allergic granulomatosis and angiitis (Churg-Strauss syndrome): Report and analysis of 30 cases. Mayo Clinic Proceedings, 52, 477-484.
- Hueto-Perez-de-Heredia, J.J., Dominguez-del-Valle, F.J., Garcia, E., Gomez, M.L. and Gallego, J. (1994) Chronic eosinophilic pneumonia as a presenting feature of ChurgStrauss syndrome. European Respiratory Journal, 7, 1006-1008.
- Golstein, M.A. and Steinfeld, S. (1996) Chronic eosinophilic pneumonia followed by Churg-Strauss syndrome. Revue de Rhumatisme (English Edition), 63, 624-628.
- Tillie-Leblond, I. and Tonnel, A.B. (2005) Allergic bronchopulmonary aspergillosis. Allergy, 60, 1004-1013. http://dx.doi.org/10.1111/j.1398-9995.2005.00887.x
- Gupta, R.K., Chandr, A. and Gautam, P.B. (2012) Allergic bronchopulmonary aspergillosis—A clinical review. Journal of the Association of Physicians of India, 60, 46- 51.
- Crestani, B., Valeyre, D., Roden, S., Wallaert, B., Dalphin, J.C. and Cordier, J.F. (1998) Bronchiolitis obliterans organizing pneumonia syndrome primed by radiation therapy to the breast. American Journal of Respiratory and Critical Care Medicine, 158, 1929-1935. http://dx.doi.org/10.1164/ajrccm.158.6.9711036
- Cordier, J.F. (2000) Organising pneumonia. Thorax, 55, 318-328. http://dx.doi.org/10.1136/thorax.55.4.318
- Xie, L.X., Mo, G.X., Chen, L.A. and Liu, Y.N. (2006) Chronic eosinophilic pneumonia with mucous plugs: Case report. Chinese Medical Journal (English Edition), 119, 262-264.
ABBREVIATIONS
CEP = Chronic eosinophilic pneumonia;
ESR = Erythrocyte sedimentation rate;
PCT = Procalcitonin;
CRP = C-reactive protein;
6MWT = Six-minute walk test;
BALF = Bronchial alveolar lavage fluid;
FEV1 = Forced expiratory volume in one second;
FVC = Forced vital capacity;
VC = Vital capacity;
ICS/LABA = inhaled corticosteroids/long-acting β2 agonists;
TBLB = Transbronchial lung biopsy;
AEP = Acute eosinophilic pneumonia;
CSS = Churg-strauss syndrome;
ABPA = Allergic bronchopulmonary aspergillosis;
COP = Cryptogenic organizing pneumonia;

