Open Journal of Internal Medicine
Vol.1 No.3(2011), Article ID:16537,8 pages DOI:10.4236/ojim.2011.13013
Impact of relative contraindications on the use, benefits, and risks of anticoagulant prophylaxis in atrial fibrillation: analysis of a claims database
1Johnson & Johnson Pharmaceutical Services, LLC, Raritan, USA;
2Janssen Scientific Affairs, LLC, Raritan, USA.
Email: rmills@its.jnj.com
Received 13 September 2011; revised 30 September 2011; accepted 27 October 2011.
Keywords: RCI; Anticoagulant; Atrial Fibrillation; Prophylaxis; Stroke; Claims Database
ABSTRACT
Background: Many individuals with atrial fibrillation (AF) do not receive recommended anticoagulant prophylaxis for stroke prevention. The study investigators attempted to assess whether the presence of relative contraindications (RCIs) to anticoagulation with warfarin might contribute to this, and to assess the risks and benefits of prophylaxis in patients with RCIs. Methods: Study investigators identified patients with established non-valvular AF and flutter in a claims database. Operationally defined RCIs included, in order of clinical severity: (1) prior intracranial hemorrhage; (2) gastrointestinal bleeding or esophageal varices; (3) neurological disorder; and (4) dizziness. Nonfatal events were attributed to warfarin if patients had an appropriate claim in the previous month. Results: A total of 67,082 AF patients were eligible for analysis, including 50,485 (75.3%) in the prevalent cohort. Warfarin exposure during the study period was 68% in the prevalent cohort. At baseline, 50.5% of prevalent cohort patients had one or more RCIs. Patients with RCI had higher prevalence of stroke risk factors (congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, and prior stroke or transient ischemic attack) compared to those without RCI. Patients with RCIs often received warfarin and had lower rates of ischemic stroke than those who did not. Conclusions: These results suggest that RCIs do not account for underutilization of anticoagulant prophylaxis in AF patients. Further, the benefit/risk aspects of anticoagulation for stroke prophylaxis may be favorable for many patients with RCIs.
1. INTRODUCTION
Stroke prophylaxis in patients with atrial fibrillation (AF) requires weighing the risk of stroke vs the risk of bleeding due to relative contraindications (RCIs) to anticoagulation. Several stroke risk stratification schemes have been developed recently [1,2]. The CHADS2 system, which combined 2 existing predictor models (Atrial Fibrillation Investigators [AFI] and Stroke Prevention in Atrial Fibrillation [SPAF]), is widely used [3]. The risk factors in CHADS2 include congestive heart failure (C), hypertension (H), age ≥75 years (A), diabetes mellitus (D), and prior stroke or transient ischemic attack (S2).
In contrast to stroke risk, the evidence base for the risk associated with RCIs to warfarin is limited and has been established primarily by clinical practice [4-8]. Cirrhosis, unsupervised dementia, and a history of seizures are substantial contraindications to anticoagulant therapy [9]. In addition, increasing age, prior intracranial hemorrhage (ICH), prior gastrointestinal bleeding (GIB), and neurological diseases associated with increased risk of falling are widely accepted as RCIs to warfarin prophylaxis.
Despite evidence-based recommendations supporting anticoagulant prophylaxis for stroke prevention in patients with AF [10], observational data suggest that many individuals with AF do not receive appropriate prophylaxis [11]. For example, the NABOR program (National Anticoagulation Benchmark Outcomes Report), an evaluation of anticoagulation in US hospitals to determine treatment gaps and predictors of the use of warfarin in patients with AF, showed that the use of warfarin in patients with high and moderate risk of stroke was 54.7% and 54.1%, respectively [12].
We hypothesized that underutilization of anticoagulation in AF may, in part, reflect clinical concerns about the role of prophylaxis in patients with RCIs [8,13,14]. Therefore, we studied a “real-world” population of AF patients using a commercial insurance claims database. We analyzed the frequency of risk factors for cardioembolic stroke, the frequency of RCIs to warfarin, and the rates of nonfatal cardioembolic and bleeding events per patient-month.
2. METHODS
2.1. Design and Database
This was a retrospective ad hoc analysis of a commercial insurance claims database, PharMetrics, the IMS LifeLink™ Health Plan Claims Database (PharMetrics, Inc., Watertown, MA, USA). PharMetrics included ~60 million individuals and over 85 insurance plans, geographically representative of the US population, from January 2005 to September 2008. Because patient data were de-identified, the study protocol was not submitted for institutional review board approval and written informed consent was not considered necessary.
Data from January 2005 through September 1, 2006 were used to establish a “medical history” of >12 and ≤21 months (Figure 1). The database contained enrollment information, inpatient and outpatient data (based on ICD-9 diagnosis and procedure codes) [15] and CPT procedure codes [16], pharmacy data, and laboratory claims for services billed to the insurance provider. Only prescription medication claims were available; therefore, use of over-the-counter medications, such as aspirin, likely used in this patient population [17], could not be assessed. Claims for laboratory services could be linked to other data, but laboratory results were unavailable. Deaths were not recorded in the database.
2.2. Study Populations
Patients were included in, or excluded from, the analysis according to criteria similar to the AnTicoagulation and Risk Factors In Atrial Fibrillation (ATRIA) cohort. Patients ≥20 years of age with chronic, non-valvular AF and having a claim with a diagnosis code for AF over the study period (9/1/2006 - 9/1/2008) were selected (Figure 1) [15]. Only individuals continuously enrolled during the study period were included. Individuals with <12 months
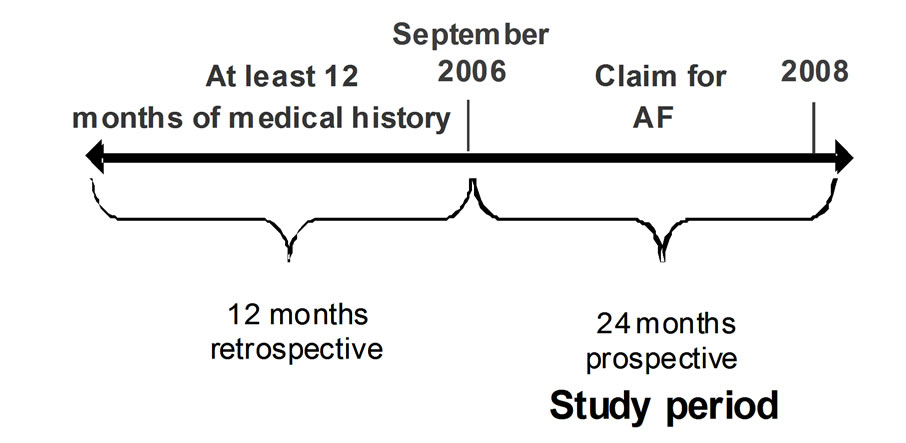
Figure 1. Atrial fibrillation (AF) algorithm for the PharMetrics analysis.
of study information after first diagnosis of AF were excluded. Patients with hyperthyroidism preceding the diagnosis of AF or with isolated AF after a cardiac procedure (transient AF) were excluded (Appendix 1) [15]. Those with an ICD-9 code for mitral stenosis or valve repair were also excluded. Finally, patients for whom prophylaxis is not currently recommended, those <65 years of age at the time of first AF diagnosis with no history of heart disease or stroke (“lone” AF), were excluded [7,18].
Establishing an incident and a prevalent cohort allowed detailed information; however, management patterns are more stable in a prevalent cohort. This analysis focuses primarily on the prevalent cohort, i.e., those patients having established AF at the onset of the observational period. “Baseline” was defined as the study period beginning September 1, 2006 for the prevalent cohort (Figure 1).
2.3. Analytic Methods
Risk factors for ischemic stroke
Using available claims, patient records were evaluated for each of the CHADS2 risk factors based on ICD-9 codes. We used the ICD-9 codes used in the ATRIA cohort [7,18,19] for baseline coronary heart disease (CHD), ischemic stroke, diabetes, and hypertension, and a subset of the ATRIA codes for congestive heart failure. ICD-9 codes for transient ischemic attack (TIA) were taken from a study of stroke and AF in the general Medicare population [11].
Relative contraindications
The methodological constraints of this analysis required an operational definition of RCIs in terms of preexisting conditions that could be identified by ICD-9 codes [11] in the claims history as of September 2006 (prevalent cohort). In this analysis, these included: 1) ICH, 2) GIB or esophageal varices, 3) neurological disorders impairing gait or station, such as Parkinson’s disease or dementia, and 4) conditions predisposing to falls, including dizziness or orthostatic hypotension. RCIs were then ranked based on their perceived clinical severity to achieve mutually exclusive patient groups (Table 1).
Effect of warfarin use
Warfarin exposure was based on a prescription claim for warfarin, on international normalized ratio (INR) procedure claims, or on diagnosis codes of warfarin (Coumadin®) therapy. In a given month, warfarin use was determined using the following algorithm:
• Any warfarin prescription claim in the current or previous month OR
• An INR test or warfarin visit code in the current or previous month, if the patient had a warfarin claim and/or 2 or more INR tests and/or warfarin visits within
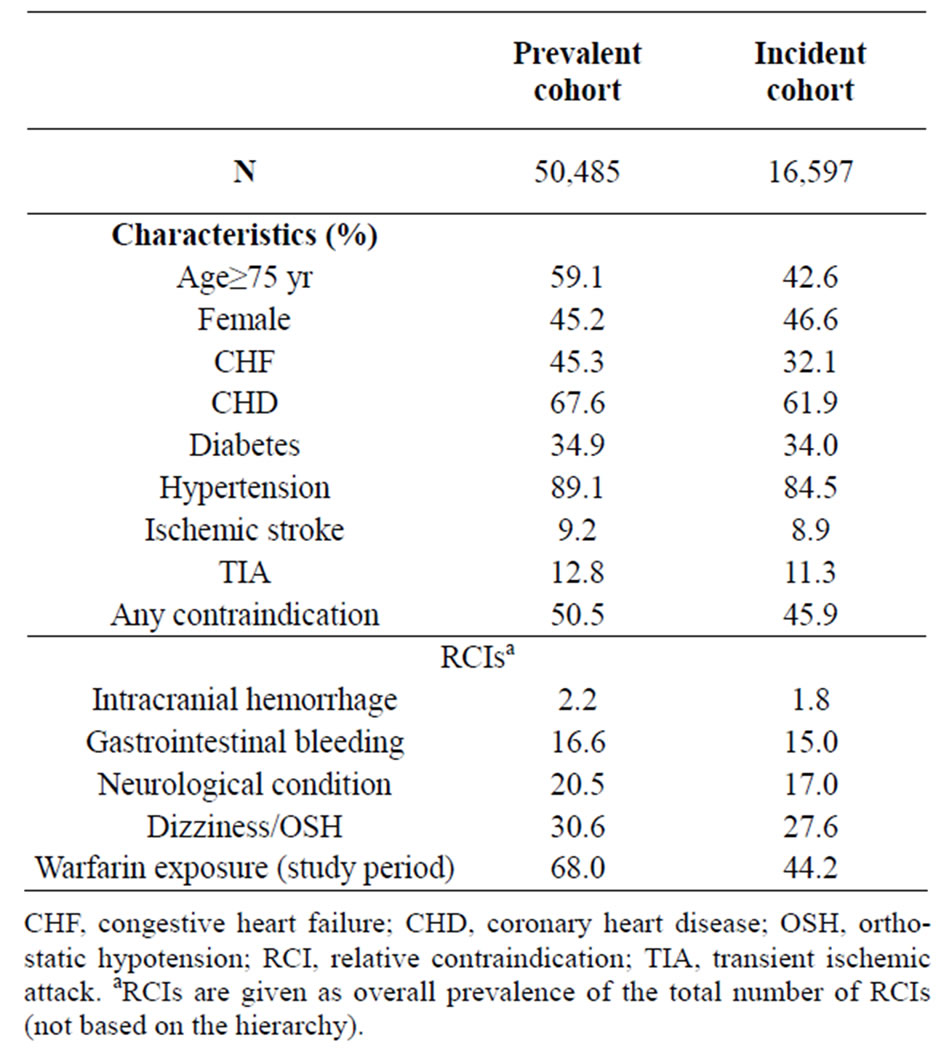
Table 1. Patient demographics for prevalent and incident cohorts.
60 days of one another. Events of interest occurring in any given month were attributed to warfarin use or nonuse in the immediately preceding month; this approach limited the observation of events of interest to 23 of the 24 months in the study period.
Outcome measures
Nonfatal hospitalization event rates of ischemic stroke, TIA, hemorrhagic stroke, and GIB were determined for both cohorts and within RCI patient groups over the study period. Hospitalization events were captured on a monthly basis so that multiple hospitalizations for the same event and the same patient within a 1-month time frame were counted only once. Outcome events were attributed to warfarin exposure if the patient met the definition for monthly warfarin use in the month prior to the event. Person-time over the study period was classified as either exposed or not exposed to warfarin based on monthly 1) ICH; 2) GIB or esophageal varices; 3) neurological disorders impairing gait or station, such as Parkinson’s disease or dementia; and 4) conditions predisposing to falls, including dizziness or orthostatic hypotension warfarin use. Primary and repeat events were measured and presented as rate per 100 person—years.
2.4. Statistical Analysis
Descriptive statistics were generated for demographics and clinical characteristics of the prevalent and incident cohorts. These included gender, age (≥75 years), history of CHF, CHD, diabetes mellitus, hypertension, ischemic stroke, or TIA, RCIs (ICH, GIB, prior neurological conditions without ICH and GIB with or without dizziness/ orthostatic hypotension [NEURO], or dizziness/orthostatic hypotension only [FDO]) to warfarin use, and the percentage of months in which there was warfarin exposure.
For each cardioembolic risk factor, the Cochran-Armitage trend test [19] was conducted to determine whether there was an association between the percentage of patients with the risk factor and the order of the RCI. Modified Ridit scores were used to assign values to each RCI. The statistical analyses were conducted with Proc FREQ of SAS® software.
Ischemic strokes, TIAs, hemorrhagic strokes, and GIB events while patients were either exposed or not exposed to warfarin were summarized as the number of events per 100 person–years of observation. For each event outcome, overall and by RCI category, a Poisson regression model [20,21] was constructed in which the covariates for each patient were gender, age ≥ 75 years, history of CHF, CHD, diabetes mellitus, ischemic stroke, or TIA, and RCI (ICH, GIB, NEURO, FDO, or none) to warfarin use. The model included, for each month of the study period, whether an event had occurred, whether the patient had used warfarin, and the indication of RCI and warfarin use. Generalized estimating equations were used to incorporate the correlation of warfarin usage results (spanning months) for each patient. Least square means, along with the associated 95% confidence intervals, were computed to obtain event rates per 100 person— years of observation while on warfarin or not on warfarin, adjusted for the specified covariates, for patients within each RCI cohort. Results of the preliminary analyses for each event outcome indicated no statistically significant impact of warfarin by RCI interaction; therefore, analyses were conducted without the interaction to obtain adjusted event rates across the RCI cohorts. These statistical analyses were conducted with Proc GENMOD of SAS® software, version 9.2 (SAS Institute, Inc., Cary, NC).
3. RESULTS
3.1. Demographics
A total of 67,082 patients were included in the study; 50,485 (75.3%) in the prevalent cohort and 16,597 (24.7%) in the incident cohort. Table 1 describes the demographics of both cohorts. The majority was male; in the prevalent cohort, 59% were ≥75 years old. Mean age (± SD) was 75.8 ± 10.0 years in the prevalent cohort. Hypertension was the most common cardioembolic risk factor in both cohorts (>80%). All other risk factors occurred more frequently in patients in the prevalent cohort compared with the incident cohort. Warfarin exposure during the 24-month study period was 68% and 44% in the prevalent and incident cohorts, respectively.
3.2. Relative Contraindications
At baseline, 50.5% of patients in the prevalent cohort had one or more identifiable RCIs. Dizziness/orthostatic hypotension was the most common, followed by neurological conditions, GIB or esophageal varices, and ICH (Table 2). There were no major differences in stroke rates and bleeding events in the incident cohort relative to rates found among prevalent patients (data not shown).
Table 2 shows the demographics of the prevalent cohort by RCIs and CHADS2 risk factors. A total of 2.2% of patients had a history of ICH, with or without other RCIs, and 16.1% had a history of GIB/varices; 17.0% had FDO as the only RCI. The prevalence of CHADS2 risk factors for stroke (congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, and prior stroke or TIA) was significantly higher in patients with an RCI, compared with those with no RCI (P < 0.0001). Patients with no RCI were younger, more often male, and had a lower prevalence of all cardioembolic risk factors compared with those with 1 or more RCIs (all trends were significant). There was a significant decreasing trend in the occurrence of CHADS2 risk factors across categories of decreasing severity of RCIs for all prognostic factors, except age and gender (P < 0.0001).
3.3. Effects of Warfarin in the Prevalent Cohort
Table 3 shows demographics of the prevalent cohort by RCI and warfarin use. Approximately two-thirds of patients in the prevalent cohort experienced some warfarin exposure during the 24-month study period (Table 1 and Figure 2). The proportion exposed to warfarin increased as the clinical severity of the RCI decreased: among patients with prior ICH, 58.0% were exposed to warfarin, whereas 69.7% of those with only FDO were exposed to warfarin, similar to those with no RCI (Figure 2). Across all RCIs, patients exposed to warfarin were more likely to be male, younger, and with a higher prevalence of cardioembolic risk factors (Table 3). As shown in
Figure 3, the adjusted rates of ischemic stroke and TIA were lower among those exposed to warfarin in the month prior to the event, relative to those not exposed to warfarin. Adjusted rates of GIB were higher in patients exposed to warfarin than in those not exposed. Hemorrhagic stroke was the least common event, and rates were higher in patients exposed to warfarin, compared with those who were not exposed (0.29% vs 0.18%, respectively).
3.4. Effects of Warfarin in RCI Patients
Table 4 shows the effects of warfarin on the rates of cardioembolic and bleeding events by RCI group (adjusted event rates per 100 patient—years). Rates of ischemic stroke and TIA among patients with RCI were numerically higher than those without RCI, irrespective of warfarin status. Hemorrhagic stroke rate was highest (P = NS) among patients with a history of ICH. Patients with ICH as an RCI who had received warfarin had lower rates of ischemic stroke than those who had not (1.4% vs 2.1%, respectively), while rates of hemorrhagic stroke (0.6% vs 0.5%, respectively) and GIB (1.0% for both groups) were similar in both groups (Table 4).
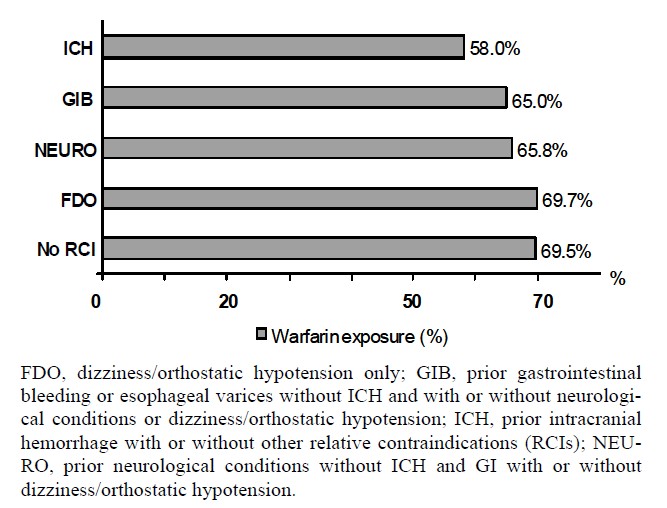
Figure 2. Use of warfarin in patients with relative contraindications.
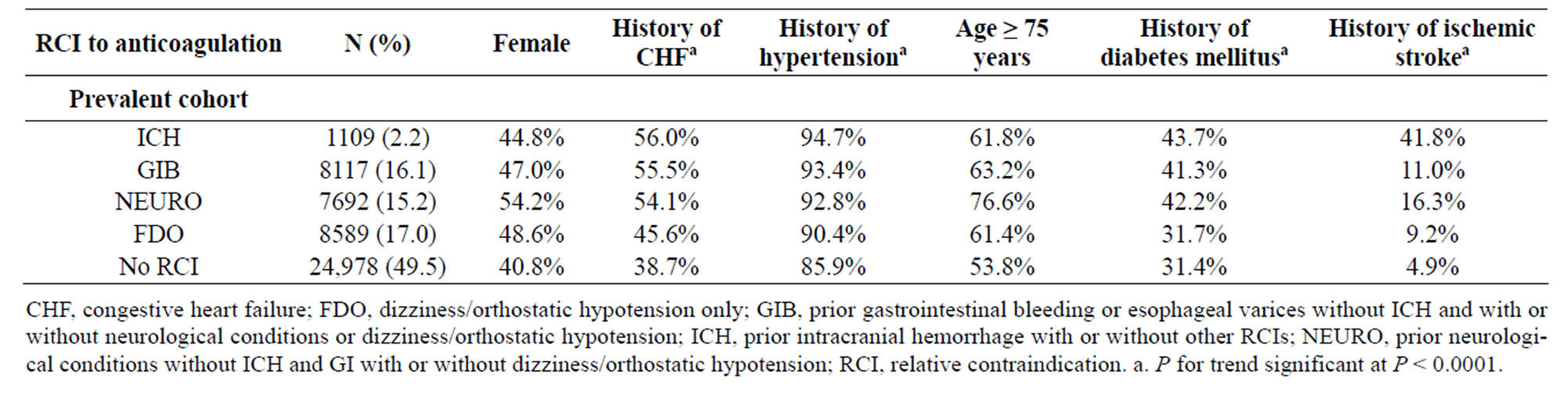
Table 2. Demographics in the prevalent cohort by CHADS2 risk factors and relative contraindications.
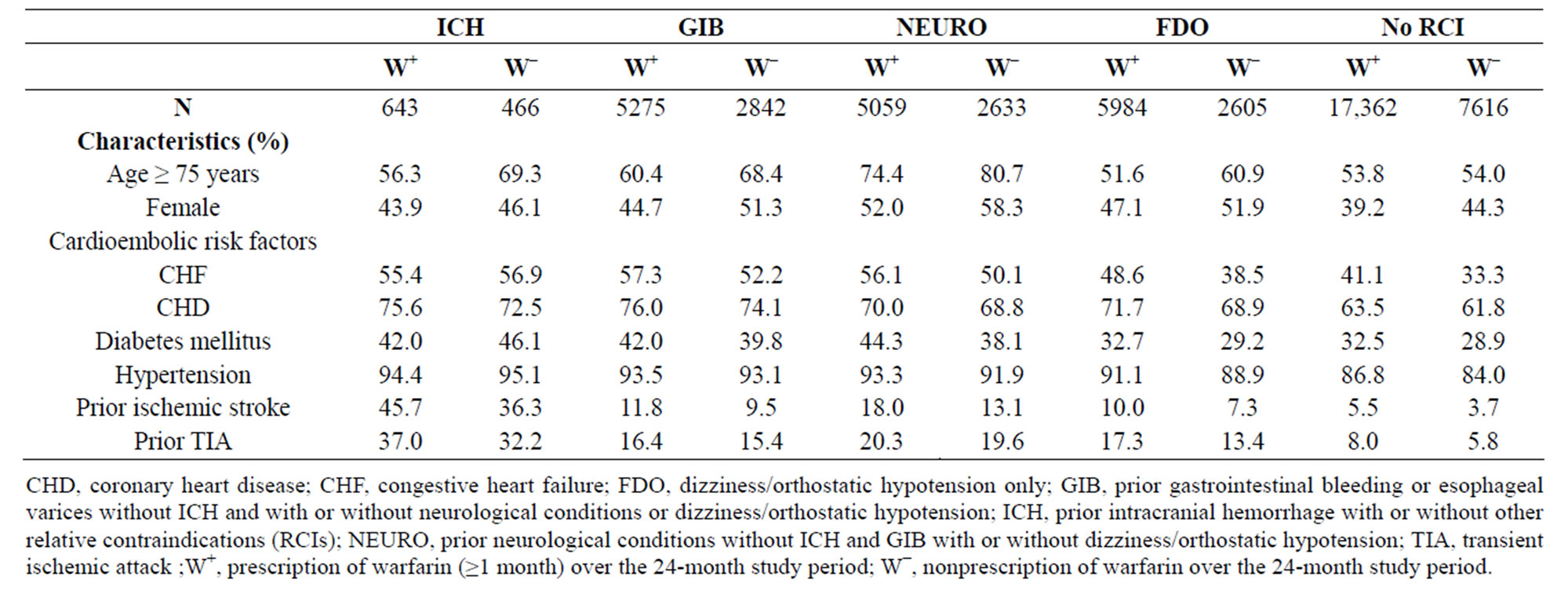
Table 3. Demographics in the prevalent cohort according to relative contraindications and warfarin use.
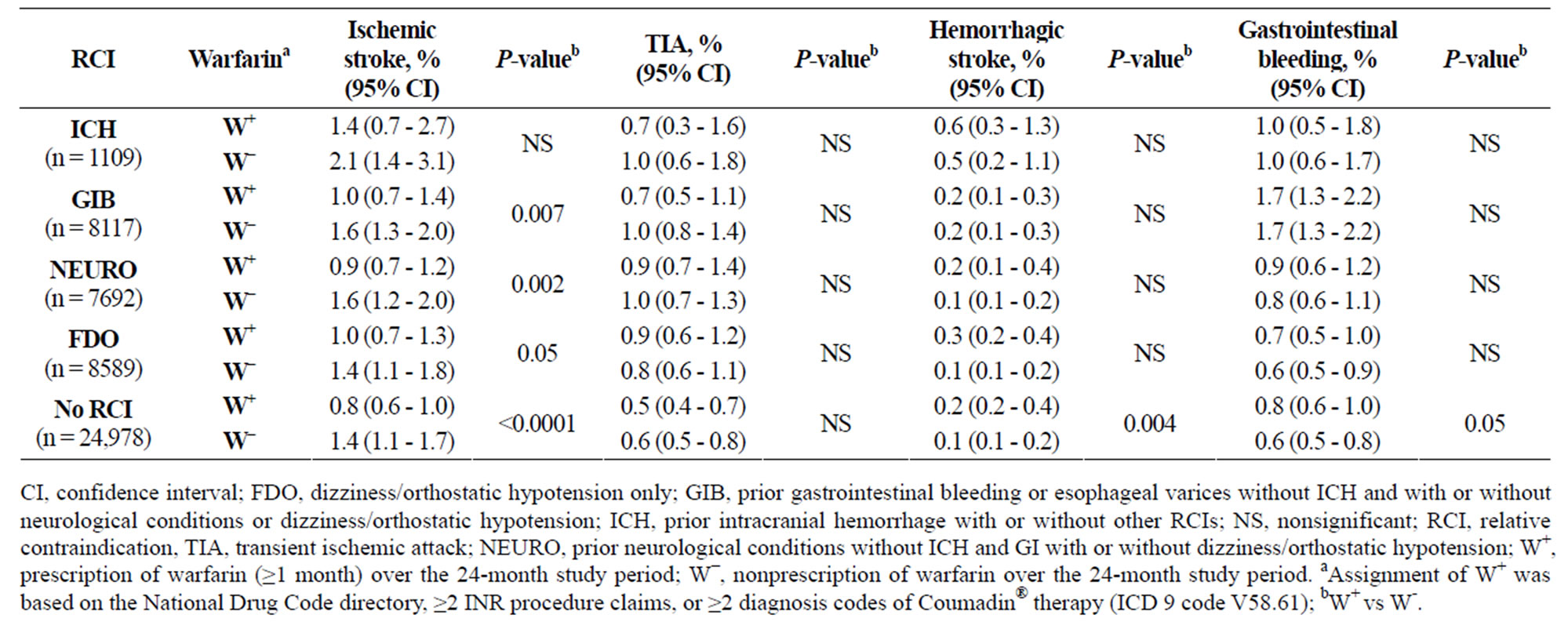
Table 4. Effect of warfarin on the rates of cardioembolic and bleeding events in the prevalent cohort (adjusted event rates).
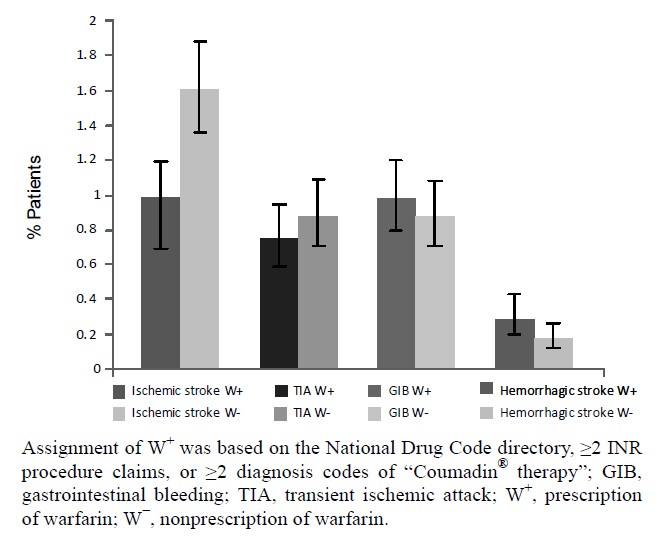
Figure 3. Effect of warfarin on the rates of cardioembolic events and bleeding by all sample rates.
Significantly fewer warfarin-receiving patients with either GIB or NEURO as an RCI had an ischemic stroke, compared with similar patients who had not received warfarin (Table 4). No significant differences in the incidence of GIB events based on warfarin exposure were observed in patients with or without an RCI. Hemorrhagic stroke occurred more frequently with warfarin exposure among those with FDO as their only RCI (P = NS) and among those with no RCIs (P < 0.004), compared with similar patients not exposed to warfarin (Table 4).
4. DISCUSSION
4.1. Findings
This analysis of a large commercial insurance claims database demonstrated that: 1) only 68% of the prevalent AF cohort received warfarin, similar to the results of other contemporary studies [22,23], 2) more than half of the patients who had prevalent AF also had 1 or more of 4 “relative contraindications” to warfarin, 3) prevalence of CHADS2 risk factors for stroke were significantly higher in these patients compared to the no-RCI group; rates of ischemic stroke and TIA were numerically higher among these patients compared to those without RCI, and 4) 58% - 70% of patients with specific RCIs received warfarin and had fewer strokes.
These results are consistent with contemporary studies showing that many eligible patients do not receive anticoagulants and that many “ineligible” patients are prescribed prophylaxis [22,23]. Similar findings were reported in a study of Medicare patients with AF conducted from 1992-2002; 40% of eligible patients (without bleeding or fall risk factors) were not prescribed warfarin, whereas 49% of those with risk factors for bleeding/falls did receive prescriptions [11].
This analysis focused on the prevalent cohort, with 24 months of complete patient follow-up. Compared with findings of a claims study in younger patients (36% of patients ≥75 years of age vs 55% in the present study), cardioembolic risk factors were more common in our prevalent cohort [9]. CHADS2 risk factors were more common in patients with RCIs than in those without. Patients with RCIs were often managed with warfarin, and those with ICH, GIB, and NEURO who were exposed to warfarin had fewer ischemic strokes compared with similar patients not exposed to warfarin. The rate of hemorrhagic stroke in the FDO group exposed to warfarin was not significantly higher than those not exposed to warfarin. Warfarin exposure was associated with significantly higher rates of hemorrhagic stroke and GIB in the no-RCI group. Two factors might contribute to these observations. First, some physicians are undoubtedly quite skillful at managing anticoagulant prophylaxis in patients with RCIs. Second, medical management of hypertension and acid-peptic disease have improved dramatically since the introduction of warfarin in clinical practice more than 60 years ago [24]. Agents such as angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, β-blockers, H2-blockers, and protonpump inhibitors, which substantially reduce the complications of hypertension and GI disease, likely mitigate these relative contraindications to anticoagulant prophylaxis.
The data in this study have important implications for medical practice, particularly in light of the expected introduction of new agents for stroke prophylaxis in AF [25]. As entry criteria for recent trials indicate [26,27], active bleeding remains a contraindication to anticoagulation. However, observational evidence suggests that the benefit/risk aspects of anticoagulation for stroke prophylaxis may be favorable for many patients who have RCIs to warfarin. Clinicians managing AF patients should weigh an individual’s cardioembolic risk against the specific contraindications for anticoagulant prophylaxis based on up-to-date information.
4.2. Limitations
This study had important limitations, some of which are inherent in using commercial insurance claims data. The investigators could not assess mortality; fatal strokes or fatal bleeding, and other deaths possibly associated with AF, such as death from heart failure, could not be evaluated. One-year mortality is high (~35% - 55%) after cardioembolic strokes associated with AF [28,29]; however, the absolute number of fatal strokes was probably not high enough to meaningfully impact the data in these subgroups. Although patients over 65 years of age with private insurance (with or without Medicare) were represented in the data set, data concerning services not billed to the private insurer (such as Veterans Administration health services) were not available. Because the investigators had no access to clinical records, they were required to define RCIs in operational terms that could be observed in a claims database. The risk associated with a tendency to fall is controversial, but clinicians often cite unstable gait as a reason not to prescribe anticoagulation. Because only the occurrence/nonoccurrence of a cardioembolic or bleeding event within a month was recorded, rather than the number of such events per patient in a given month, rates of events that could occur more than once a month may be underestimated in this analysis. The investigators did not have access to laboratory data, and evidence of warfarin use is a less satisfactory indicator of effective stroke prevention in patients with AF than time-in-therapeutic range. Nonetheless, there was a meaningful reduction in ischemic stroke rates in patients receiving prescriptions for warfarin. The study investigators did not have access to clinical data that might have differentiated subsets of ICH into hemorrhagic transformation of ischemic stroke vs primary ICH. Finally, although data were available on prescribed concomitant therapies, no data were available on aspirin use, which could be a confounding factor.
5. CONCLUSIONS
The presence of RCIs probably does not account for underutilization of anticoagulant stroke prophylaxis in patients with AF. Results of study data underscore the importance of emphasizing recognition and management of comorbid conditions in therapeutic guidelines. Improved therapy for comorbidities may have substantially reduced the complications of anticoagulant prophylaxis. The advent of new oral agents will offer the opportunity to test this hypothesis with data from rigorous randomized controlled trials.
6. ACKNOWLEDGEMENTS
All listed authors had full access to the data and collaborated in writing this manuscript. The authors wish to thank Michael A. Craig. MSc, and Ruth Sussman, PhD, for editorial assistance with funding from Janssen Scientific Affairs, LLC.
REFERENCES
- Stroke Risk in Atrial Fibrillation Working Group (2008) Comparison of 12 risk stratification schemes to predict stroke in patients with nonvalvular atrial fibrillation. Stroke, 39, 1901-1910. doi:10.1161/STROKEAHA.107.501825
- Lip, G.Y., Nieuwlaat, R., Pisters, R. , Lane, D.A. and Crijns, H.J. (2010) Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on atrial fibrillation. Chest, 137, 263-272. doi:10.1378/chest.09-1584
- Gage, B.F., Waterman, A.D., Shannon, W., Boechler, M., Rich, M.W. and Radford, M.J. (2001) Validation of clinical classification schemes for predicting stroke: Results from the National Registry of Atrial Fibrillation. Journal of the American Medical Association, 285, 2864-2870. doi:10.1001/jama.285.22.2864
- Coumadin. Bristol-Myers Squibb, Princeton, 2010. http://packageinserts.bms.com/pi/pi_coumadin.pdf
- Aspinall, S.L., DeSanzo, B.E., Trilli, L.E. and Good, C.B. (2005) Bleeding risk index in an anticoagulation clinic. Assessment by indication and implications for care. Journal of General Internal Medicine, 20, 1008-1013. doi:10.1111/j.1525-1497.2005.0229.x
- Gage, B.F., Yan, Y., Milligan, P.E., et al. (2006) Clinical classification schemes for predicting hemorrhage: Results from the National Registry of Atrial Fibrillation (NRAF). American Heart Journal, 151, 713-719. doi:10.1016/j.ahj.2005.04.017
- Go, A.S., Hylek, E.M., Phillips, K.A., Chang, Y., Henault, L.E., Selby, J.V., et al. (2001) Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: The AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. Journal of the American Medical Association, 285, 2370-2375. doi:10.1001/jama.285.18.2370
- Lin, P. (2005) Reviewing the reality: Why we need to change. European Heart Journal, 7, E15-E20.
- Walker, A.M. and Bennett, D. (2008) Epidemiology and outcomes in patients with atrial fibrillation in the United States. Heart Rhythm, 5, 1365-1372. doi:10.1016/j.hrthm.2008.07.014
- Singer, D.E., Albers, G.W., Dalen, J.E., et al. (2008) Antithrombotic therapy in atrial fibrillation: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition). Chest, 133, 546S-592S. doi:10.1378/chest.08-0678
- Lakshminarayan, K., Solid, C.A., Collins, A.J., Anderson, D.C. and Herzog, C.A. (2006) Atrial fibrillation and stroke in the general Medicare population: A 10-year perspective (1992 to 2002). Stroke, 37, 1969-1974. doi:10.1161/01.STR.0000230607.07928.17
- Waldo, A.L., Becker, R.C., Tapson, V.F., Colgan, K.J. (2005) Hospitalized patients with atrial fibrillation and a high risk of stroke are not being provided with adequate anticoagulation. Journal of the American College of Cardiology, 46, 1729-1736. doi:10.1016/j.jacc.2005.06.077
- Deplanque, D., Leys, D., Parnetti, L., et al. (2004) Stroke prevention and atrial fibrillation: reasons leading to an inappropriate management. Main results of the SAFE II study. British Journal of Clinical Pharmacology, 57, 798-806. doi:10.1111/j.1365-2125.2004.02086.x
- Lip, G.Y. and Tse, H.F. (2007) Management of atrial fibrillation. Lancet, 370, 604-618. doi:10.1016/S0140-6736(07)61300-2
- Centers for Disease Control and Prevention (2011) Classification of Diseases, Functioning, and Disability. http://www.cdc.gov/nchs/icd.htm
- American Medical Association (2009) CPT 2010 Standard Edition (Current Procedural Terminology (CPT) Standard). American Medical Association Press, Chicago.
- Hart, R.G., Pearce, L.A. and Aguilar, M.I. (2007) Metaanalysis: Antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Annals of Internal Medicine, 146, 857-867.
- Go, A.S., Hylek, E.M., Borowsky, L.H., Phillips, K.A., Selby, J.V. and Singer, D.E. (1999) Warfarin use among ambulatory patients with nonvalvular atrial fibrillation: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study. Annals of Internal Medicine, 131, 927-934.
- Fleiss, J., Levin, B. and Paik, M. (2003) Statistical methods for rates and proportions. 3rd Edition, John Wiley & Sons Inc., Hoboken. doi:10.1002/0471445428
- Koch, G., Atkinson, S. and Stokes, M. (1986) Poisson regression. In: Johnson, N.L. and Kotz, S., Eds. Encyclopedia of Statistical Sciences. John Wiley & Sons, Inc., Hoboken, 32-41.
- Stokes, M., Davis, C., Koch, G. (2009) Categorical data analysis using the SAS system. 2nd Edition, SAS Institute, Carey.
- Kowey, P.R., Reiffel, J.A., Myerburg, R., et al. (2010) Warfarin and aspirin use in atrial fibrillation among practicing cardiologist (from the AFFECTS Registry). American Journal of Cardiology, 105, 1130-1134. doi:10.1016/j.amjcard.2009.11.047
- Nieuwlaat, R., Capucci, A., Lip, G.Y., et al. (2006) Antithrombotic treatment in real-life atrial fibrillation patients: a report from the Euro Heart Survey on Atrial Fibrillation. European Heart Journal, 24, 3018-3026. doi:10.1093/eurheartj/ehl015
- Ansell, J., Hirsh, J., Hylek, E., Jacobson, A., Crowther, M. and Palareti, G. (2008) Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition). Chest, 133, 160S-198S. doi:10.1378/chest.08-0670
- Weitz, J.I., Hirsh, J. and Samama, M.M. (2008) New antithrombotic drugs: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition). Chest, 133, 234S-256S. doi:10.1378/chest.08-0673
- Connolly, S.J., Ezekowitz, M.D., Yusuf, S., et al. (2009) Dabigatran versus warfarin in patients with atrial fibrillation. New England Journal of Medicine, 361, 1139- 1151. doi:10.1056/NEJMoa0905561
- Patel M.R. (2010) Rivaroxaban-once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation: Rationale and design of the ROCKET AF study. American Heart Journal, 159, 340-347. doi:10.1016/j.ahj.2009.11.025
- Kolominsky-Rabas, P.L., Weber, M., Gefeller, O., Neundoerfer, B. and Heuschmann, P.U. (2001) Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long-term survival in ischemic stroke subtypes: A population-based study. Stroke, 32, 2735-2740. doi:10.1161/hs1201.100209
- Petty, G.W., Brown, R.D., Jr., Whisnant, J.P., Sicks, J.D., O’Fallon, W.M. and Wiebers, D.O. (2000) Ischemic stroke subtypes: A population-based study of functional outcome, survival, and recurrence. Stroke, 31, 1062-1068. doi:10.1161/01.STR.31.5.1062
APPENDIX
Table 1. CD-9 Diagnosis codes.
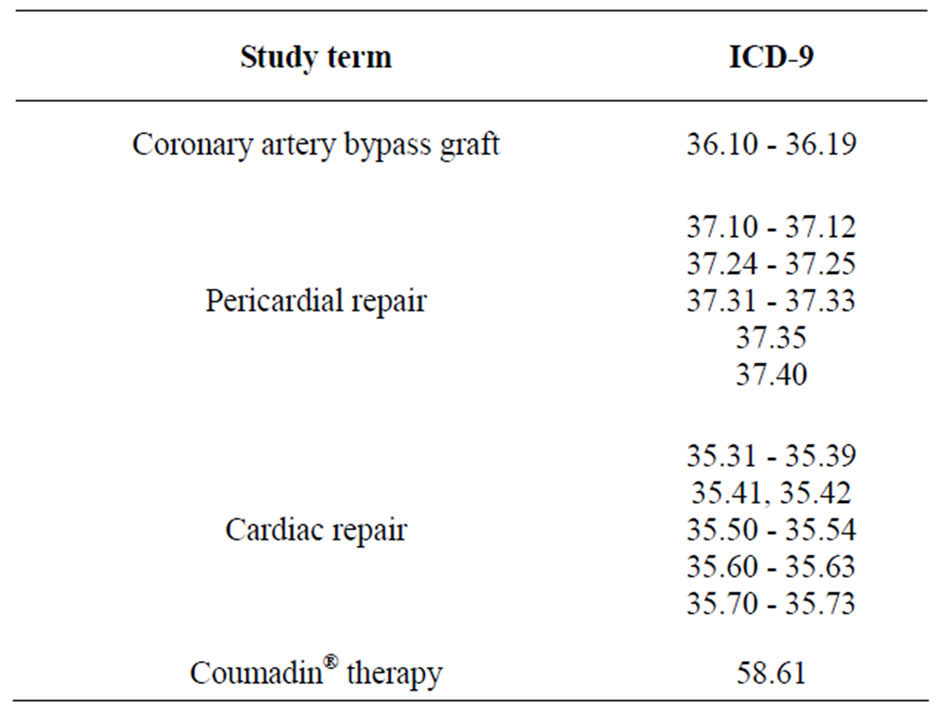
Table 2. ICD-9 procedure codes.

