Advances in Entomology
Vol.2 No.1(2014), Article ID:41792,6 pages DOI:10.4236/ae.2014.21003
Antifeedant and insecticidal activities of selected plant extracts against Epilachna beetle, Henosepilachna vigintioctopunctata (Coleoptera: Coccinellidae)
1PG & Research Department of Zoology, Arignar Anna Government Arts College, Musiri, India
2PG & Research Department of Botany, Arignar Anna Government Arts College, Musiri, India
3Department of Advanced Zoology and Biotechnology, Government Arts College (Autonomous), Chennai, India; *Corresponding Author: professorelumalai@gmail.com
Copyright © 2014 Alagarmalai Jeyasankar et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In accordance of the Creative Commons Attribution License all Copyrights © 2014 are reserved for SCIRP and the owner of the intellectual property Alagarmalai Jeyasankar et al. All Copyright © 2014 are guarded by law and by SCIRP as a guardian.
Received 15 August 2013; revised 20 September 2013; accepted 2 October 2013
KEYWORDS
Antifeedant; Insecticidal; Henosepilachna vigintioctopunctata; Plant Extracts; Achyranthes aspera
ABSTRACT
Six different indigenous plants were screened for antifeedant and insecticidal activity against fourth instar larvae of Epilachna beetle, Henosepilachna vigintioctopunctata, which is a severe pest on brinjal. Among the plants screened, Achyranthes aspera showed higher activity against the selected pest. Ethyl acetate extracts of A. aspera showed higher antifeedant index and insecticidal activity against fourth instar larvae of H. vigintioctopunctata. Preliminary phytochemical analysis revealed that the presence of alkaloid and quinines in the ethyl acetate extracts indicate higher percentage of activities. Hence, it may suggest its use for controlling the vegetables insect pest, H. vigintioctopunctata.
1. INTRODUCTION
Applications of chemical pesticides minimize the threat from pest manifestation by rapid knock-down effect, albeit with little consideration to the quality (nutritional contents) of the crop and agro-residues. Many workers reported that the indiscriminate use of chemical pesticide over a long period has not only been proved to be harmful to soil microflora, animals and human life, but also contributed to a number of side effects, viz. development of resistance by the insects/weeds/pests resurgence and outbreak of new pests, toxicity to non-target organism, presence of non permissible level of pesticide residues on seeds, vegetables, fruits, border alteration in dynamics of pest species population, cumulatively causing poor soil fertility and hazardous effects on environment endangering the sustainability of ecosystem [1,2]. Due to higher dose and repeated frequency of application, every year one million people suffer from pesticide poisoning [3]. The use of botanical pesticides for protecting crops from insect pests has assumed greater importance all over the world due to growing awareness of harmful effects of indiscriminate use of synthetic pesticides.
Vegetables play a vital role in providing essential protective nutrients like vitamins and minerals and are used as selective diets by everybody. Brinjal or egg plant (Solanum melongena Linn.) is one of the most important vegetable crops grown all over India. Brinjal is heavily infested by a number of insects/pests among Henosepilachna vigintioctopunctata (Coleoptera: Coccinellidae), which is also one of the most destructive pests extensively found all over India and in other countries [4-6]. It is a polyphagous pest which shows its presence on brinjal and other economically important solanaceous and cucurbitaceous crops. Henosepilachna vigintioctopunctata (F.) (Coleoptera: Coccinellidae) is one among the most injurious pests of solanaceous crops in India, especially Solanum melongena and S. tuberosum.
Henosepilachna beetle causes considerable economic losses to many crops including brinjal depending on place and season for variations of prevailing environmental conditions [7-9]. It is highly destructive at both, adult and larval stages which feed on the epidermal tissues of leaves, flowers, and fruits by scrapping the chlorophyll content and cause a big yield loss [10-12]. It is fairly common throughout the country and causes considerable damage to a number of solanaceous, cucurbitaceous and leguminous crops. The neonate larva initially attacks the foliage of the plants and the later stage feeds whole leaf by scraping. This is the serious pest of various economically important vegetable crops and also the developed resistance in almost all commercially available chemical pesticides. In the present investigation, screening and evaluation of various solvent plants’ crude extracts tested against Epilachna beetle.
2. MATERIALS AND METHODS
2.1. Collection and Extraction of Plant Material
Different plants species were collected from in and around the Trichy District, Tamil Nadu, India. The collected plant species were identified by Fr. John Brido, Rabinat Herbarium, St. Jopesh’s College, Trichy, Tamilnadu, India. Voucher specimens were deposited at Department of Zoology, Arignar Anna Govt. Arts College, Musiri, Trichydt, Tamilnadu.
The plant materials were thoroughly washed with tap water and shade dried under room temperature (27.0˚C ± 2˚C) at Department of Zoology, Government Arts College, Musiri. After complete drying the plant materials were powdered using electric blender and sieved through kitchen strainer. 1000 g of plant powder was extracted with hexane, diethyl ether and ethyl acetate, sequentially with increasing polarity of solvents and filtered through Whatman’s No. 1 filter paper. The solvents from the crude extract were evaporated to air dryness at room temperature. The crude extracts were collected in clean borosil vials and stored in the refrigerator at 4˚C for subsequent bioassay against Henosepilachna vigintioctopunctata.
2.2. Rearing of Epilachna Beetle, Henosepilachna vigintioctopunctata
The larvae were collected from Brinjal vegetables field at Anaipatti, Musiri, Trichy district, Tamilnadu. Larvae were reared in laboratory condition at the Department of Zoology, Government Arts College, Musiri, Tamilnadu, India. These laboratory-reared larvae were used for bioassays and the cultures were maintained throughout the study period.
2.3. Antifeedant Activity
Antifeedant activity of crude extracts was studied using leaf disc with no choice method [13]. The stock concentration of crude extracts (5%) was prepared by dissolving in acetone and mixing with dechlorinated water. Polysorbate 20 (Tween 20) at 0.05% was used as emulsifier [14]. Fresh brinjal leaf discs of 3-cm diameter were punched using cork borer and dipped with 0.625%, 1.25%, 2.50% and 5.0% concentrations of crude extracts, individually. Leaf discs treated with acetone and without solvent (water) were considered as control. After airdrying, each leaf disc was placed in petridish (1.5 cm × 9 cm) containing wet filter paper to avoid early drying of the leaf disc and a single 2 hrs pre-starved, H. vigintioctopunctata was introduced. For each concentration five replicates were maintained. Progressive consumption of leaf area by the larva after 24 hrs feeding was recorded in control and treated discs using graph sheet method. Leaf area consumed in plant extract treatment was corrected from the control. The percentage of antifeedant index was calculated using the formula of [15].
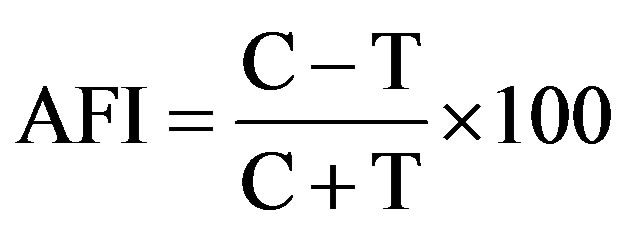
where AFI = Antifeedant Index;
C = Area protected in control leaf disc;
T = Area protected in treated leaf disc.
2.4. Insecticidal Activity
Fresh castor leaves were treated with different concentrations (as mentioned in antifeedant activity) of crude extracts. Castor leaves treated with acetone and without solvent were considered as control. Petioles of the potato leaves were tied with wet cotton plug (to avoid early drying) and placed in round plastic trough (29 cm × 8 cm). In each concentration 10 pre-starved (2 hrs) H. vigintioctopunctata were introduced individually and covered with muslin cloth. Five replicates were maintained for all concentrations and the number of dead larvae was recorded after 24 hrs up to pupation. Percentage of larval mortality was calculated and corrected by Abbott’s formula [16].
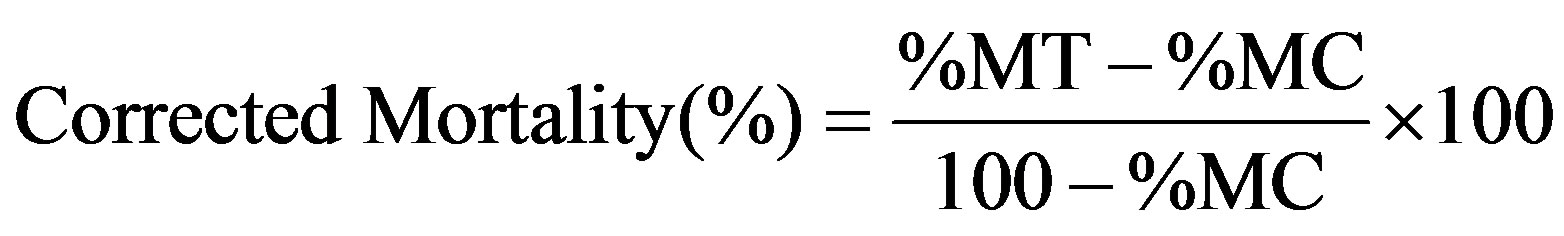
where %MT = % larval mortality in treatment;
%MC = % larval mortality in control.
2.5. Statistic Analysis
Data analysis was carried out using Microsoft Excel 2007. Two-Way ANOVA was performed for all the experimental data from that Least Significant Difference was calculated and the significant differences were marked with different alphabet.
3. RESULT AND DISCUSSION
Botanicals are a rich source of organic chemicals on earth. Already 10,000 secondary metabolites have been chemically identified. In nature many plants have unpalatable substances like high content of phenols, alkaloids, flavanoids, terpenes, quinone, coumarin etc., which play a defensive role against particularly agriculture insect pests. Identifying sources with useful biological activity is only the starting point in the long process of development of a botanical pest management product. Success of botanical in the field depends on number of factors such as, ongoing availability of the natural resources, adequate biomass to justify extraction, the feasibility of extraction near the harvest site and the stability of the extract in storage after preparation [17].
Different indigenous plants were collected and screened for biological activity against Epilachna beetle. Among the plants screened, Achyranthes aspera showed promising results (Table 1). Solvent crude extracts prepared from Achyranthes aspera using solvents of hexane, chloroform and ethyl acetate and their bioactivities were tested at different concentrations against Henosepilachna vigintioctopunctata.
3.1. Antifeedant Activity of Crude Extracts
Antifeedant activity of the crude extracts of seeds of A. aspera was studied at different concentrations and the results are presented in Table 2. Antifeedant activity of solvent extracts was assessed based on antifeedant index. Higher antifeedant index normally indicates decreased rate of feeding. In the present study irrespective of concentration and solvents used for extraction the antifeedant activity varied significantly. The antifeedant activity of the extract of leaves and seeds were tested at different concentrations. Data pertaining to the above experiment
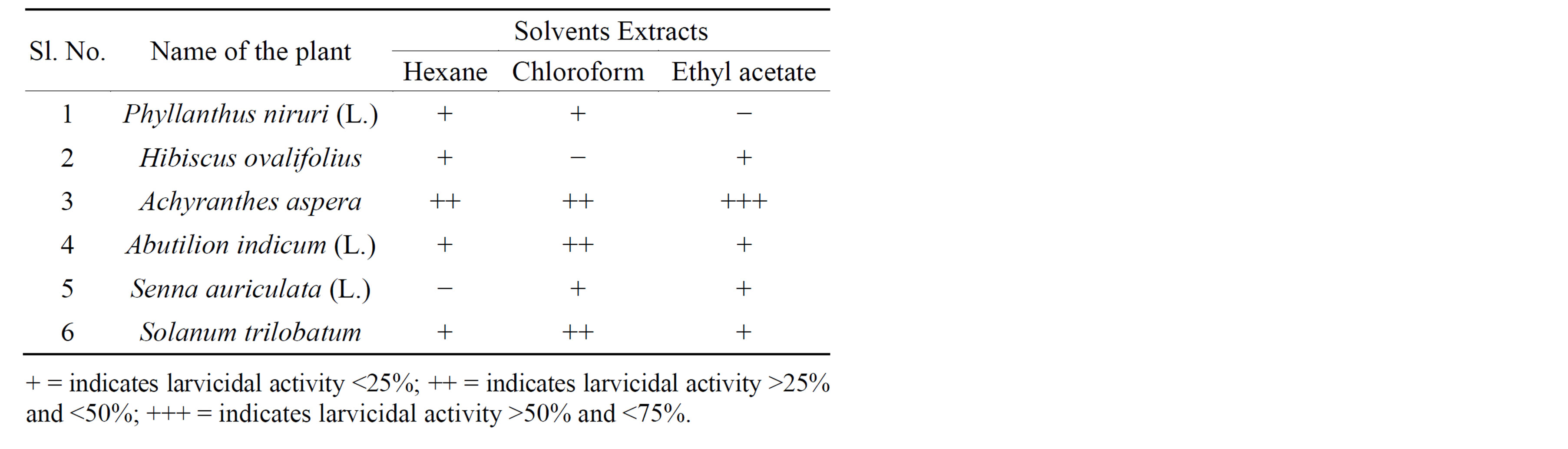
Table 1. Screening of indigenous plants for insecticidal activity against Henosepilachna vigintioctopunctata.
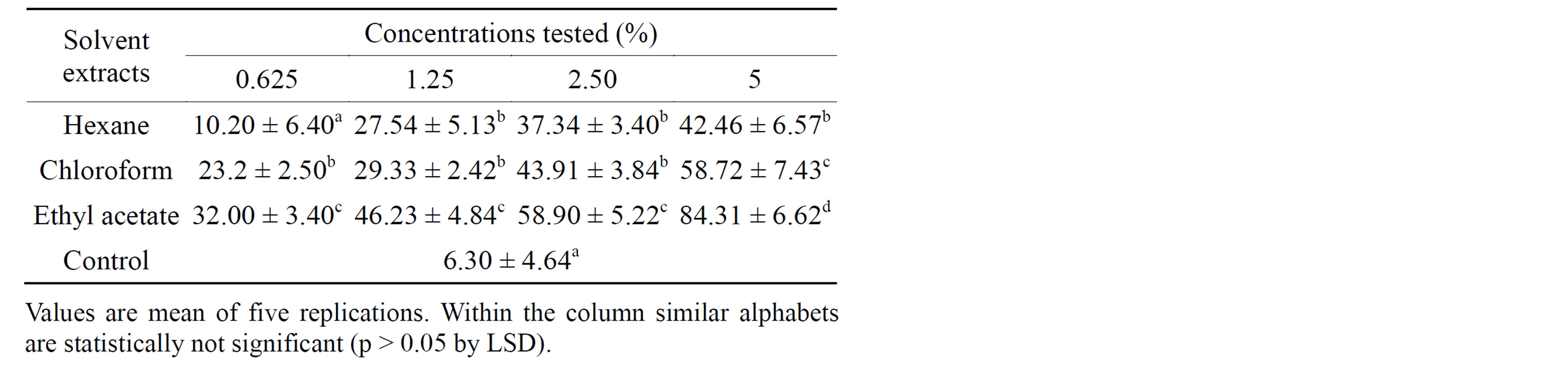
Table 2. Antifeedant activity of crude extracts of Achyranthes aspera against fourth instar larvae of H. vigintioctopunctata.
clearly revealed that maximum antifeedant activity was recorded in ethyl acetate extract (84.31%) and at 5% concentration compared to control. One-way analysis of variance (ANOVA) followed by least significant difference (LSD) test showed statistical significance (p < 0.05) compared to control.
Antifeedant is defined as a chemical that inhibits feeding without killing the insect directly, while the insect remains near the treated foliage and dies through starvation. Most potent insect antifeedants are quinoline, indole alkaloids, sesquiterpene lactones, diterpinoids, and triterpinoids [18]. The present study, ethyl acetate extract of A. aspera was promising in reducing feeding rate of H. vigintioctopunctata. The rate of feeding significantly varied depending on the concentration of the plant extracts. This indicates that the active principles present in the plants inhibit larval feeding behaviour or make the food unpalatable or the substances directly act on the chemosensilla of the larva resulting in feeding deterrence. Several authors have reported that plant extracts possess similar type of antifeedant activity against lepidopteran pests [19-24].
Antifeedant chemicals play a major role in the unsuitability of non-host plants as food for insects. Isolation and structure elucidation of these chemicals is important not only for understanding the ecological aspects of insect pests relationship, but also for their potential in insect pests control [25]. In the present study, preliminary phytochemical analysis revealed that triterpenoids, flovonoids, alkaloids and quinines present in the ethyl acetate extracts indicate that higher percentage of antifeedant activity. These findings are in agreement with the earlier reports of [26]. They have reported that quinone, remirol and cyperquinone isolated from the plants of the family cyperaceae had strong antifeedant activity against S. litura.
3.2. Insecticidal Activity of Crude Extracts
Insecticidal activity of crude extracts of A. aspera was studied at different concentrations and the results are presented in Table 3. Insecticidal activity of solvent extracts was calculated based on larval mortality after
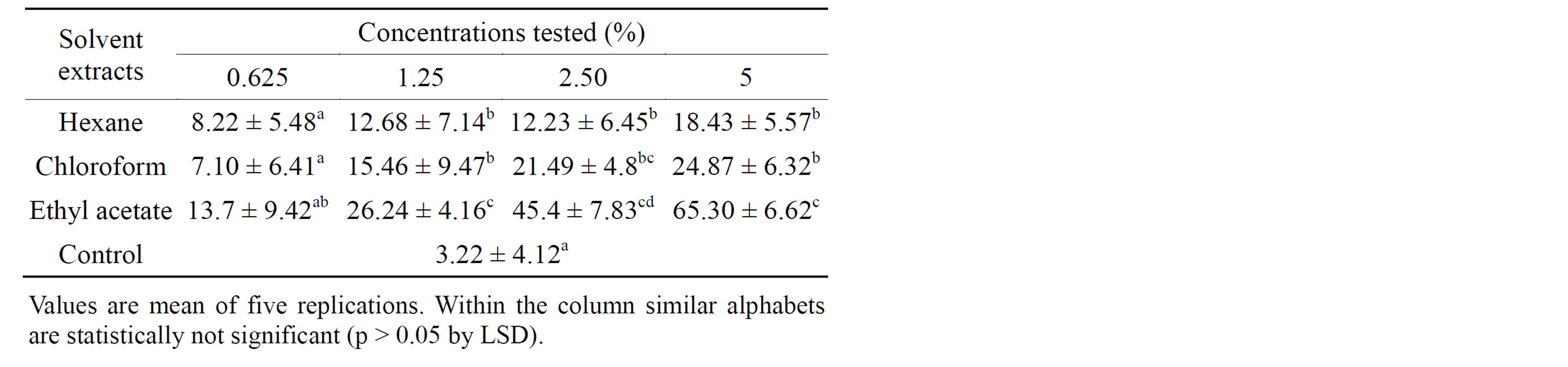
Table 3. Insecticidal activity of crude extracts of Achyranthes aspera against Epilachna beetle H. vigintioctopunctata.
treatment. High larval mortality normally indicates potential insecticidal activity of plant extracts. In the present study irrespective of concentration and solvents used for extraction the insecticidal activity varied significantly. Data pertaining to the insecticidal activity clearly revealed that maximum insecticidal activity was recorded in ethyl acetate extract (65.30%) at 5% concentrations compared to control. One-way analysis of variance (ANOVA) followed by least significant difference (LSD) test showed statistical significance (p < 0.0) compared to control.
Screening plant extracts for deleterious effects on insects is one of the approaches used in the search for novel botanical insecticides [27]. Secondary plant compounds act as insecticides by poisoning per se or by production of toxic molecules after ingestion. These compounds also deter or possibly repel an insect from feeding [28]. In the present study ethyl acetate extract from A. aspera exhibited significant insecticidal activity at 5% concentration. It is possible that the insecticidal property present in the selected plant compound may arrest the various metabolic activities.
In the present study preliminary phytochemical analysis revealed that alkaloid and quinines (Table 4) present in the ethyl acetate extract indicate that higher percentage of insecticidal activity observed in leaves extract of A. aspera. Similar works have already reported insecticidal activity of many plants and their compounds against different groups of insects [29-36].
4. CONCLUSION
In conclusion, ethyl acetate extract of seeds of A. aspera showed higher insecticidal and growing inhibition activities against H. vigintioctopunctata. Hence, it may be suggested that the leaves extracts of A. aspera can be used for controlling the insect pest, H. vigintioctopunctata.
ACKNOWLEDGEMENTS
The authors are thankful to Prof. K. Sundararasu, HOD (PG & Research Department of Zoology), Arignar Anna Govt. Arts College, Musiri, Tamil Nadu, India, for their support and suggestions. This work
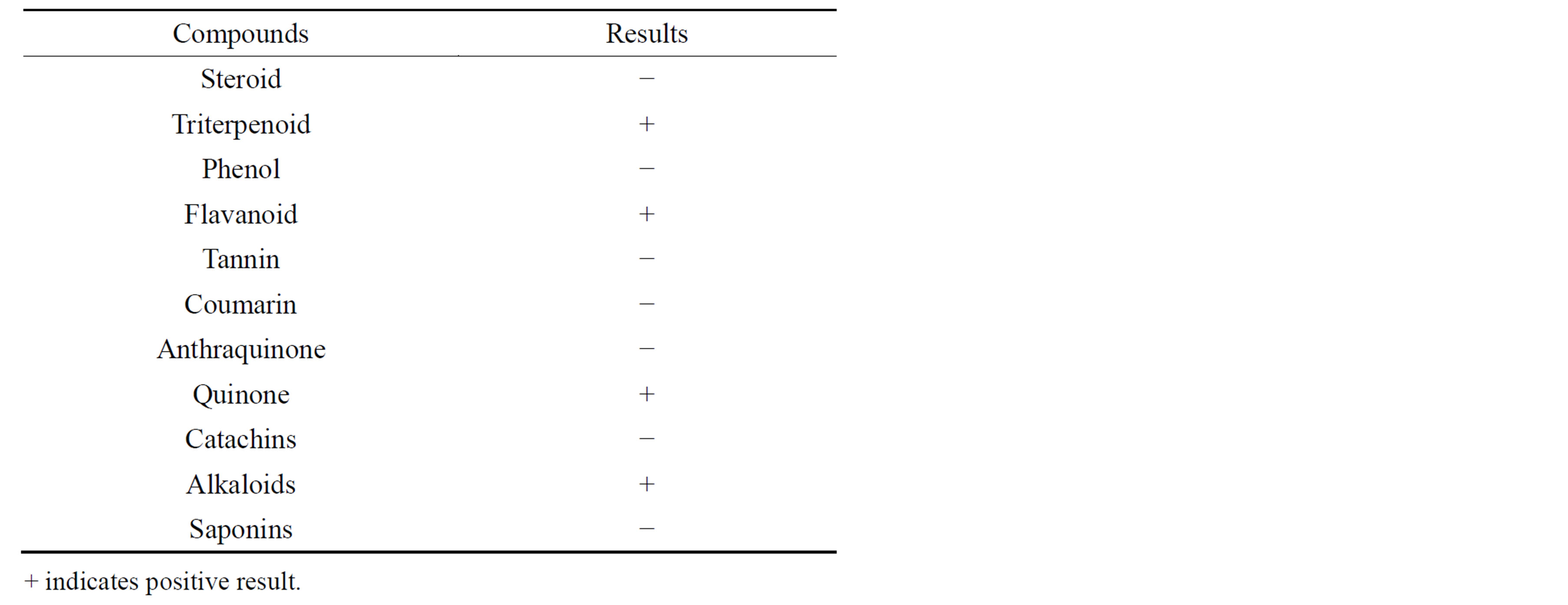
Table 4. Preliminary qualitative phytochemical analysis of ethyl acetate extracts Achyranthes aspera.
was conducted in the laboratory which is financially supported by University Grant Commission (Ref. No. 42-570/2013 SR).
COMPETING INTERESTS
The authors of this work have no competing interests.
REFERENCES
- Katyal, T. and Satake, M. (1996) Environmental pollution. In: Rajkumar, Ed., Introduction to Environmental Pollution, Anamol Publications, New Delhi, 1-6.
- Kannaiyan, S. (2002) Insect pest management strategies: current trends and future prospectus In: Ignacimuthu, S. and Jeyaraj, S., Eds., Strategies in Integrated Pest Management, Phoenix Publishing House, New Delhi, 1-13.
- Bami, H.L. (1997) Pesticide use in India-Ten questions. Chemical Week, 4, 7-10.
- Anam, M., Ahmed, M. and Haque, M.A. (2006) Efficacy of neem oil on the biology and food consumption of epi laichna beetle, Epilachna dodecastigma (Wied.). Journal of Agriculture and Rural Development, 4, 132-136.
- Rahaman M.A, Prodhan M.D.H and Maula A.K.M. (2008) Effect of botanical and synthetic pesticides in controlling Epilachna beetle and the yield of bitter gourd. International Journal of Sustainable Crop Production, 3, 23-26.
- Sharma, A. and Saxena, R. (2012) Bioactivity of some indigenous plants for the control of hadda beetle, Henosepilachna vigintioctopunctata infesting brinjal. Journal of Biopesticides, 5, 100-106.
- Rajagopal, D. and Trivedi, T.P. (1989) Status, biology and management of epilachna beetle, Epilachna vigintioctopunctata Fab. (Coleoptera: Coccinellidae) on potato in India. Tropical Pest Management, 35, 410-413. http://dx.doi.org/10.1080/09670878909371418
- Bhagat, K.C. and Munshi, S.K. (2004) Host preference of spotted leaf eating beetle, Henosepilachna vigintioctopunctata (Fabr.) on different brinjal varieties. Pest Management and Economic Zoology, 12, 77-81.
- Islam, K., Islam, S. and Ferdousi, Z. (2011) Control of Epilachna vigintioctopunctata Fab. (Coleoptera: Coccinellidae) using some indigenous plant extracts. Journal of Life and Earth Science, 6, 75-80.
- Imura, O. and Ninomiya, S. (1978) Quantitative measurement of leaf area consumption by Epilachna vigintioctopunctata (Fabricius) (Coleoptera: Coccinellidae) using image processing. Appllied Entomological Zoological, 33, 491-495.
- Srivastava, K.P. and Butani, D. (1998) Pest management in vegetable. Research Periodical and Book Publishing House, 197-225.
- Ghosh, S.K. and Senapati, S.K. (2001) Biology and seasonal fluctuation of Henosepilachna vigintioctoctopunctata Fabr. On brinjal under terai region of West Bengal. Indian Journal of Agricultural Research, 35, 149-154.
- Isman, B., Koul, O., Lucyzynski, A. and Kaminski, J. (1990) Insecticidal and antifeedant bioactivities of neem oils and their relationship to Azadirachtin content. Journal of Agricultural and Food Chemistry, 38, 1407-1411. http://dx.doi.org/10.1021/jf00096a024
- Subramonithangam, T. and Kathiresan, K. (1988) Toxic effect of mangrove plant extracts on mosquito larvae Anopheles stephensi L. Current Science, 57, 914-915.
- Ben Jannet, H., Skhiri, H.F., Mighri, Z., Simmonds, M.S.J. and Blaney, W.M. (2000) Responses of Spodoptera littoralis larvae to Tunisian plant extracts and to neoclerodane diterpenoids isolated from Ajuga pseudoiva leaves. Fitoterapia, 71, 105-112. http://dx.doi.org/10.1016/S0367-326X(99)00146-X
- Abbott, W.S. (1925) A method of computing the effectiveness of an insecticide. Journal of Economic Entomology, 18, 265-266.
- Isman, M.B., Gunning, P.J. and Spollen, K.M. (1997) Tropical timber species as sources of botanical insecticides. In: Hedin, P.A., Hollingworth, R.M., Masler, E.P., Miyamoto, J. and Thomson, D.G., Eds., Phytochemicals for Pest Control, ACS Symposium Series, 658, 27-37.
- Schoonhoven, L.M. (1982) Biological aspects of antifeedants. Entomologia Experimentalis et Applicata, 31, 57- 69. http://dx.doi.org/10.1111/j.1570-7458.1982.tb03119.x
- Jeyarajan, S., Babu, P.C.S., Srimannarayana, G. and Geethanjali, Y. (1990) Antifeedant and morphogenetic effects of azadirachtin rich fractions on Spodoptera litura F. Botanical Pesticides in Integrated Pest Management, 13, 376-380.
- Sahayaraj, K. (1998) Antifeedant effect of some plant extracts on the Asian armyworm, Spodoptera litura (Fabricius). Current Science, 74, 523-525.
- Morimoto, M., Tanimoto, K., Saktani, A. and Komai, K. (2002) Antifeedant activity of an anthrquinone aldehydes in Galium aparine L. against Spodoptera litura F. Phytochemistry, 60, 163-166. http://dx.doi.org/10.1016/S0031-9422(02)00095-X
- Jeyasankar, A., Raja, N. and Ignacimuthu, S. (2010) Antifedant and growth inhibitory activities of crude extracts and fractions of Syzygium lienare (Myrtaceae) against Spodoptera litura (Fab.). Current Research Journal of Biological Sciences, 2, 173-177.
- Jeyasankar, A., Raja, N. and Ignacimuthu, S. (2011) Insecticidal compound isolated from Syzygium lienare (Myrtaceae) against Spodoptera litura (Fab.). Soudi Journal Biological Sciences, 18, 329-332. http://dx.doi.org/10.1016/j.sjbs.2011.01.003
- Jeyasankar, A., Premalatha, S. and Elumalai, K. (2012) Biological activities of Solanum pseudocapsicum (Solanaceae) against cotton bollworm, Helicoverpa armigera Hübner and armyworm, Spodoptera litura Fabricius (Lepidoptera: Noctuidae). Asian Pacific Journal of Biomedicine, 2, 981-986. http://dx.doi.org/10.1016/S2221-1691(13)60010-6
- Yasui, H., Kato, A. and Yazawa, M. (1998) Antifeedants to armyworm, Spodoptera litura and Pseudaletia separata, from bitter gourd leaves, Momordica charantia. Journal of Chemical Ecology, 24, 803-813. http://dx.doi.org/10.1023/A:1022317432674
- Morimoto, M., Fujii, Y. and Komai, K. (1999) Antifeedants in Cyperaceae: Coumaran and quinines from Cyperus spp. Phytochemistry, 51, 605-608. http://dx.doi.org/10.1016/S0031-9422(99)00098-9
- Isman, M.B., Wan, A.J. and Passreiter, C.M. (2001) Insecticidal activity of essential oils to the tobacco cutworm, Spodoptera litura. Fitoterapia, 72, 65-68. http://dx.doi.org/10.1016/S0367-326X(00)00253-7
- Lajide, L., Escoubas, P. and Mizutani, J. (1993) Antifeedant activity of metabolites of Aristolochia albida against the tobacco cutworm, Spodoptera litura. Journal of Agricultural and Food Chemistry, 41, 669-673. http://dx.doi.org/10.1021/jf00028a031
- Rajam, M.V. (1991) Insecticidal activity of inhibitors of polyamine synthesis on Spodoptera litura F. larvae. Indian Journal of Experimental Biology, 29, 881-882.
- Bohnenstengel, F.J., Wray, V., Write, L., Srivastava, R.P. and Prokach, P. (1999) Insecticidal Meliascarpins (C-seco limonoids) from Melia azedarach. Phytochemistry, 50, 977-982. http://dx.doi.org/10.1016/S0031-9422(98)00644-X
- Isman, M.B. (2000) Plant essential oils for pest and disease management. Crop Protection, 19, 603-608. http://dx.doi.org/10.1016/S0261-2194(00)00079-X
- Supratman, U., Fujita, T., Akiyama, K. and Hayashi, H. (2001) Insecticidal compounds from Kalanchoe daigremontiana X tubiflora. Phytochemistry, 58, 311-314. http://dx.doi.org/10.1016/S0031-9422(01)00199-6
- Hashim, M.S. and Devi, K.S. (2003) Insecticidal action of the polyphenilic rich fractions from the stem bark of Streblus asper on Dysdercus cingulatus. Fitoterapia, 74, 670-676. http://dx.doi.org/10.1016/S0367-326X(03)00186-2
- Leatemia, J.A. and Isman, M.B. (2004) Insecticidal activity of crude seed extracts of Annona spp., Lanium domesticum and Sandoricum koetjape against Lepidopteran larvae. Phytoparasitica, 32, 30-37. http://dx.doi.org/10.1007/BF02980856
- Jeyasankar, A. and Alexander Jesudasan, R.W. (2005) Insecticidal properties of novel botanicals against a few lepidopteran pests. Pestology, 29, 42-44.
- Jeyasankar, A, (2012) Antifeedant, insecticidal and growth inhibitory activities of selected plant oils against black cutworm, Agrotis ipsilon Hufnagel (Lepidoptera: Noctuidae). Asian Pacific Journal of Tropical Disease, 2, S347- S351. http://dx.doi.org/10.1016/S2222-1808(12)60179-0

