Optics and Photonics Journal
Vol.05 No.05(2015), Article ID:56581,6 pages
10.4236/opj.2015.55018
Metal Nano Layer Coating for Improving the Detection and Recognition of Micro-Poisons Using Reflection Spectroscopic Measurement
Amir Abramovich*, Alexander Shulzinger, Meir Ochana, David Rotshild
Department of Electrical and Electronic Engineering, Ariel University, Ariel, Israel
Email: *amir007@ariel.ac.il
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 23 March 2015; accepted 22 May 2015; published 25 May 2015
ABSTRACT
Metal nano layer coating for increasing the sensitivity of spectroscopic measurements is proposed and experimentally demonstrated in this paper. The metal nano layer will attract the micro- poisons from any measured aqueous sample increasing the concentration of the micro-poison in the vicinity of the surface and significantly improves the sensitivity of the spectroscopic measure- ment. The demonstration was carried out using Fourier Transform Infra-Red (FTIR) operating in the MIR 400 cm−1 - 4000 cm−1 and 5 nm Gold layer which was grown on silicon oxide substrate. In the experimental demonstration Malathion organophosphate pesticide was used as micro-poison. The spectroscopic measurement proves that Malathion was attracted to the metal nano layer. Fur- thermore, the absorption lines of Malathion were detected and recognized. This proof of principle can be applied to any Internal Reflection Elements (IRE) and it can be used to purify any aqueous solutions and atmosphere from micro-poisons which will be attracted to the metal Nano layer.
Keywords:
FTIR Spectroscopy, Internal Reflection Element (IRE), Metal Nano Layer Coating, Detection of Micro-Poisons, Organophosphates

1. Introduction
Pollution of the environment caused by industrialization, domestic waste, massive use of insecticides, pesticides and herbicides, antibiotics remains and microorganisms, results in increasing the numbers of environment contaminations. On the other hand, deliberate contamination as act of terror should be taken into account. Those contaminations are potentially dangerous to humans and to the ecosystem. Recent investigations indicate that the influence of micro poisons, especially Organophosphates, on humans can be expressed as long term damage and diseases [1] [2] , part of them can cause death [3] . Thus, the need for real time and sensitive monitoring system capable of detecting and identification of micro poisons is obvious.
Organophosphates which are widely used micro poisons in agriculture and domestic use, can be detected in aqua solutions samples using very sensitive gas or liquid chromatographic [4] [5] . However, the dynamic nature of aquatic sources which changes the micro poisons concentrations (spatially and temporally), can be misled for those kind of devices. Consequently, there is a necessity for continuously operating in situ sensing devices, which are able to monitor organophosphates or other micro poisons in aqueous solution especially in drinking water. Furthermore, gas or liquid chromatographic devices are time consuming and are very expensive.
Pre-concentration is well known method [6] for concentration of an analyte from an aqueous sample before analysis. There are two types of pre-concentration: 1) solid-phase microextraction (SPME), which involves the use of a fiber coated with an extracting phase, that can be a liquid (polymer) or a solid (sorbent), which extracts different kinds of analytes [7] . And 2) sorbent trapping, which is a material used to absorb or adsorb liquids or gases [8] . Both methods used to increase the detectivity of the analyte.
Biosensors are another group of micro poisons detectors in which the bioreceptor is designed to interact with the specific analyte to produce a measurable signal. This can be carried out using enzymes [9] , acetyl cholinesterase (AChE) and organophosphate hydrolase (OPH) modified amperometric [10] . AChE inhibition-based biosensors are sensitive and useful as single-use disposable sensors for environmental monitoring. Biosensors based on organophosphorus hydrolase (OPH), an enzyme that catalyzes hydrolysis of a range of organophosphate pesticides and chemical warfare agents have been reported [11] . However, these biosensors are not suitable for continuous and real time monitoring application. Fiber-optic [12] schemes have already been successfully implemented. Nevertheless, the relatively high detection limits of fiber-optic biosensors and instabilities make them not suitable for environmental monitoring.
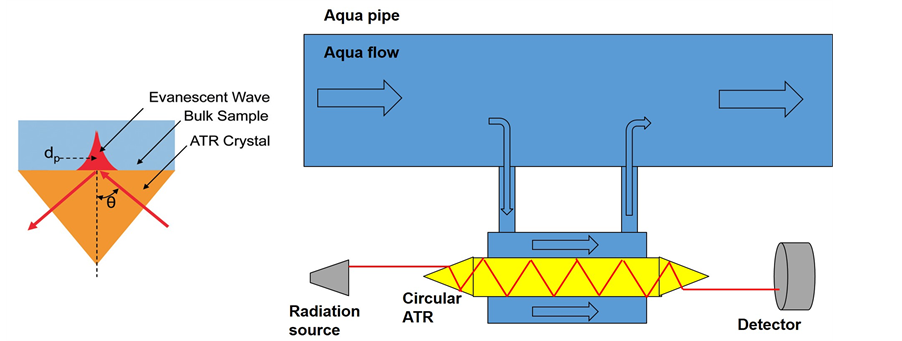
Optical sensors based on spectroscopic properties of the micro-poisons are of particular interest compared with chemical sensing mentioned above due to: 1) uniqueness of spectral signature in MIR and Far Infrared (FIR) spectral region enabling multicomponent detection; 2) robustness; 3) remote sensing capability; and 4) can be contentious and real time. Attenuated Total Reflection (ATR) which is an Internal Reflection Element (IRE), is well known and it is wildly used in infrared spectroscopic measurements and spectroscopic research [13] . In ATR measurement method, a beam of infrared light is passed through the ATR crystal in such a way that it reflects back from the internal surface in contact with the sample provided that incidence angle, q, is greater or equal to the critical angle, qc (see Figure 1) [13] [14] . At the interface, an evanescent wave is generated which penetrates into the sample (see Figure 1 left). The main advantage of the ATR is that the optical path into the sample is very small, in the order of 1 micron in the infrared band. This avoids strong attenuation due to sample high absorption compared with transmission measurement especially where the absorption of the sample is very high. Thus ATR method is used in aqueous solutions where the absorption is high. Only the evanescent wave penetrates into the sample as can be seen in Figure 1 left.
The penetration depth, dp, of the evanescent wave into the sample is given by [13] [14] :
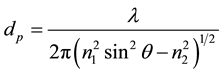 (1)
(1)
where l is the beam wavelength, q is the angle of the incidence beam and n1 and n2 are the indices of refraction of the ATR crystal and the sample respectively. The combination of Fourier Transform Infra-Red (FTIR) system [15] with ATR accessory [13] [16] yields a very sensitive diagnostic tool based on measurement of the spectroscopic signature of samples in Medium Infra-Red (MIR) and Far Infra-Red (FIR) regime. Coating the ATR crystal with organically modified sol-gels as recognition layers for in situ detection of micro-poisons in aqueous media was demonstrated and found to be very sensitive [17] . However, Sol-gels have an absorption line in the MIR which limit their use to narrow band and for selected micro poisons [17] .
In order to detect and recognize a micro-poison in aqueous solution, a good knowledge of its absorptions spectral lines (spectroscopic signature) is required. Using a radiation source and a detector operating in the
Figure 1. (Left) the evanescent wave penetration into the sample in ATR measurements (right) circular ATR configuration for identification and recognition of micro-poisons in aqueous solutions.
absorption line wavenumber (see Figure 1 right), can indicate the presence of the micro-poison in the aqueous solution if the detected signal is suddenly reduced. The configuration of Figure 1 right can be used for several absorption wavenumbers simultaneously, continuously and real time. In order to compensate the radiation attenuation inside the ATR crystal, high power radiation sources can be used. Further increase in sensitivity performance can be achieved using metal nano layer coating on the circular ATR configuration shown in Figure 1 right. This can improve significantly the detection and recognition performance of spectroscopic systems due to attraction of organophosphate to the metal nano layer.
An example of using metal nano particles in spectroscopic measurements is the Surface-Enhanced Infrared Absorption (SEIRA) method [18] . In this method, Silver nano particles on germanium substrate were used to en- hance the MIR absorption in spectroscopic measurement. Improvement of detection performance was achieved for optimal conditions. The disadvantage of this method is its strong dependence on environmental factors such as temperature and the surface preparation quality.
2. Metal Nano Layer Preparation
Nano particles research has intense scientific interest due to many potential application in versatile fields such as biomedical, optics and electronic fields [19] . One of the interesting technologies regarding the metal nano particles is thin coatings and layers of materials [20] . In order to grow those thin coatings and layers, sputtering machines are used [21] . Sputtering is a process in which atoms are ejected from a solid target material due to bombardment by energetic particles (usually ions) in vacuum environment. It requires that the kinetic energy of the incoming particles is much higher than conventional thermal energies (≫1 eV). This process is commonly utilized for thin-film growing, etching and analytical techniques [21] . The ejected atoms from the solid target (Gold or Aluminum in this case) deposit on all the surface of the substrate. The time duration of this process determines the thickness of the nano layer. In the frame of this work we prepared several Gold and Aluminum nano layer coating on standard silicon oxide substrates using sputtering and deposition process. In order to glue and fix the Gold Nano particles layers to the silicon oxide substrate, thin layer (5nm) of chrome is required. The Aluminum Nano particles does not require thin Chrome layer. The thickness of the deposited layers were 15 nm and 5 nm of both metals (Gold and Aluminum). The samples were kept on sealed cell until the use in the experiment. If a cylindrical substrate such as Silicon Circular ATR is needed to be coated with metal Nano particle layer, a special rotating holder is used during the sputtering process.
3. Experimental Results
In order to demonstrate this method of pre-concentration using metal nano layer coatings, we choose micro- poison Malathion from the organophosphate (OP) group [22] to be the detected and recognized micro-poison. Malathion is an insecticide which is widely used in agriculture, residential landscaping and public recreation areas. On the other hand, Malathion is known for his toxicity to humans [23] and thus it is important to monitor and control its concentration in drinking water, agriculture products and the environment.
The First step of the demonstration includes ATR-FTIR spectroscopic signature analysis of the pure Malathion. The measurements were conducted in the MIR range using ISF 113 FTIR (Bruker Inc.) configured to operate with Globar source, Ge/KBr beam-splitter and DTGS detector. The ATR accessory was MVP-pro STAR ATR (Harrick Inc.). Figure 2 shows the ATR FTIR spectral measurement of pure Malathion in MIR band for two different ATR crystal materials; silicon and diamond.
The second step in the demonstration is to investigate the interaction between metal nano particles coatings and electromagnetic radiation in the MIR. This investigation was carried out using thin metal nano layer coating on silicon oxide substrate. Thin metal nano layers with different thickness were grown using sputtering machine on a regular silicon oxide substrates. Those samples were experimentally tested using the FTIR system in the MIR band for their spectroscopic signature. Figure 3 shows the MIR spectral signature of metal nano particles. The silicon oxide sample with thickness of 0.3 mm was used as a substrate, metal nano particle layer thickness was 5 nm.
From Figure 3 it can be seen that the metal nano particle coatings on the silicon substrate influence the spectral signature of the pure silicon. The metal nano particles didn’t change the basic spectral signature of the silicon substrate, they increased the reflection from the silicon substrate and decreased the transmittance as was expected from metals. Unlike the Sol-gels coatings [17] mentioned above, that add absorption line to spectral signature. Thus, the metal nano thin layers on the silicon substrate can be used in spectroscopic measurements.
In order to demonstrate the attraction of Malathion organophosphate to the metal nano particles and the feasibility of detecting and recognizing it using Circular ATR, we prepared two silicon oxide substrates. One of them was coated using sputtering machine with 5 nm layer of Gold nano particles and the second without coating that was used as control. A drop of pure Malathion was put on each of those two silicon substrates for two days in close cell until the drops were dried. After two days a FTIR reflection measurement in MIR band was carried out for the two substrates. The FTIR beam was directed to the location of the dried Malathion drops on the substrate surface. The results are shown in Figure 4.
From the spectral signature results of Figure 4, it can be seen clearly that the silicon substrate with the nano Gold particles coating attracted the Malathion molecules. The spectral signature of absorption lines of Malathion was recognized easily and they are exactly the same as the pure Malathion given in Figure 2. Note that Figure 2 describe an ATR FTIR measurements and Figure 4 describes FTIR reflection measurements but the absorption lines are on the same wavenumbers. On the other hand, the spectral measurement of the control silicon substrate almost no similarity to pure Malathion was found.
4. Conclusion
The concept of pre-concentration using Circular ATR crystal coated with Gold nano particles was presented and demonstrated in this study. The result of Figure 4 proofs two important principles. The first is that metal nanoparticles are attracts micro poisons in aqua solutions. The second is that the micro poison that is attracted to the Gold nano particle can be detected and recognized by ATR FTIR measurement. In this case, no sample
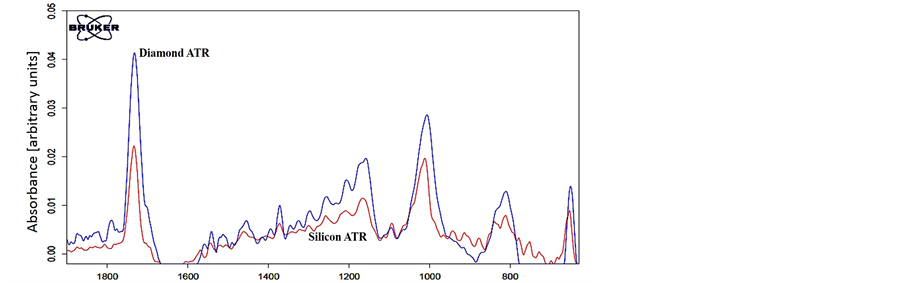
Figure 2. ATR-FTIR spectral signature analysis in the MIR band of Malathion for two ATR crystal materials; Diamond (blue) and Silicon (red).
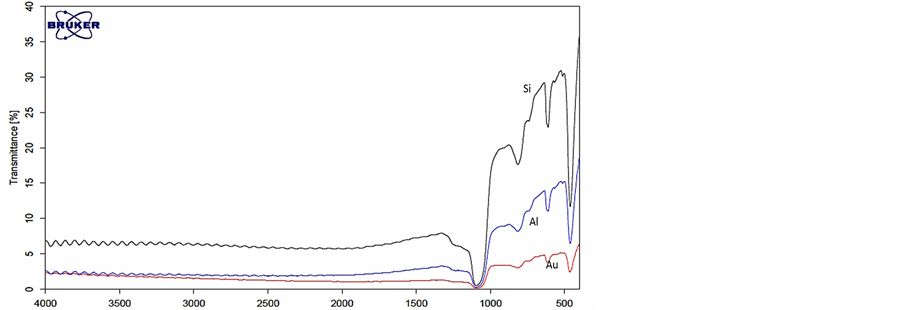
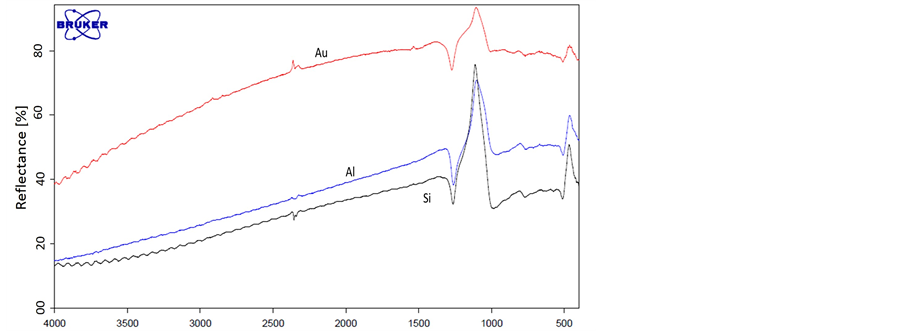
Figure 3. Transmittance (top) and reflection (bottom) spectral signature of: 1) pure silicon substrate (black), 2) Aluminum nano particle coating on silicon substrate (Blue) and 3) Gold nano particles coating on silicon substrate (red).
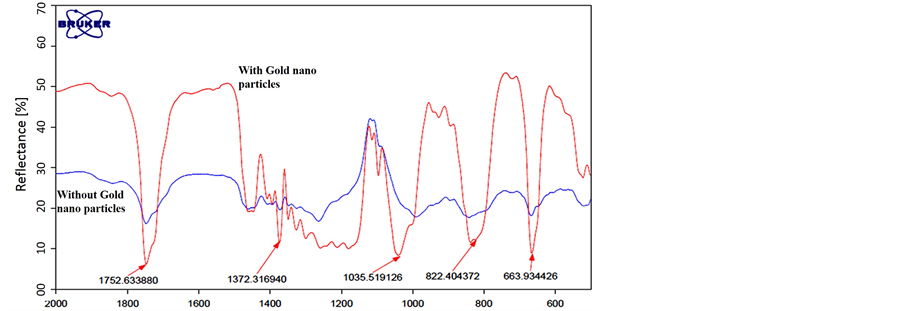
Figure 4. FTIR reflection measurements of dried drop of Malathion on silicon substrates for two cases; with Gold nano particles coating (red) and without coating (blue).
preparations are needed and the results are in real time. Thus, this new concept can be used to control and monitor drinking water and/or other aqua solutions and/or agriculture food products. Remote control spectral measurements can be carried out using the above experimental set-up and the circular ATR configuration (with nano metal coating) shown in Figure 2 right.
Acknowledgements
The authors would like to thanks Dr Amos Sharoni from the Nano technology center of Bar Ilan University (BIU) for is help with the preparation of metal Nano particles coatings and discussions.
References
- Prüss-Ustün, A., Vickers, C., Haefliger, P. and Bertollini, R. (2011) Known and Unknowns on Burden of Disease Due to Chemicals: A Systematic Review. Environmental Health, 10, 9. http://dx.doi.org/10.1186/1476-069X-10-9
- Briggs, D. (1991) Environmental Pollution and the Global Burden of Disease. British Medical Bulletin, 68, 1-24. http://dx.doi.org/10.1093/bmb/ldg019
- Chompton, J.A. (1988) Military Chemical and Biological Agents. Telford Press, NJ, 135.
- Yao, S., Meyer, A., Henze, G. and Fresenius, J. (1991) Comparison of Amperometric and UV-Spectrophotometric Monitoring in the HPLC Analysis of Pesticides. Journal of Analytical Chemistry, 339, 207-211. http://dx.doi.org/10.1007/BF00325738
- Sherma, J. (1993) Pesticides. Journal of Analytical Chemistry, 65, R40. http://dx.doi.org/10.1021/ac00060a004
- Leyden, D.E. and Wegscheider, W. (1981) Pre-Concentration for Trace Element Determination in Aqueous Samples. Journal of Analytical Chemistry, 53, 1059A-1065A. http://dx.doi.org/10.1021/ac00232a731
- Spietelun, A., Pilarczyk, M., Kloskowski, A. and Namieśnik, J. (2010) Current Trends in Solid-Phase Microextraction (SPME) Fiber Coatings. Chemical Society Reviews, 39, 4524. http://dx.doi.org/10.1039/c003335a
- Harper, M. (2000) Sorbent Trapping of Volatile Organic Compounds from Air. Journal of Chromatography A, 885, 129-151. http://dx.doi.org/10.1016/S0021-9673(00)00363-0
- Mulchandani, A., Chen, W., Mulchandani, P., Wang, J. and Rogers, K.R. (2001) Biosensors for Direct Determination of Organophosphate Pesticides. Biosensors and Bioelectronics, 16, 225. http://dx.doi.org/10.1016/S0956-5663(01)00126-9
- Palchetti, I., Cagnini, A., Del Carlo, M., Coppi, C., Mascini, M. and Turner, A.P.F. (1997) Determination of Anticholinesterase Pesticides in Real Samples Using a Disposable Biosensor. Analytica Chimica Acta, 337, 315. http://dx.doi.org/10.1016/S0003-2670(96)00418-7
- Mulchandani, A., Mulchandani, P., Kaneva, I. and Chen, W. (1998) Biosensor for Direct Determination of Organopho- sphate Nerve Agents Using Recombinant Escherichia coli with Surface-Expressed Organophosphorus Hydrolase. 1. Potentiometric Microbial Electrode. Analytical Chemistry, 70, 4140-4145, http://dx.doi.org/10.1021/ac9805201
- Mulchandani, A., Kaneva, I. and Chen, W. (1998) Biosensor for Direct Determination of Organophosphate Nerve Agents Using Recombinant Escherichia coli with Surface-Expressed Organophosphorus Hydrolase. 2. Fiber-Optic Microbial Biosensor. Analytical Chemistry, 70, 5042-5046. http://dx.doi.org/10.1021/ac980643l
- Harrick, N.J. (1967) Internal Reflection Spectroscopy. Wiley, New York.
- Li-Chan, E., Chalmers, J. and Griffiths, P. (2010) Applications of Vibrational Spectroscopy in Food Science. Wiley, New York.
- Griffiths, P.R. and de Haseth, J.A. (1986) Fourier Transform Infrared Spectrometry, Wiley, New York.
- Singh, B.R. and Fuller, M.P. (1991) FT-IR in Combination with the Attenuated Total Reflectance Technique: A Very Sensitive Method for the Structural Analysis of Polypeptides. Applied Spectroscopy, 45, 1017-1021. http://dx.doi.org/10.1366/0003702914336174
- Janotta, M., Karlowatz, M., Vogt, F. and Mizaikoff, B. (2003) Sol-Gel Based Mid-Infrared Evanescent Wave Sensors for Detection of Organophosphate Pesticides in Aqueous Solution. Analytica Chimica Acta, 496, 339-348. http://dx.doi.org/10.1016/S0003-2670(03)01011-0
- Yang, J. and Griffiths, P.R. (2007) Preparation and Characterization by Surface-Enhanced Infrared Absorption Spectroscopy of Silver Nanoparticles Formed on Germanium Substrates by Electroless Displacement. Analytical and Bioanalytical Chemistry, 388, 109-119. http://dx.doi.org/10.1007/s00216-006-1109-7
- Taylor, R., Coulombe, S., Otanicar, T., Phelan, P., Gunawan, A., Lv, W., Rosengarten, G., Prasher, R. and Tyagi, H. (2013) Small Particles, Big Impacts: A Review of the Diverse Applications of Nanofluids. Journal of Applied Physics, 113, Article ID: 011301. http://dx.doi.org/10.1063/1.4754271
- Park, J., Yang, R.D., Colesniuc, C., Sharoni, A., Jin, S., Schuller, I.K., Trogler, W.C. and Kummel, A.C. (2008) Bilayer Processing for an Enhanced Organic-Electrode Contact in Ultrathin Bottom Contact Organic Transistors. Applied Physics Letters, 92, Article ID: 193311. http://dx.doi.org/10.1063/1.2918121
- Behrisch, R. (1981) Sputtering by Particle Bombardment. Springer, Berlin. http://dx.doi.org/10.1007/3-540-10521-2
- Howard, P.H. (1991) Handbook of Environmental Fate and Exposure Data: For Organic Chemicals. Taylor & Francis Group, UK.
- Bonner, M.R., Coble, J., Blair, A., et al. (2007) Malathion Exposure and the Incidence of Cancer in the Agricultural Health Study. American Journal of Epidemiology, 166, 1023-1034. http://dx.doi.org/10.1093/aje/kwm182
NOTES
*Corresponding author.