Stem Cell Discovery
Vol.3 No.2(2013), Article ID:29930,9 pages DOI:10.4236/scd.2013.32014
Influence on proliferation and inducing capacity of bone mesenchymal stem cells during myocardial differentiation and construction of engineered myocardium-like tissue in vitro
![]()
1Department of Cardiothoracic Surgery, The Second Hospital of Shanxi Medical University, Taiyuan, China; *Corresponding Author: xwhcardia@sina.com
2Department of Internal Medicine, People’s Hospital of Jiexiu City, Jiexiu, China
Copyright © 2013 Wanhong Xing et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 28 November 2012; revised 31 December 2012; accepted 20 January 2013
Keywords: Mesenchymal Stem Cells; Proliferation; 5-Azacitidine; Cardiomyocytes; Myocardium; Tissue Engineering
ABSTRACT
The study is to investigate BMSCs ability to differentiate cardiomyocytes, especially discussed cell generations and 5-Aaz concentration influence on BMSCs capability of proliferation and differentiation into cardiomyocytes during constructing the engineered myocardium-like tissue in vitro. The results have demonstrated that the different concentration of 5-Aza has the influence on proliferation rate of different generations of BMSCs, the second generation BMSCs was superior to the sixth, and tenth generation in proliferation capacity after being induced, and 5-Aza had some influence on proliferation capacity. Rat BMSCs could be differentiated into cardiomyocytes-like cells, which have a good biocompatibility with acellular bovine pericardium, and myocardium-like tissue could be engineered with BMSCs and acellular bovine pericardium in vitro. In conclusion, BMSCs could be induced and differentiated into cardiomyocytes-like cells in vitro. Different generations and different 5-Aza density have influence on the rate of increase of BMSCs, and the engineered myocardium-like tissue could be constructed with BMSCs and acellular bovine pericardium in vitro.
1. INTRODUCTION
Congenital heart disease (CHD) causes a high morbidity, and maybe one of the leading cause of death for infants, especially neonates [1]. In the last few years, nearly all the cardiovascular congenital malformation could be surgically corrected with the advancement of surgical techniques, monitoring devices and treatments taken after the operation. At present, the repairing materials frequently used in the heart operation are artificial high polymer materials such as polytetrafluoroethylene (ePTFE) and Dacron, which lack contractility and growth potential, and are prone to thrombus formation [2]. In addition, biomaterials like pericardium have the disadvantage of calcification [3]. Because of the disadvantages mentioned above, searching for the ideal materials in CHD surgery has been a hotspot, but cardiac tissue engineering has afforded a new idea and strategy. Mesenchymal stem cells (MSCs) reside in many tissues, especially abundant in bone marrow [4-6]. They have the potential of multi-orientation differentiation in certain conditions. Wakitani and colleagues [7] have confirmed that bone mesenchymal stem cells (BMSCs) could be induced to myocytes by 5-Azacitidine (5-Aza). Tomita and colleagues [8] reported that BMSCs cultured with 5-Aza differentiated into cardiac-like muscle cells in vitro, formed myotubules which stained positively for troponin I and myosin heavy chain, and improved myocardial function in vivo. Xu [9] reported that human MSCs could be induced into cardiomyocytes in vitro by 5-Aza. BMSCs have many advantages such as having no immunogenicity with self source, drawing the materials by bone marrow aspiration with little damage, sufficient sources, and strong expanding capability. Accordingly, MSCs could be one of the ideal resources in cardiac tissue engineering.
Cardiac tissue engineering aims to construct engineered cardiac tissue with characteristics similar to those of the native tissue. Bioengineered cardiac grafts are created by combining autologous cell transplantation with a degradable scaffold as a temporary extracellular matrix [10]. At present, myocardium-like tissue has been constructed with embryonic stem cells [11,12] or newly born cardiomyocytes [13-15] in vitro, but it is very difficult to practice it clinically for immunologic rejection, ethics and so on. This study discussed cell generation and 5-Aaz concentration influence on BMSCs’ capability of proliferation and differentiation into cardiomyocytes, and attempted to construct the engineered myocardium-like tissue with BMSCs and acellular bovine pericardium in vitro. Our results may provide the step on the long road toward engineered myocardial tissue for repairing the nonfunctional myocardium or defect in CHD, such as ventricular septal defect or tetralogy of Fallot.
2. MATERIALS AND METHODS
2.1. Isolation of BMSCs and Myocardial Differentiation
4 weeks old male Sprague Dawley rats, weighing 100 - 120 grams were provided by the Institutional Experimental Animal Center of Shanxi medical university. Animal treatment corresponded to Management and use of laboratory Guide issued by US National Institutes of Health. The study protocol was reviewed and approved by the Institutional Animal Care and Use Committee.
Rats were executed by suffocation with carbon dioxide, and were then immersed in 75% alcohol for 5 minutes. Under the condition of no destroying rat thigh bone, two hind legs of the rat were dissected, and the fur, muscle and tendon of the legs were wiped out. Thigh bone was immersed in 75% alcohol for 3 minutes. It was then rinsed 2 times with PBS, and immersed in complete medium-LG-DMEM. The thigh bone was broken, and the marrow cavity was washed repeatedly 3 times with LG-DMEM. Slowly adding washing fluid which was obtained from marrow cavity as has been shown above on the surface of 5 ml Percoll Liquid Separation (1.077 g/ml), the mixture was centrifuged at centrifugal force of 1100 g/min for 30 minutes, and the stratum intermedium of mononuclear cells was sucked. The cells at the density of 2.5 × 105 cells/cm2 were inoculated in the culture plate which contained 10% Fetal Bovine Serum (FBS) and Low Glucose Dulbecco’s modified Eagle medium (LGDMEM). The cells above were cultured in the incubator which contained 5% CO2 at 37˚C. Primary passage changed the medium at the fourth day, and then changed the culture medium every 3 days. When the attached cells got near confluence, they were digested by 0.25% pamcreatin-EDTA. The cells were transferred to culture at the ratio of 1:3 when passaged. The cell morphology was observed with Inverted phase contrast microscope (CKX 41, Olympus Company).
2.2. BMSCs Proliferation and Growth Curve
The second generation of BMSCs was digested by 0.25% pamcreatin-EDTA,cell density was adjusted to 3 × 104 cells/ml, and the cells were inoculated in nine 96 well plates. 200 μl of cell suspension was added in each well. Got one of the plates every day after their adherence, and 20 μl MTT (5 mg/ml) was added into every well. The cells were incubated at ±37˚C for 4 hours, and supernatant in every well was then discarded. 150 μl DMSO was added into each well, then the plate was placed on the concentrator for 10 minutes at low-speed oscillation in order to dissolve the purple crystal sufficiently. The absorbance (OD) of each well was measured with ELISA apparatus (680, Bio-Rad) at 490 nm wavelength, the results were recorded, and the growth curve was drawn.
2.3. Myocardial Differentiation
When the BMSCs had been passaged for second, sixth, or tenth generation, they were incubated by 5 μmol/L, 10 μmol/L, and 15 μmol/L 5-Aza for 24 hours respectively. The culture medium which contained 5-Aza was sucked. Then they were cultured for 4 weeks. Untreated cells acted as negative control group. Cell morphology were monitored by Inverted phase contrast microscope (CKX41, Olympus Company).
2.4. Different Generations of BMSCs and Different 5-Aza Density Influence on Rate of Increase
BMSCs at the second, sixth, and tenth generation were selected, and were inoculated in a 96 well plate at a cell density of 3 × 104 cells/ml respectively. Three plates were inoculated for each generation. Each plate was divided into 4 groups, each group included 23 wells, and the remaining 4 wells were set up for zero holes. Three groups in every 96 well plate acted as induced groups, and 5 μmol/L, 10 μmol/L, 15 μmol/L 5-Aza was used to inoculate BMSCs for 24 hours respectively. 23 wells acted as a control group in every 96 well plate, and were inoculated in complete medium for 24 hours. All groups were then incubated with complete medium for 5 days. Absorbance (OD) was measured with MTT at the first, third and fifth day after being induced with 5-Aza.
2.5. Immunocytochemistry for Cardiac Specific Proteins
BMSCs at the second, sixth and tenth generation were detected with Desmin after being induced with the different concentrations, i.e. 5 μmol/L, 10 μmol/L and 15 μmol/L 5-Aza for 4 weeks. The second generation BMSCs were detected with troponin T, α-Actin expression after being induced with 10 μmol/L 5-Aza for 4 weeks, The normal rat cardiomyocytes acted as a positive control group, and the untreated BMSCs acted as negative control group.
Cells that grown on labteck chamber slides (50 × 104 cells/well) (Nunc) were washed with PBS (0.01 mmol/L, pH 7.4), fixed with 4% paraformaldehyde for 20 min and permeabilized with 0.1% Triton X-100/PBS (Sigma) for 15 min. Endogenous peroxidase was blocked with 3% H2O2—methanol for 15 min. Each manipulation was preceded by washing the cells three times in PBS with 0.05% Tween 20 (Sigma). After blocking in PBS-BSA 5% (Sigma) for 1 h, the cells were incubated overnight at 4˚C with the primary antibodies: Rabbit polyclonal to Desmin (Desmin, crossreactivity with mouse) 1:300, Shanghai Bluegene Biotech CO., LTD; Mouse antiRabbit cardiac Troponin T (cTnT, crossreactivity with mouse), 1:200, Sigma; Rabbit polyclonal to alpha smooth muscle Actin (α-Actin, crossreactivity with mouse), 1:300, Sigma. Bound primary antibodies were visualized by Peroxidase labeled streptomycin avidin staining reagents (Shanghai Bluegene Biotech CO., LTD). Slices were mounted, and were observed with Inverted microscope system (CKX 41, Olympus Company). The same protocols were followed for staining of the normal rat cardiomyocytes and the untreated BMSCs.
2.6. Reverse Transcription-Polymerase Chain Reaction (RT-PCR) for Cardiac Specific Genes
Total RNA was extracted from the second passage BMSCs which have been induced with 5-Aza for 4 weeks using Trizol reagent (Invitrogen, Carlsbad, CA). RNA samples were treated with Superscript III RNase H-reverse transcriptase (Invitrogen, Carlsbad, CA) to converting to cDNA in a 20 μL reaction mixture. The sequences of PCR primers for GATA-4, Nkx 2.5 and TDGF-l were as follows: GATA-4, 130 bp, 5’-GAC TGA GTT CTG GGC ATCT-3’ and 5’-CAA TCT TTA GGC TCT GGTTT-3’; Nkx 2.5, 102 bp, 5’-CGC CAA CAG CAA CTT CGT-3’ and 5’-ATG CCG TGC AGC GTG GAGAC-3’; TDGF-l, 183 bp, 5’-GCA ACT GTG AGC ACG ATG-3’ and 5’-GTG GAG TCC TGG ATA CCTT-3’. RNA of untreated third passage BMSCs was used as negative control. The PCR products were sizefractionated by 1% agarose gel electrophoresis.
2.7. Preparation for Acellular Bovine Pericardium Biomaterial
Fresh adult bovine pericardium was collected and transported in ice brine protection. Residual fat and connective tissue was removed from the pericardial surface. The bovine pericardium was cut into pieces of 0.5 cm × 0.5 cm, put in 0.1% bromogeramine liquid for 30 minutes, then washed repeatedly with PBS to remove bromogeramine from the bovine surface. Acellular bovine pericardium was obtained by using the method of detergent-enzyme digestion [16], and it was crosslinked with 0.625% genipin solution (2 ml/cm2) for 72 hours. Then the acellular bovine pericardium was rinsed with PBS. It was placed on a Petri dish, thoroughly disinfected by ultraviolet light for 20 minutes, immersed in 75% alcohol for 30 minutes, and rinsed 3 times with PBS. After this procedure, it was placed in LG-DMEM for 6 hours before seeded by BMSCs.
2.8. Construct the Engineered Myocardium-Like Tissue
After BMSCs at the third generation induced by 10 μmol/L 5-Aza had overgrown the 100 millimeter-sized culture dish, they were digested by 0.25% pancreatinEDTA, then centrifuged with 1500 rpm × 10 min. The supernatant was thrown away and the appropriate amount culture medium was added to form cell suspension. Cells with a density of 4.0 - 5.0 × 106 cells/cm2 were implanted on acellular bovine pericardium which had been treated with Poly-L-Lysine at a concentration of 2 mg/180 ml. After 4 hours incubation +37˚C, 5% CO2, the sufficient cell culture medium was added, supplemented with 10 μmol/L Basic fibroblast growth factor (bFGF). The complex of cells and scaffold had been cultured for 2 weeks in the Incubator +37˚C, 5% CO2. During this period, the culture medium was changed every two days. After it had been seeded for 2 weeks, histological detection was made.
2.9. Statistical Analysis
The sample data was analyzed with SPSS 13.0. Data description was indicated with mean ± standard deviation (X ± SD). The difference among groups was analyzed with Analysis of variance (ANOVA). Comparison between two groups was tested with the student-Newman-Keuls (SNK) method. Test level is α = 0.05, and P < 0.05 is significant difference.
3. RESULTS
3.1. BMSCs Isolation, Culture and Morphology Observation
The BMSCs during the primary culture period looked like small, short spindle or triangular cells after they had been seeded in the complete medium for 48 hours, and the cells had a good refractional capability. The percoll particles could be seen on the surface of the cells. After they had been seeded for 72 hours, the BMSCs looked like slender spindle cells. From the fourth day, the BMSCs had a significant proliferation, companied with many double refraction bodies, and formed island-like clones. Cells were arranged irregularly when cell density was lower, and triangular cells could be seen. Cells formed 70% - 80% confluence at the seventh-tenth day, then began to arrange regularly, and appeared as bundle, whirlpool or fish-like shapes. BMSCs’ morphology tended to be a unanimous appearance when passaged to the second generation (Figure 1).
Some of the cells at every generation died after being induced with 5 μmol/L, 10 μmol/L or 15 μmol/L 5-Aza for 24 hours. Cell mortality was increased with higher 5-Aza concentration, but generations didn’t seem to have an influence on cell mortality. After being induced with 5-Aza for 24 hours, some of the adherent cells became flat or short rod shape. Cells began to proliferate after being induced for 3 days, some presented the slender spindle shape, and others appeared triangular or polygonnal. After being cultured for 4 weeks, 80% - 90% cells presented mainly with a myofiber shape, though a few looked polygonal or flagstone-like shape, and cells grew
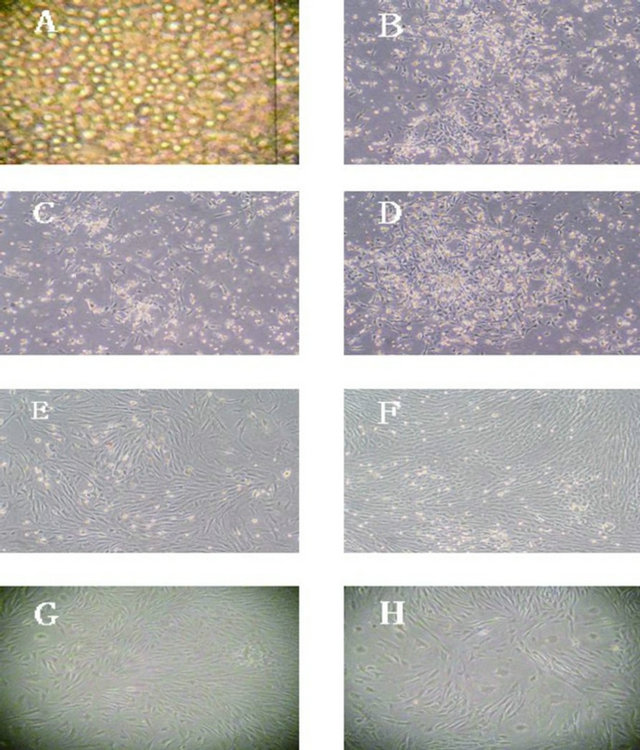
Figure 1. Different generations BMSCs. Note: (A) Primary generation BMSCs after percoll separation (100×); (B) Primary generation BMSCs after separation and culture 48 hours (40×); (C) Primary generation BMSCs after separation and culture 72 hours (40×); (D) Primary generation BMSCs after separation and culture 4 days (40×); (E) Primary generation BMSCs after separation and culture 7 days (40×); (F) The second generation BMSCs (40×); G: The 6th generation BMSCs (40×); (H) The 10th generation BMSCs (40×).
in an aggregation-like method. No spontaneous beating cells could be observed for different generations of BMSCs or different concentrations of 5-Aza (Figure 2).
3.2. Rat BMSCs Proliferation and Growth Curve
BMSCs subculture incubation period continued 1 or 2 days. By the third or fourth day, they had begun the logarithmic growth phase. At the sixth or seventh day, they entered into the platform period (Figure 3).
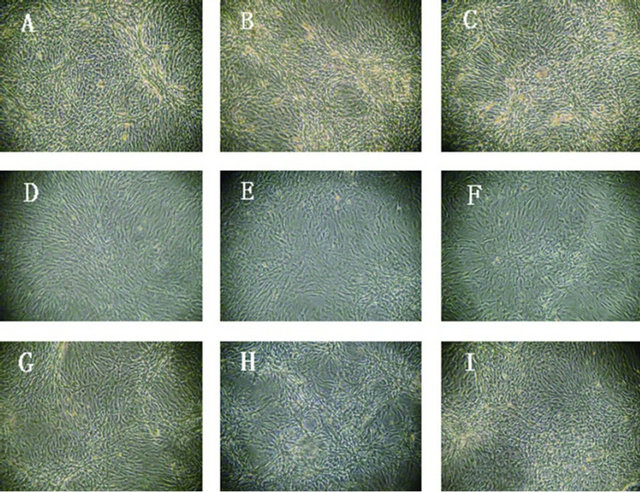
Figure 2. The second, sixth and tenth generation BMSCs after induced with 5, 10 or 15 μmol/L 5-Aza for 4 weeks (40×). Note: (A) The second generation BMSCs induced with 5 μmol/L 5-Aza for 4 weeks; (B) The second generation BMSCs induced with 10 μmol/L 5-Aza for 4 weeks; (C) The second generation BMSCs induced with 15 μmol/L 5-Aza for 4 weeks; (D) The sixth generation BMSCs induced with 5 μmol/L 5-Aza for 4 weeks; (E) The sixth generation BMSCs induced with 10 μmol/L 5-Aza for 4 weeks; (F) The sixth generation BMSCs induced with 15 μmol/L 5-Aza for 4 weeks; (G) The tenth generation BMSCs induced with 5 μmol/L 5-Aza for 4 weeks; (H) The tenth generation BMSCs induced with 10 μmol/L 5-Aza for 4 weeks; (I) The tenth generation BMSCs induced with 15 μmol/L 5-Aza for 4 weeks.
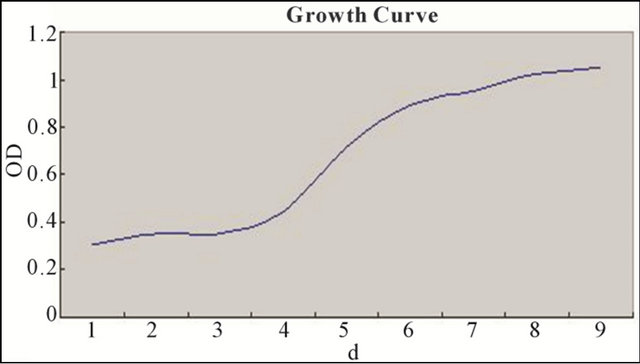
Figure 3. BMSCs subculture incubation period continued 1 or 2 days. By the third or fourth day, they had begun the logarithmic growth phase. At the sixth or seventh day, they entered into the platform period.
3.3. Different Concentration 5-Aza Influence on Proliferation Rate of Different Generations of BMSCs
BMSCs at different generations induced with different concentrations of 5-Aza were detected with MTT. It has been demonstrated that the second generation BMSCs was superior to the sixth, and tenth generation in proliferation capacity after being induced, but there was no statistical difference between the sixth and tenth comparison. In the control group, having no induction with 5-Aza, the second and sixth generation BMSCs showed no statistical difference in proliferation capacity, but all were superior to the tenth generation. 5-Aza had some influence on proliferation capacity, but with the increase of generations, this influence had a weakening trend (Figures 4-6 and Tables 1 and 2).
3.4. Immunocytochemistry
BMSCs at the second, sixth and tenth generation were
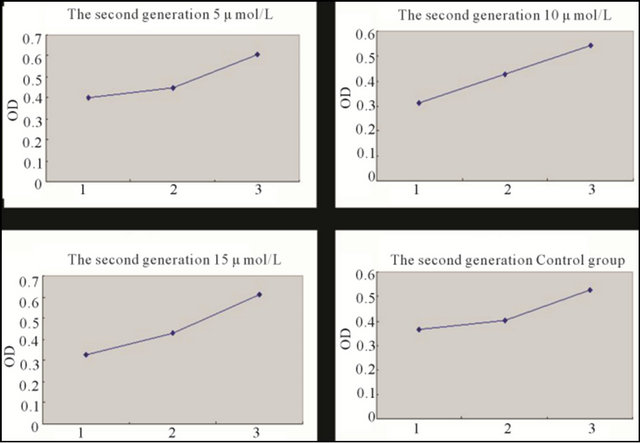
Figure 4. Growth curve of the second generation BMSCs induced with different concentration 5-Aza for 5 days and the control group BMSCs. Note: *1: First day; 2: Third day; 3: Fifth day.
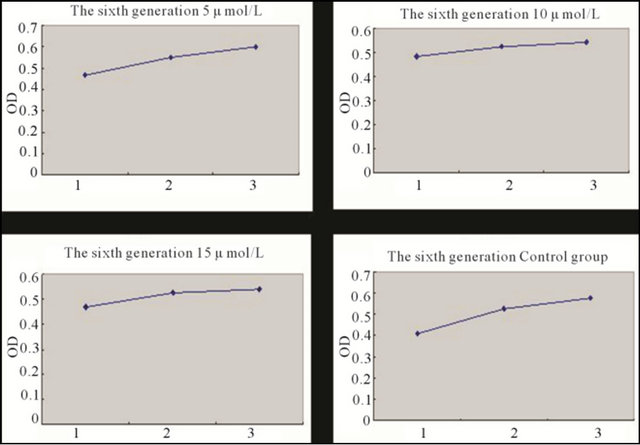
Figure 5. Growth curve of the sixth generation BMSCs induced with different concentration 5-Aza for 5 days and the control group BMSCs. Note: *1: First day; 2: Third day; 3: Fifth day.
detected with immunocytochemistry for Desmin after being induced with the different concentrations, i.e. 5 μmol/L, 10 μmol/L and 15 μmol/L 5-Aza for 4 weeks. Cells in all groups had positive expression for desmin. It was demonstrated that BMSCs at the second, sixth and tenth generation treated with the three different concentrations above had been induced and transformed to the myocardial orientation, and expressed cardiac-specific protein (Figure 7).
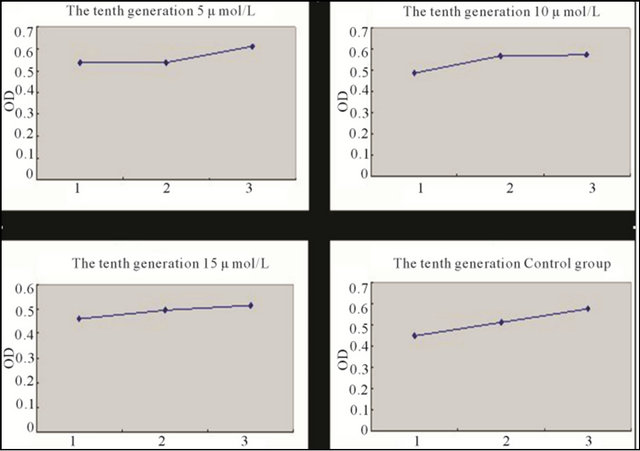
Figure 6. Growth curve of the tenth generation BMSCs induced with different concentration 5-Aza for 5 days and the control group BMSCs. Note: *1: First day; 2: Third day; 3: Fifth day.
Table 1. The proliferation rate of the second, sixth and tenth BMSCs induced with the concentration 5-Aza for 5 days and control group BMSCs.
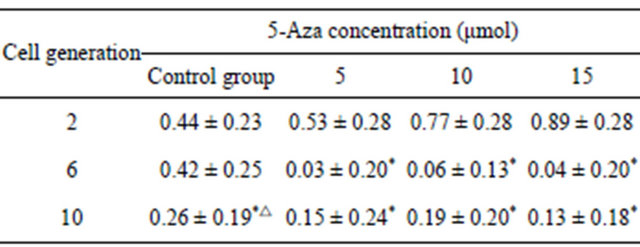
Note: *indicate P < 0.05 when compared with the second generation, △indicate P < 0.05 when compared with the 6th generation.
Table 2. The proliferation rate of the same passage BMSCs induced with the different concentration 5-Aza for 5 days and control group BMSCs.
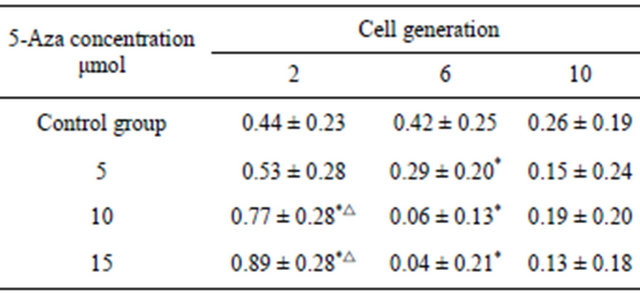
Note: *indicate P < 0.05 when compared with the control group, △indicate P < 0.05 when compared with the group induced with 5 μmol 5-Aza.
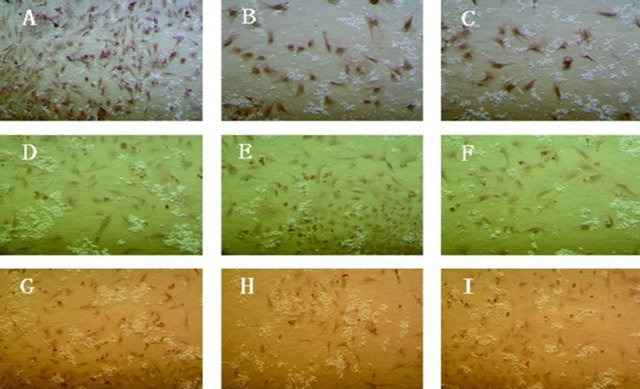
Figure 7. Cardiac specific protein Desmin expression of the second, sixth and tenth generation BMSCs induced with different concentration 5-Aza in vitro for 4 weeks (40×). Note: (A) The second generation BMSCs induced with 5 μmol/L 5-Aza for 4 weeks; (B) The second generation BMSCs induced with 10 μmol/L 5-Aza for 4 weeks; (C) The second generation BMSCs induced with 15 μmol/L 5-Aza for 4 weeks; (D) The sixth generation BMSCs induced with 5 μmol/L 5-Aza for 4 weeks; (E) The sixth generation BMSCs induced with 10 μmol/L 5-Aza for 4 weeks; (F) The sixth generation BMSCs induced with 15 μmol/L 5-Aza for 4 weeks; (G) The tenth generation BMSCs induced with 5 μmol/L 5-Aza for 4 weeks; (H) The tenth generation BMSCs induced with 10 μmol/L 5-Aza for 4 weeks; (I): The tenth generation BMSCs induced with 15 μmol/L 5-Aza for 4 weeks.
After the second generation BMSCs being induced with 10 μmol/L 5-Aza for 4 weeks, the induced group, negative group and positive group were detected with immunocytochemistry for troponin T, α-Actin expression. BMSCs in the induced group and positive group showed expression, but BMSCs in the negative group had no expression. It was demonstrated that BMSCs treated with 5-Aza have been induced and transformed to the myocardial orientation, and expressed cardiac-specific protein (Figure 8).
3.5. Associated Gene Expression with Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
The results of RT-PCR indicated that BMSCs induced with 5-Aza expressed GATA-4, Nkx 2.5 and TDGF-l. The induced group had an obvious stripe at 130 pb, 102 pb and 183 pb. It was demonstrated that BMSCs in the induced group had expressed the early myocardial forming transcription factor, such as GATA-4, Nkx 2.5, TDGF-l, etc., but none of in the control group (Figure 9).
3.6. Construction of Myocardium-Like Tissue in Vitro and Observation
Bovine pericardium after treatment with 0.625% genipin solution presented light blue. Its texture was flexible, and had a highly flexible character (Figure 10).
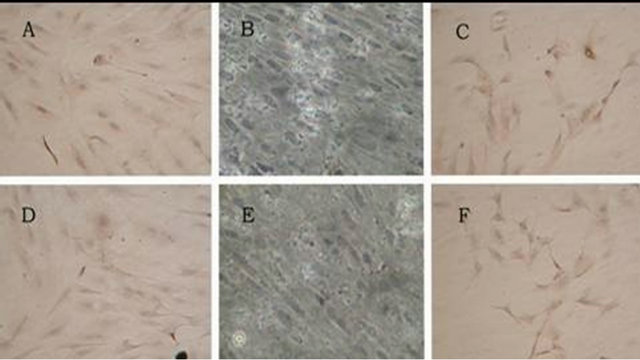
Figure 8. The troponin T and α-Actin expression of induced group, control group and positive control group (200×). Note: (A) Induced group positive expression of troponin T; (B) Control group negative expression of troponin T; (C) Positive control group positive expression of troponin T; (D) Induced group positive expression of α-Actin; (E) Control group negative expression of α-Actin; (F) Positive control group positive expression of α-Actin.
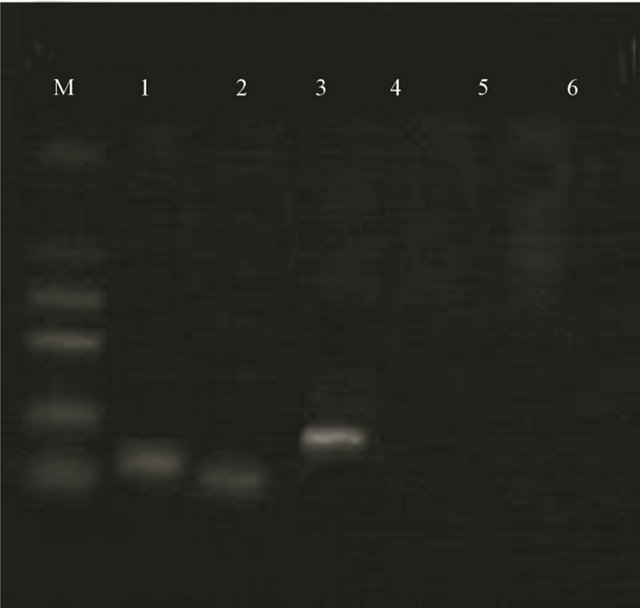
Figure 9. Expression of GATA-4, Nkx 2.5 and TDGF-l of induced group and control group detected RT-PCR. Note: M: Marker; 1. Induced group GATA-4; 2. Induced group Nkx 2.5; 3. Induced group TDGF-l; 4. Control group GATA-4; 5. Control group Nkx 2.5; 6. Control group TDGF-l.
Figure 10. Bovine pericardium after treatment with 0.625% genipin solution presented light blue.
Induced BMSCs were attached on the surface of bovine pericardium, and the levels were distinctive. It was demonstrated that induced BMSCs could be attached on the surface of acellular bovine pericardium (Figure 11).
4. DISCUSSION
At present, all kinds of repair materials applied during CHD surgery would lack contractility and growth potential, and have the risk of calcification and thrombus-poietic, which often requires repeat operations in the future [17]. In addition, effective treatment of heart failure resulting from ischemic heart disease would have been still a challenge. Unlike other tissues, myocardium has no substitute which could be used to repair myocardial defect or damaged cardiac tissue. With the development of cell culture technology in vitro and the conception of tissue engineering, myocardial regenerative medicine has become an exciting therapy for treatment of myocardial injury [18]. Construction of tissue engineered myocardium in vitro has become a challenging target in cardiovascular tissue engineering following cell transplant.
It was demonstrated that MSCs, Endothelial Progenitor Cells, Skeletal Myoblasts, Embryonic Stem Cells, etc., could be induced to Myocardial cells in vitro or in vivo [19]. BMSCs are a kind of primitive bone marrow stromal cells, and have properties such as self-renewal, amplification and muti-directional differentiation potentiality. Under certain conditions, they could be differentiated into many kinds of histiocytes [20]. MSCs have become one of the hotspots as seeding cells in cardiac tissue engineering [21]. However, MSCs content in bone marrow is very low. When it is near higher density during the period of cell culture, cells enter into platform, and the morphology converts from a spindle-like form to a bigger, flatter shape [22]. The capability of cell proliferation and differentiation could be influenced. When constructing the engineered cardiac tissue, first of all, we need sufficient cells and their good differentiation capability. Thus, a means of obtaining the sufficient cells and good differentiation capability would be necessary to rationalize MSCs use.
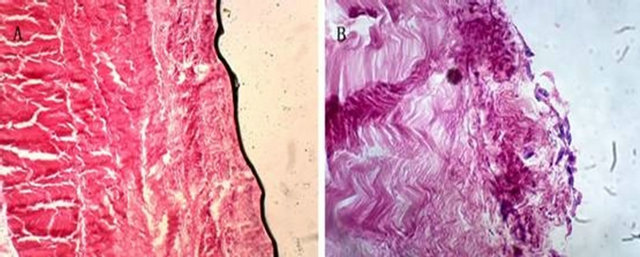
Figure 11. Acellular bovine pericardium before and after seeded with BMSCs (100×). Note: (A) Acellular bovine pericardium before seeded with induced BMSCs; (B) Acellular bovine pericardium after seeded with induced BMSCs.
In this study, we have demonstrated that cell passages had influence on BMSCs quantity, multiplication capacity, differentiation capacity and purity. The multiplication capacity of the second generation was superior to that of the sixth or tenth generation, but no difference occured between the sixth and tenth. In the same generation, such as the second, the sixth and tenth generation, different 5-Aza concentrations had no influence on multiplication capacity. With the increase of cell passages, 5-Aza concentration had a decreasing influence on multiplication capacity. BMSCs induced with 5-Aza expressed cardiac-specific protein. Qian demonstrated that the expression rate of Cardiac-specific protein in tenth generation MSCs was superior to that of the first, fourth or eighth generation after induced with 5-Aza [23]. Therefore, in the case of the same proliferation capability, there is a bigger cell quantity and stronger differentiation capacity with the increase of cell passages. In this study, we tried to use the induced BMSCs of third generation as the seeding cells for constructing myocardium-like tissue in vitro.
So far, there are several methods to induce MSCs to cardiomyocytes, including a co-culture system to mimic myocardial microenvironment [24], physical stimulation [18], and chemical compound induction. Among these methods, 5-Aza, a chemical compound, has been regarded as an effective agent for inducing differentiation of MSCs into cardiomyocytes in vitro. However, the optimal 5-Aza concentration, MSCs quantity, long-term safety and efficacy of the differentiated MSCs in vivo still need to be studied and further confirmed.
Scaffold material is another difficult point when constructing the engineered cardiac tissue in vitro [25]. In recent years, it has been found that acellular scaffold material was one kind of bio-derived material which has good properties, with a natural microenvironment and the following advantages [26]: low antigenicity, good biological chemotaxis, involved in tissue healing process, cellular and vascular forming, adjusted degradation time, etc. Chang et al. [27] has demonstrated that acellular bovine pericardium crosslinked with genipin, a natural cross-linking agent, had lesser inflammatory reaction than with the traditional methods when it was implanted in vivo, and it could afford a good natural microenvironment for cell migration and tissue regeneration.
Bovine pericardium is mainly made of I type collagen, which reaching up to 95%. Bovine pericardium is characterized by good flexibility, little antigenicity, good biocompatibility, etc. MSCs adherence with acellular bovine pericardium scaffolds have two types, non-specific adhesion and specific adhesion. Non-specific adhesion is decided by physical property of bio-material and cell surface. It is called specific adhesion if it is involved in the specific interactions of surface molecules among them. As far as we know, there are 4 types of cell surface adhesion molecules: Cadherin, Integrin, Immunoglobulin superfamily adhesion molecule and Select lectin [28]. It was demonstrated that cell adherence could be increased with scaffolds preloaded with polylysine, bFGF, etc., which promoted cell growth and proliferation also [29].
In this study, we have discussed BMSCs could be induced and differentiated into cardiomyocytes-like cells via treated with 5-Aza in vitro, and cell generations and 5-Aaz concentration influence on BMSCs’ capability of proliferation and differentiation into cardiomyocytes. We have optimized the method of the cellular seeding on scaffolds. We used third generation BMSCs as the seeding cells, and used the classical 5-Aza induction method, then bFGF in culture media to promote BMSCs differentiation to cardiomyocytes. We seeded induced BMSCs in the static inoculation on the acellular bovine pericardium which had been coated with Poly-L-Lysine when constructing the engineered caidiac tissue in vitro. The experimental method was reliable, and the procedure was simple. Induced BMSCs could express cardiac-specific proteins such as troponin T, α-Actin and Early myocardial transcription factors such as GATA-4, Nkx 2.5, TDGF-l. Furthermore, we have preliminarily confirmed the possibility of constructing the engineered cardiac tissue with adult stem cells and the acellular bovine pericardium in vitro, but still had some limitations such as no cultivation in vivo and no further detection of engineered myocardium-like tissue. However, this study has established the basis for further constructing tissue engineered myocardium and repairing the damaged heart in the future.
5. ACKNOWLEDGEMENTS
In this study, Wanhong Xing has duty of research design, data analysis and interpretation. Lingping Tian has afforded some concept about the research, and done the Statistics for data. Ning Wang and Jianhong Zhang have done cell culture, differentiation and construction of engineered cardiac tissue in vitro. Ping Chen has done the research of acellular bovine pericardium biomaterial and constructed the engineered cardiac tissue in vitro. Jie Ma has done the Critical revision of this article. Research Project Supported by Shanxi Scholarship Council of China (2012091) and Shanxi Science and Technology Agency, China (20120313021-3) is gratefully acknowledged.
REFERENCES
- Ding, W.-X. (2000) The review and prospect of pediatric heart surgery in China. Chinese Journal of Pediatric Surgery, 21, 266.
- Sakai, T., Li, R.K., Weisel, R.D., Mickle, D.A., Kim, E.T., Jia, Z.Q. and Yau, T.M. (2001) The fate of a tissue-engineered cardiac graft in the right ventricular outflow tract of the rat. The Journal of Thoracic and Cardiovascular Surgery, 121, 932-942. doi:10.1067/mtc.2001.113600
- Mirsadraee, S., Wilcox, H.E., Watterson, K.G., Kearney, J.N., Hunt, J., Fisher, J. and Ingham, E. (2007) Biocompatibility of acellular human pericardium. Journal of Surgical Research, 143, 407-414. doi:10.1016/j.jss.2007.01.026
- Young, H.E., Mancini, M.L., Wright, R.P., Smith, J.C., Black Jr., A.C., Reagan, C.R. and Lucas, P.A. (1995) Mesenchymal stem cells reside within the connective tissues of many organs. Developmental Dynamics, 202, 137-144. doi:10.1002/aja.1002020205
- Prockop, D.J. (1997) Marrow stromal cells as stem cells for nonhematopoietic tissues. Science, 276, 71-74. doi:10.1126/science.276.5309.71
- Kern, S., Eichler, H., Stoeve, J., Klüter, H. and Bieback, K. (2006) Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells, 24, 1294-1301. doi:10.1634/stemcells.2005-0342
- Wakitami, S., Saito, T. and Caplan, A.I. (1995) Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve, 18, 1417- 1426. doi:10.1002/mus.880181212
- Tomita, S., Li, R.K., Weisel, R.D., Mickle, D.A., Kim, E.J., Sakai, T. and Jia, Z.Q. (1999) Autologus transplantation of bone marrow cells improves damaged heart function. Circulation, 100, 247-256.
- Xu, W., Zhang, X., Qian, H., Zhu, W., Sun, X., Hu, J., Zhou, H. and Chen, Y. (2004) Mesenchymal stem cells from adult human bone marrow differentiate into a cardiomyocyte phenotype in vitro. Experimental Biology and Medicine, 229, 623-631.
- Ishii, O., Shin, M., Sueda, T. and Vacanti, J.P. (2005) In vitro tissue engineering of a cardiac graft using a degradable scaffold with an extracellular matrix-like topography. The Journal of Thoracic and Cardiovascular Surgery, 130, 1358-1363. doi:10.1016/j.jtcvs.2005.05.048
- Guo, X.M., Zhao, Y.S., Chang, H.X., Wang, C.Y., E, L.L., Zhang, X.A., Duan, C.M., Dong, L.Z., Jiang, H., Li, J., Song, Y. and Yang, X.J. (2006) Creation of engineered cardiac tissue in vitro from mouse embryonic stem cells. Circulation, 113, 2229-2237. doi:10.1161/CIRCULATIONAHA.105.583039
- Capi, O. and Gepstein, L. (2006) Myocardial regeneration strategies using human embryonic stem cell-derived cardiomyocytes. Journal of Controlled Release, 116, 211- 218. doi:10.1016/j.jconrel.2006.06.027
- Cheng, M., Park, H., Engelmayr, G.C., Moretti, M. and Freed, L.E. (2007) Effects of regulatory factors on engineered cardiac tissue in vitro. Tissue Engineering, 13, 2709-2719. doi:10.1089/ten.2006.0414
- Leor, J., Aboulafia-Etzion, S., Dar, A., Shapiro, L., Barbash, I.M., Battler, A., Granot, Y. and Cohen, S. (2000) Bioengineered cardiac grafts: A new approach to repair the infarcted myocardium. Circulation, 102, 56-61.
- Ishii, O., Shin, M., Sueda, T. and Vacanti, J.P. (2005) In vitro tissue engineering of a cardiac graft using a degradable scaffold with an extracellular matrix-like topography. The Journal of Thoracic and Cardiovascular Surgery, 130, 1358-1363. doi:10.1016/j.jtcvs.2005.05.048
- Courtman, D.W., Pereira, C.A., Kashef, V., McComb, D., Lee, J.M. and Wilson, G.J. (1994) Development of a pericardial acellular matrix biomaterial: Biochemical and mechanical effects of cell extraction. Journal of Biomedical Materials Research, 28, 655-666. doi:10.1002/jbm.820280602
- Mirensky, T.L. and Breuer, C.K. (2008) The development of tissue-engineered grafts for reconstructive cardiotho racic surgical applications. Pediatric Research, 63, 559- 568. doi:10.1203/01.pdr.0000305938.92695.b9
- Ge, D., Liu, X., Li, L., Wu, J., Tu, Q., Shi, Y. and Chen, H. (2009) Chemical and physical stimuli induce cardiomyocyte differentiation from stem cells. Biochemical and Biophysical Research Communications, 381, 317-321. doi:10.1016/j.bbrc.2009.01.173
- Yuasa, S. and Fukuda, K. (2008) Cardiac regenerative medicine. Circulation Journal, 72, 49-55. doi:10.1253/circj.CJ-08-0378
- Pittenger, M.F., Mackay, A.M., Beck, S.C., Jaiswal, R.K., Douglas, R., Mosca, J.D., Moorman, M.A., Simonetti, D.W., Craig, S. and Marshak, D.R. (1999) Multilineage potential of adult human mesenchymal stem cells. Science, 284, 143-147. doi:10.1126/science.284.5411.143
- Psaltis, P.J., Zannettino, A.C., Worthley, S.G. and Gronthos, S. (2008) Concise review: Mesenchymal stromal cells: Potential for cardiovascular repair. Stem Cells, 26, 2201-2210. doi:10.1634/stemcells.2008-0428
- Sekiya, I., Larson, B.L., Smith, J.R., Pochampally, R., Cui, J.G. and Prockop, D.J. (2002) Expansion of human adult stem cells from bone marrow stroma: Conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells, 20, 530-541. doi:10.1634/stemcells.20-6-530
- Qian, H.Y. (2007) Experimental study of cardiomyocyte differentiation from porcine bone marrow mesenchymal stem cell in vitro and optimized transplantation to treat acute myocardial infarction in vivo. Ph.D. Thesis, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing.
- Yoon, J., Shim, W.J., Ro, Y.M. and Lim, D.S. (2005) Transdifferentiation of mesenchymal stem cells into cardiomyocytes by direct cell-to-cell contact with neonatal cardiomyocyte but not adult cardiomyocytes. Annals of Hematology, 84, 715-721. doi:10.1007/s00277-005-1068-7
- Akhyari, P., Kamiya, H., Haverich, A., Karck, M. and Lichtenberg, A. (2008) Myocardial tissue engineering: The extracellular matrix. European Journal CardioThoracic Surgery, 34, 229-241. doi:10.1016/j.ejcts.2008.03.062
- Kanematsu, A., Yamamoto, S., Ozeki, M., Noguchi, T., Kanatani, I., Ogawa, O. and Tabata, Y. (2004) Collagenous matrices as release carriers of exogenous growth factors. Biomaterials, 25, 4513-4320. doi:10.1016/j.biomaterials.2003.11.035
- Chang, Y., Tsai, C.C., Liang, H.C. and Sung, H.W. (2002) In vivo evaluation of cellular and acellular bovine pericardia fixed with a naturally occurring crosslinking agent (genipin). Biomaterials, 23, 2447-2457. doi:10.1016/S0142-9612(01)00379-9
- Wang, S. and Li, W.-B. (2009) Current situation and prospect in the study of acellular tissue engineering heart valve. China Medical Device Information, 15, 13-21.
- Salacinski, H. J., Tiwari, A., Hamilton, G. and Seifalian, A.M. (2001) Cellular engineering of vascular bypass grafts: role of chemical coatings for enhancing endothelial cell attachment. Medical & Biological Engineering & Computing, 39, 609-618. doi:10.1007/BF02345431

