Journal of Encapsulation and Adsorption Sciences
Vol.07 No.01(2017), Article ID:74558,27 pages
10.4236/jeas.2017.71003
Adsorption-Desorption of BTX (Benzene, Toluene and O-Xylene) on Fe, Fe-Al Pillared Clay
Zohra Mèçabih
Laboratory of Materials and Catalysis, Department of Chemistry, Faculty of Exact Sciences, University of Djillali Liabes, Sidi Bel Abbes, Algeria

Copyright © 2017 by author and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: January 5, 2017; Accepted: March 3, 2017; Published: March 6, 2017
ABSTRACT
The studies are conducted in laboratory to determine the adsorption-desorp- tion behavior of BTX (benzene, toluene and o-xylene) in gas phase on Fe, Fe-Al pillared clays adsorbents. In experimental conditions of constant atmospheric pressure, initial concentrations with an increasing volume (0.5 - 2 ml) injected benzene (2.25), toluene (1.89) and o-xylene (1.66) μmol/L at T (40˚C, 60˚C and 80˚C), and the adsorption increases with increase of temperature, indicating that the adsorption process would be a chemical adsorption rather than physical one. The results are shown that the BTX adsorption data fitted very well (R2 > 0.999) to the both equations Langmuire and Elovitch for the three samples: bentonite (B), Fe-bentonite ( ) and Fe-Al/bentonite (
) and Fe-Al/bentonite (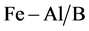 ). At 80˚C, the BTX adsorption capacity increased in the following order:
). At 80˚C, the BTX adsorption capacity increased in the following order: . The maximum adsorption capacity (
. The maximum adsorption capacity ( ) at 80˚C is 175.13, 171.84 and 171.81 μg/g respectively for benzene, toluene and o-xylene for
) at 80˚C is 175.13, 171.84 and 171.81 μg/g respectively for benzene, toluene and o-xylene for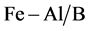 ; the last is a good adsorbent of BTX removal. The benzene diffuses faster than toluene and o-xylene. Thermodynamic parameters, such as
; the last is a good adsorbent of BTX removal. The benzene diffuses faster than toluene and o-xylene. Thermodynamic parameters, such as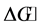 ,
, 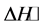 and
and 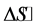 are also discussed and the results suggested that the BTX adsorption on all samples used is a spontaneous and endothermic process. Desorption studies show that BTX is very easily desorbed with
are also discussed and the results suggested that the BTX adsorption on all samples used is a spontaneous and endothermic process. Desorption studies show that BTX is very easily desorbed with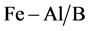 .
.
Keywords:
Fe, Al-Pillared, Benzene, Toluene, O-Xylene, Adsorption

1. Introduction
The natural gas and oil industry activities are known for some time to create harmful air emissions that emit volatile organic compounds (VOCs) and oxides of nitrogen (NOX), which are precursors to tropospheric ozone. The major VOCs, the benzene, toluene and xylene (BTX) volatile compounds are significant environmental concern and are listed as priority pollutants by the United States Environmental Protection Agency (US EPA) [1] because of their toxic and carcinogenic effects on humans. Besides, the benzene is already known as the leukemia agent in humans [2] . Another, VOCs characterized by their photochemical activity could undergo a series of photochemical reaction to from the secondary organic aerosol, which is one of the major components of airborne fine particles. The VOCs cause environmental concerns about their toxicity and malodor, even at very low concentrations, due to the obvious impacts on atmosphere and human health; it is necessary to limit and control this air emission. The difficulty, for decreasing the VOCs in gas phase at very low concentrations, requires a highly optimized process. Various processes can be used for abatement of VOCs which are broadly classified into two types: destruction (biofiltration, thermal oxidation, catalytic oxidation, reverse flow reactor) and recovery (adsorption, condensation, membrane separation) [3] . The adsorption by solid adsorbents is one of the best solutions for this treatment; the choice of adsorbent depends on these adsorptive properties and availability. Granular or powdered actived carbon is the most widely used adsorbent [4] , but their use is usually limited due to their high cost. Over several decades, many researchers show their interests in searching for low-cost adsorbents with excellent adsorption characteristics, such as zeolites [5] , organokaolinite [6] , smectite [7] , hectorite [8] , organosilica [9] , and montmorillonite [10] [11] . The pillared interlayer clay (PILC) attracts attention of many researchers, and constitutes one of the most widely studied series among the microporous materials with a wide range of potential applications in adsorption processes. PILCs are formed by insertion of polynuclear inorganic cation into their interlayer space, followed by calcinations to give stable metal oxide pillars (e.g. Al2O3, Fe2O3 etc.) having larger micropores. The purpose of this paper is to present the effectiveness of (Fe, Fe-Al)-pillared bentonite clay adsorbents to reduce the concentration of BTX and determine behavior of BTX with evaluating the influence of the temperature on BTX adsorption. Adsorption isotherm is measured at three different temperatures: 40˚C, 60˚C and 80˚C.
2. Materials and Methods
2.1. Materials
The natural clay used in this work is a bentonite type from Maghnia (west Algerian). It is supplied by the Algeria Bentonite Company (ENOF). The natural bentonite is purified in laboratory [12] [13] , using a sedimentation method to obtain the < 2 μm montmorillonite rich faction. The carbonates are removed by sodium acetate/chloridric acid, iron oxide by sodium thiosulfate/sodium chloride and organic materials by hydrogen peroxide (30% vol.). To ensure complete transformation into the sodium from all samples, they are washed several times with 0.5 M NaCl. The exchange capacity or CEC 91 meq/100g (by methylene bleu exchange).
2.2. Preparation of Hydroxyl-Al
The pillaring solution of Al and Fe polycation are prepared separately [13] . 0.207 M NaOH solution is added slowly while stirring to a 0.207 M AlCl3 solution until it reached an  molar ratio of 2.5 in the mixture. The mixture is aged at room temperature during 6 days at room temperature.
molar ratio of 2.5 in the mixture. The mixture is aged at room temperature during 6 days at room temperature.
2.3. Preparation of Hydroxyl-Fe
Fe polycations solution is prepared by slowly adding a 0.1 M NaOH solution to 0.1 M FeCl3 solution under vigorous stirring, until the  ratio reaches the value 2.5. The mixture is aged for two weeks at room temperature [13] .
ratio reaches the value 2.5. The mixture is aged for two weeks at room temperature [13] .
2.4. Preparation of Fe-Al Pillared Bentonite
The pillaring solution containing hydroxyl-Al oligocations and hydroxyl-Fe oligocations are slowly added under vigorous stirring into the suspension purified bentonite while, until the mass ratio of M3+ (M3+ = Fe3+, Al3+)/caly reached 6.25% [13] . The solids are filtered and washed with deionized water until it are free of Cl− ions. The solids B, 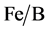 and
and 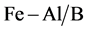 are calcined for 2 h at 300˚C ground and sieved.
are calcined for 2 h at 300˚C ground and sieved.
High-purity BTX: benzene (99 wt.%, Aldrich), toluene (99.5 wt.%, Aldrich) and o-xylene (99 wt.%, Aldrich) are used absorbate.
2.5. Characterization
The Analysis of the chemical composition of the purified bentonite is obtained fluorescence X. The surface area is measured with a Micromeritics ASAP 2010 instrument by adsorption of nitrogen at 77 K. Before measurement, the samples are degassed under vacuum of 20.8 Pa at 120˚C for 2.
2.6. BTX Adsorption Kinetics
1 g of the samples in the nacelle is placed in glass enclosure (10 L), closed and thermostated degassed for 2 h using the means of a water-jet pump. Then, 2 ml of BTX (benzene, toluene and o-xylene) containing respectively, 2.25, 1.89 and 1.66 μmol/L is sprayed into the enclosure by injection (Figure 1). After, the nacelles are removed from the enclosure and the samples are weighed. The experiments are carried out at 40˚C, 60˚C and 80˚C in a temperature controlled bath (Figure 1).
2.7. Equilibrium Isotherm
1 g of the samples in the nacelle is placed in glass enclosure (10 L), closed and thermostated degassed for 2 h using the means of a water-jet pump. Then, different volume ranging from (0.5 - 2 ml) is sprayed into enclosure by injection of liquid BTX: benzene, toluene and o-xylene with initial concentration respectively 2.25, 1.89 and 1.66 μmo/L. The experiments are carried out at 40˚C, 60˚C and 80˚C in a temperature controlled bath (Figure 1). After reaching the adsorption equilibrium, the nacelles are removed from the enclosure and the samples
Figure 1. Experimental set-up for the adsorption of the BTX.
weighed by Sartorius 1219 MP balance type (accuracy ± 10−3 g). The amount of adsorbed BTX on adsorbents (qe μg/g) is calculated as follows:
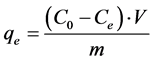 (1)
(1)
where 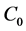 and
and 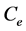 are the initial and equilibrium BTX concentrations (μg/L), respectively; V is initial liquid volume (L) equal to glass enclosure volume; and m is the adsorbent weight (g).
are the initial and equilibrium BTX concentrations (μg/L), respectively; V is initial liquid volume (L) equal to glass enclosure volume; and m is the adsorbent weight (g).
2.8. Desorption Experiments
For desorption experiments, the nacelle in glass enclosure is subjected before to degassing for 2 h at constant pressure. 1 g of the samples are saturated in benzene, toluene and o-xylene of concentration 2.25, 1.89 and 1.66 μmol/L respectively are desorbed for 2 h and at temperature T (40˚C, 60˚C and 80˚C) (Figure 1). The amounts of BTX retained are obtained from the difference between the initial concentration (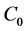 ) and the final concentration (
) and the final concentration (

3. Results and Discussion
3.1. Characterization of the Adsorbent
The chemical composition of purified bentonite by X-ray fluorescence is reported in Figure 2, the results collected in Table 1 showed the silica to alumina ration (
The N2 adsorption/desorption isotherm of purified bentonite is shown in Figure 3. The adsorption isotherm is of type IV according to (B.D.D.T) classification, which is generally associated with capillary condensation in mesopore structures, with a well-defined H4 hysteresis loop. This behavior is the indication of a mono-multilayer adsorption on slit-shaped pores among plate-like particles [14] . The opening behaviors of the hysteresis loop indicated the formation
Figure 2. Spectrum of purified bentonite by X-ray fluoresence.
Table 1. Chemical analyses of purified bentonite by X-rays fluorescence.
of irregular shape pores. This opening demonstrated the presence of mesopores in the purified bentonite. The inset of Figure 3 is the pore size distribution of the bentonite purified, in which different volume is plotted against pore size for the desopriton branches of the N2 adsorption/desorption isotherms according to the BJH model [15] . The results are given in Table 2, for purified bentonite the total pore volume and micropore volume are 0.103 and 0.027 cm3/g, respectively. The increase of the specific surface area (Table 2) after pillaring with Fe, Fe-Al polycation solutions, suggest also the increasing of the micropores [16] .
3.2. Adsorption Kinetics of BTX on Purified and Pillared Bentonite
Figure 4 presents the kinetic curves of all samples used at different temperatures.
Figure 3. N2 adsorption/desorption isotherms and pore size distribution of the purified bentonite.
Table 2. Textural properties of the samples used calcined at 300˚C.
Figure 4. Adsorption kinetics of BTX onto samples used at various temperature.
It can be seen that the adsorption capacity increased with contact time, quickly in the first 15 min and then increased gradually with increasing contact time until the adsorption reached adsorption equilibrium at 3 h. It can also be observed that the lower the temperature is, the lower the saturated adsorption capacity is. When the adsorption reached the equilibrium at 80˚C, the adsorption capacity (


The results showed that the adsorption capacity increased with an increase of temperature, indicating the adsorption process is endothermic and the adsorption process would be a chemical adsorption rather than a physical one.
3.3. Equilibrium Isotherms
For to assess efficacies for the three adsorbents: B, 


where Ce (μg/L) is the equilibrium concentration; qe (μg/g) is the equilibrium amount of BTX adsorbed; qm (μg/g) is a maximum adsorption capacity, KL (L/μg/) is the adsorption equilibrium constant. Equation (4) can be linearized into the form as follows:

The results obtained by the applying the Freundlich model is not presented because the low values correlation coefficients (R2 < 0.99) show poor agreement of Freundlich isotherm with the experimental data.
The adsorption isotherms are presented in Figure 5, from the results shown in Table 3 where, the Langmuir constants 







Figure 5. Equilibrium adsorption of BTX onto samples used at various temperature.
Table 3. Langmuir and Elovitch isotherms constants at different temperatures for the adsorption of BTX onto samples used.
Figure 6. Linear plot Langmuir isotherm of BTX onto samples used at various temperature.
Table 4. Thermodynamic parameters for the adsorption of BTX onto samples used.
strong interaction between all samples used and the molecular structure of these aromatic hydrocarbons compounds. Also, at 80˚C, the maximum capacity 






In multilayer adsorption, it is supposed that molecules are adsorbed in several layers on the adsorption surface. One of the equations that predicted multilayer adsorption with unlimited layers is Elovich equation [13] : Equation (5):

where 




The values of Elovitch maximum adsorption capacity (





3.4. Desorption of BTX
Figure 8 presented the desorption rate. The results shown that desorption are increased with increase the temperature, at 80˚C desorption of 


3.5. Adsorption Thermodynamics
In any adsorption process, namely free energy (




Figure 7. Linear plot Elovitch isotherm of BX onto samples used at various temperature.
Figure 8. Desorption of BTX onto samples used.
of an adsorption, considering the adsorption equilibrium constant 


where 






After integration, the integrated form of Equation (9) becomes:

where Y is a constant Equation (10) can be rearranged to obtain;

Let:

Substituting Equation (11) and Equation (12), 

The equilibrium constant, 







4. Conclusions
The study of adsorption of aromatic BTX hydrocarbons on B, 



Figure 9. Plot of Gibbs free energy change, 
model provides the best fit to the experimental data with high correlation coefficient (
The adsorption is easy for benzene compared with toluene and o-xylene. The Gibbs free energy (

It may be concluded that pillared bentonite may be used as a low-cost, natural and abundant source for the elimination of aromatic BTX hydrocarbons.
Cite this paper
Mèçabih, Z. (2017) Adsorption-Desorption of BTX (Benzene, Toluene and O-Xylene) on Fe, Fe-Al Pillared Clay. Journal of Encapsulation and Adsorption Sciences, 7, 40-66. https://doi.org/10.4236/jeas.2017.71003
References
- 1. U.S. Environment Protection Agency Office of Air Quality (2000) National Air Toxics Program: The Integrated Urban Strategy. Report to Congress, EPA 453-R-99-007.
http://www.epa.gov/urban-air-toxics - 2. Kuran, P. and Sojak, L. (1996) Environmental Analysis of Volatile Organic Compounds in Water and Sediment by Gas Chromatography. Journal of Chromatography A, 733, 119-141.
http://10.1016/0021-9673(95)01121-8 - 3. Alice, O.R. and Emil, D. (2003) Destruction of Volatile Organic Compounds by Catalytic Oxidation. Environmental Engineering and Management Journal, 4, 273-302.
http://omicron.ch.tuiasi.ro/EEMJ/ - 4. Daifullah, A.A.M. and Girgis, B.S. (2003) Impact of Surface Characteristics of Active Carbon on Adsorption of BTEX. Colloids and Surfaces A: Physicochemical and Engineering, 214, 181-193.
https://doi.org/10.1016/S0927-7757(02)00392-8 - 5. Blocki, S.W. (1993) Hydrophobic Zeolites Adsorbent: A Proven Advancement in Solvent Separation Technology. Technology Environmental Progress, 12, 226-230.
https://doi.org/10.1002/ep.670120312 - 6. Egbuchunama, T.O., Obia, G., Okieimenb, F.E. and Tihminliogluc, F. (2016) Removal of BTEX from Aqueous Solution Using Organokaolinite. International Journal of Applied Environmental Sciences, 11, 505-513.
http://www.ripublication.com - 7. Carvalho, M.N., Da Motta, M., Benachour, M., Sales, D.C.S. and Abreu, C.A.M. (2012) Evaluation of BTEX and Phenol Removal from Aqueous Solution by Multi-Solute Adsorption onto Smectite Organoclay. Journal of Hazardous Materials, 240, 95-101.
https://doi.org/10.1016/j.jhazmat.2012.07.057 - 8. Jaynes, W.F. and Vances, G.F. (1999) Sorption of Benzene, Toluene, Ethylbenzene, and Xylene (BTEX) Compounds by Hectorite Clays Exchanged with Aromatic Organic Cations. Clays and Clay Minerals, 47, 358-365.
https://doi.org/10.1346/CCMN.1999.0470312 - 9. Moura, C.P., Vidal, C.B., Barros, A.L., Costa, L.S., Vasconcellos, L.C.G., Dias, F.S. and Nascimento, R.F. (2011) Adsorption of BTX (Benzene, Toluene, o-Xylene, and p-Xylene) from Aqueous Solutions by Modified Periodic Mesoporous Organosilica. Journal of Colloid Interface Science, 363, 626-634.
https://doi.org/10.1016/j.jcis.2011.07.054 - 10. Sharmasarkar, S., Jaynes, W.F. and Vance, G.F. (2000) BTEX Sorption by Montmorillonite Organoclay. Water Air Soil Pollution, 119, 257-273.
https://doi.org/10.1023/A:1005167524630 - 11. Nourmoradi, H., Nikaeen, M. and Khiadani, M. (2012) Removal of Benzene, Toluene, Ethylbenzene and Xylene (BTEX) from Aqueous Solutions by Montmorillonite Modified with Non-Ionic Surfactant: Equilibrium, Kinetic and Thermodynamic Study. Chemical Engineering Journal, 191, 341-348.
https://doi.org/10.1016/j.cej.2012.03.029 - 12. Méçabih, Z., Kacimi, S. and Bouchikhi, B. (2006) Adsorption des eaux usées urbaines sur la bentonite modifiée par Fe(III), Al(III) et Cu(II). Revue Sciences Eaux, 19, 23-31.
http://10.7202/012261ar - 13. Méçabih, Z., Rose, J. and Borschneck, D. (2014) Urban Wastewater Treatment by Adsorption of Organic Matters on Modified Bentonite by (Iron-Aluminium). Journal of Encapsulation and Adsorption Sciences, 4, 71-79.
https://doi.org/10.4236/jeas.2014.43008 - 14. Rauquerol, F., Rauquerol, J. and Sing, K. (1999) Adsorption by Powders and Porous Solids: Principles, Methodology and Application. Academic Press, San Diego.
- 15. Barrett, E.P., Joyner, L.G. and Halenda, P.P. (1951) The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. Journal of American Chemical Society, 73. 373-380.
https://doi.org/10.1021/ja01145a126 - 16. Bankovic, P., Milutinovic, N.A., Jovic, J.N., Dostanic, J., Cupic, Z., Loncarevic, D. and Jovanovic, D. (2009) Synthesis, Characterization and Application of AlFe-Pil- lared. Acta Physica Polonica A, 4, 811-815.
https://doi.org/10.12693/APhysPolA.115.811 - 17. Hernandez, M.A., Corona, L., Gonzalez, A.I., Rojas, F., Lara, V.H. and Silva, F. (2005) Quantitative Study of the Adsorption Aromatic Hydrocarbons (Benzene, Toluene, and p-Xylene) on Dealuminated Clinoptiloties. Industrial Engineering Chemistry Research, 44, 2908-2916
https://doi.org/10.1021/ie049276w - 18. Song, H., Cheng, W., Jing, H., Fuxing, G. and Ho, Y.S. (2009) Adsorption Thermodynamics of Methylene Blue onto Bentonite. Journal of Hazardous Materials, 167, 630-633.
https://doi.org/10.1016/j.jhazmat.2009.01.014 - 19. Fei, Y., Jie, M. and Yanqing, W. (2011) Adsorption of Toluene, Ethylbenzene and m-Xylene on Multi-Walled Carbon Nanatubes with Different Oxygen Content from Aqueous Solutions. Journal of Hazardous Materials, 192, 1370-1379.
https://doi.org/10.1016/j.jhazmat.2011.06.048



















