Journal of Encapsulation and Adsorption Sciences
Vol.4 No.1(2014), Article ID:43455,10 pages DOI:10.4236/jeas.2014.41003
Preparation of Microcapsules Containing Aqueous Solution of Azur B with Melting Dispersion Cooling Method and Application to DNA Amplification Detector
Yoshinari Taguchi, Ryohei Yamamoto, Natsukaze Saito, Masato Tanaka*
Department of Chemistry and Chemical Engineering, Niigata University, Niigata, Japan
Email: *tanaka@eng.niigata-u.ac.jp
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 30 January 2014; revised 20 February 2014; accepted 27 February 2014
ABSTRACT
Microcapsules containing the aqueous solution of Azur B of a water soluble dye were prepared with the melting dispersion cooling method and applied to the amplification detector of plant DNA. Paraffin wax with melting temperature of 75˚C was used as the shell material. In the experiment, the aqueous solution (W) of Azur B as the core material was dispersed in the melted paraffin wax (O) to form the (W/O) emulsion and then, the (W/O) emulsion was dispersed in the silicon oil (O’) as the continuous phase to form the (W/O)/O’ emulsion at 85˚C. After formation of the (W/O)/O’ emulsion, the microcapsules were prepared by cooling the (W/O)/O’ emulsion to 50˚C. The microcapsules were prepared by changing the concentration of oil soluble surfactant in the (W/O) emulsion and the volume of the (W/O) emulsion in the (W/O)/O’ emulsion. The microencapsulation efficiency increased with the concentration of oil soluble surfactant and finally became 100% under the optimum conditions. Furthermore, the microcapsules were melted down at temperature of 85˚C to reveal the sharp thermal responsibility and to release the aqueous solution of Azur B. As a result, it was found that the microcapsules were able to be applied to the amplification detector of plant DNA by utilizing the reaction between DNA and Azur B.
Keywords
Microcapsules; Azur B; DNA Amplification Detector; Melting Dispersion Cooling Method; Multiple Emulsion

1. Introduction
Microcapsules have various functions such as protection and isolation of core material, instantaneous and controlled release of core material, surface modification of core material, solidification of liquid and gaseous core materials, responsibility to various stimuli and so on [1] [2] .
Thus, the microcapsules have prepared mainly with both the chemical and the physicochemical methods and have been applied in the various fields such as cosmetics [3] , paintings [4] [5] , adhesive [6] , medical and dental supplies [7] [8] , information recording materials [9] , food materials [10] -[12] , agricultural materials [13] , drugs [14] [15] and so on.
Stimuli-responsibility of their functions is the most important one, because the core materials are able to be released according to the stimulus species such as mechanical pressure [16] , temperature [17] , pH of solution [18] [19] , specific enzyme [20] .
Azur B of a water soluble dye is known to singularly react with plant DNA and to be applied to detect plant DNA, namely, to utilize as the Amplification Detector of plant DNA [21] [22] .
If the aqueous solution of Azur B could be microencapsulated with the shell material to reveal the thermal responsibility and these microcapsules could be dispersed stably in the aqueous solution of plant DNA, the concentration of DNA in the aqueous solution should be able to be detected instantaneously by destroying the microcapsules due to heating and releasing the aqueous solution of Azur B without direct contact to the detecting system. For this purpose, it is necessary to prepare the microcapsules by the shell material with the sharp responsibility to the desired temperature and the perfect water proof.
In this study, paraffin wax is selected as the shell material to satisfy these demands and the melting dispersion cooling method is adopted to prepare the microcapsules [20] . In this microencapsulation process, it is extremely important to form the stable multiple emulsion.
In general, the (W/O)/W emulsion as the multiple emulsion has been utilized for preparing the microcapsules containing the aqueous solution [13] [16] . However, when the core material is water soluble or hydrophilic, the water phase is not suitable to the continuous phase, because the core material is easily leaked from the shell particle in the microencapsulation process. As a result, the content of core material should be decreased. Taking these things into consideration, the silicon oil as the stronger hydrophobic material is adopted as the continuous phase in this study.
The purposes of this study are to develop the method for preparing the microcapsules containing the aqueous solution of water soluble dye, to investigate the effects of the preparation conditions on the microencapsulation efficiency, the content of aqueous solution and the thermal responsibility and to apply to the DNA amplification detector.
2. Experimental
2.1. Materials
The materials used in this study are as follows. Paraffin wax (O) with melting temperature of 75˚C was used as the shell material. Azur B of a water soluble dye with molecular structure as shown in Figure 1 was used as the core material, which is known to singularly react with plant DNA.
The silicon oil (KF400, Shinetsu Chemical Co, Ltd) was used as the continuous phase (O’). As the oil soluble surfactants, Poem PR-100 and Poem J0021 (Riken Vitamin Co, Ltd) were added in the melted shell material to form the (W/O) dispersion and in the continuous phase to form the (W/O)/O’ emulsion, respectively.
2.2. Preparation of Microcapsules
Figure 2 shows the flow chart for preparing the microcapsules. The aqueous solution (W) dissolving the given amount of Azur B was dispersed in paraffin wax (O) by stirring with the rotor-stator homogenizer to form the (W/O) emulsion. Then, this (W/O) emulsion was dispersed in the continuous phase of silicon oil (O’) by stirring with the six bladed disc turbine impeller to form the (W/O)/O’ emulsion. This operation was conducted at temperature of 85˚C to melt paraffin wax.
When the (W/O)/O’ emulsion was cooled down to 50˚C, the microcapsules were prepared due to solidification of paraffin wax of the shell material. In this preparation process, the volume of aqueous solution of Azur B in paraffin wax (hold up in the (W/O) emulsion) and the concentration (Cs) of oil soluble surfactant (PoemPR- 100) in the (W/O) emulsion were changed stepwise. The experimental conditions are shown in Table 1. Here, the hold up in the (W/O) emulsion is defined as the ratio of the volume of aqueous solution to the total volume
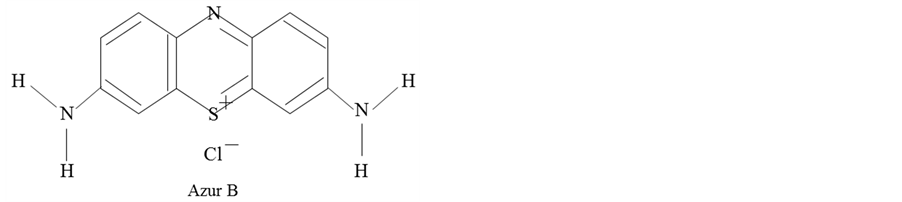
Figure 1. Molecular structure of Azur B..
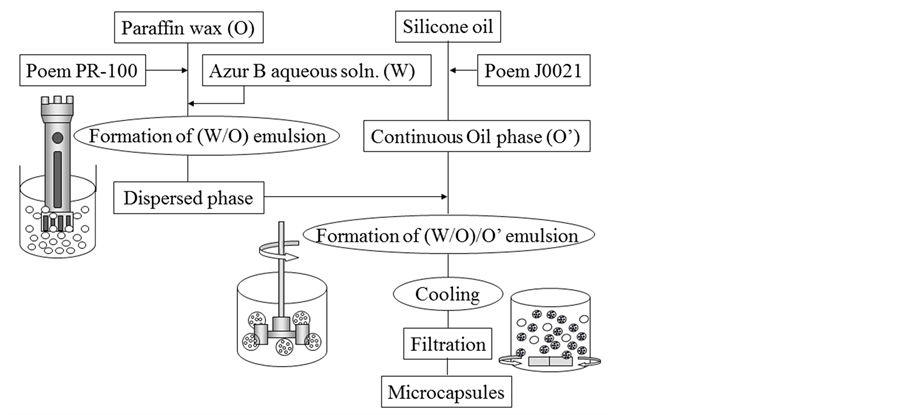
Figure 2. Flow chart for preparing microcapsules.
of paraffin wax and aqueous solution. Also, the holdup of φ = 0 means that only powder of Azur B is added in paraffin wax.
2.3. Characterization of Microcapsules
2.3.1. Content of Aqueous Solution of Azur B and Microencapsulation Efficiency
The microcapsules were destroyed in the water phase with temperature of 85˚C to release the aqueous solution of Azur B and then, the concentration of Azur B in the aqueous solution sampled out was measured by the spectrophotometry method.
For this, the correlation curve between the absorption degree and the concentration of Azur B was drawn beforehand.
The microencapsulation efficiency was estimated from the content and the amount of Azur B added by the following equation (1).
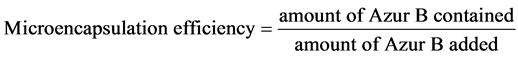 (1)
(1)
2.3.2. Leakage of Aqueous Solution of Azur B
The microcapsules of a given weight were added in the water phase. Then, the constant volume of water was sampled out at the constant time interval and the concentration of Azur B in the water phase was measured by the same method as stated just above.
The leakage ratio was obtained by the following equation (2).
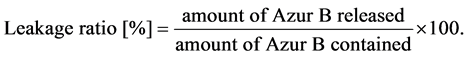 (2)
(2)
Table 1. Experimental conditions.
2.3.3. Thermal Responsibility of Microcapsules
The thermal responsibility of microcapsules was estimated by differential scanning calorimeter (DSC; Shimazu Co., DSC-50).
Namely, it was investigated whether the concentration of oil soluble surfactant affected the thermal responsibility of microcapsules or not.
2.3.4. Observation of Microcapsules
Microcapsules were observed by optical microscope (OLYMPUS Co., BX51).
2.4. Application of Microcapsules to DNA-Amplification Detector
The microcapsules thus prepared were applied to the DNA-Amplification Detector as shown in Figure 3. Namely, when a given amount of the microcapsules containing the aqueous solution of Azur B was added in the DNA aqueous solution and destroyed by heating, the color of the DNA+ aqueous solution should be changed due to the reaction of Azur B with DNA. The degree of change in color of the DNA aqueous solution is dependent on the concentration of DNA.
If the microcapsules are added in the water phase (blank), in which DNA is not dissolved, and destroyed by heating, the color of water may become blue due to releasing of Azur B.
On the other hand, if the microcapsules are broken in the DNA aqueous solution, the color of DNA aqueous solution may become light blue due to the reaction between DNA and Azur B.
3. Results and Discussion
3.1. Effect of Hold up in the (W/O) Emulsion
Figure 4 shows the dependence of the content of core material on the hold up in the (W/O) emulsion, where the microcapsules were prepared at the concentration of Cs = 2.0 wt%. In Figure 4, Φ = 0 means that powder of Azur B was directly added in the melted shell. With increasing the hold up, the content increases gradually, comes to the maximum (4.1) at Φ = 0.39 and then, decreases.
As the maximum value of content is close to that (4.3) of the content on the basis of feed, the (W/O) or the (W/O)/O’ emulsion should be stable under the preparation conditions such as the hold up and the concentrations of both surfactants.
Figure 5 shows the photographs of microcapsules prepared by changing the hold up in the (W/O) emulsion. It is found that all the microcapsules prepared at Φ = 0.39 show nearly uniform blue color due to the highest content of aqueous solution of Azur B and the microcapsules prepared under the other hold up show uneven blue color. There are a few microcapsules without the aqueous solution of Azur B at Φ = 0.56.
Figure 6 shows the transient features of the leakage ratio of Azur B from the microcapsules prepared under the various hold up in the (W/O) emulsion and the concentration of Cs = 2.0 wt% of oil soluble surfactant. The leakage ratio for all the microcapsules begins from just after immersion of the microcapsules into the water phase.
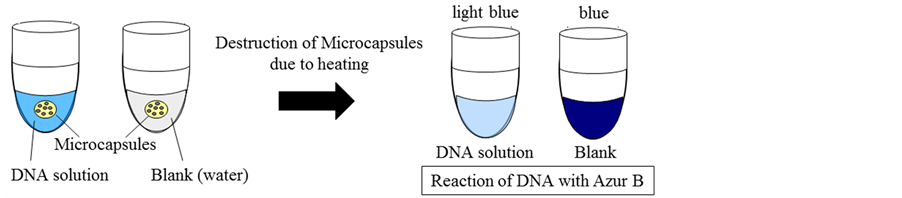
Figure 3. Application of microcapsules containing aqueous solution of Azur B to DNA detector.
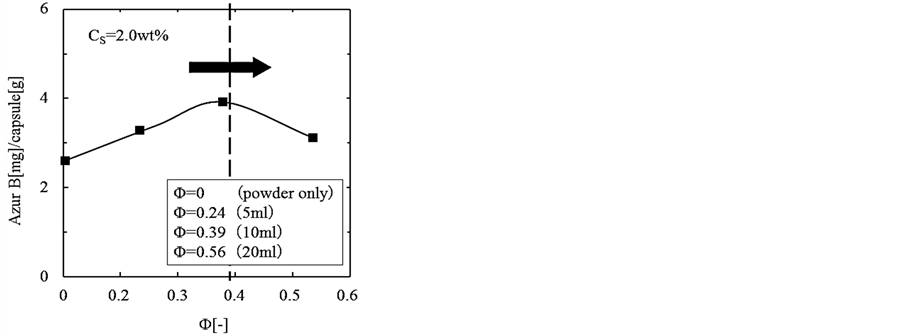
Figure 4. Dependence of content of core material on hold up in (W/O) emulsion.
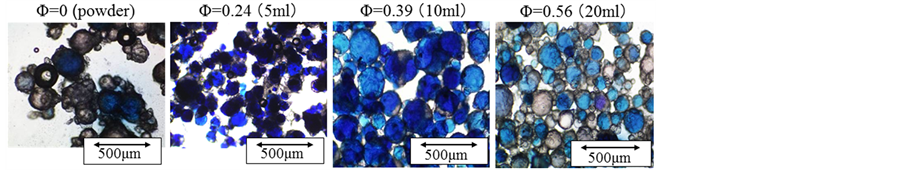
Figure 5. Photogtaphs of microcapsules (effect of hold up in (W/O) emulsion).
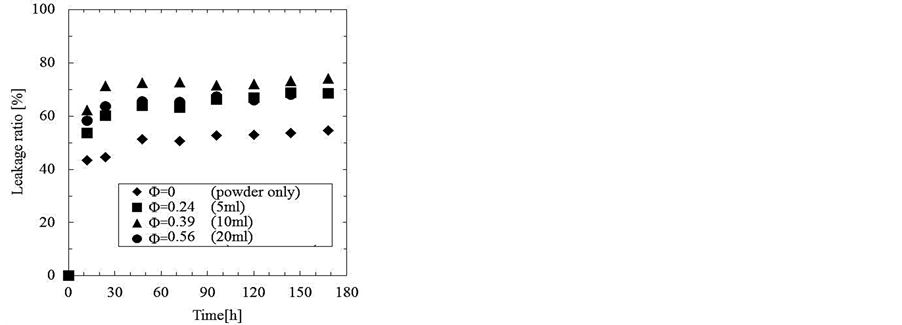
Figure 6. Transient features of leakage ratio of Azur B from microcapsules prepared with various hold up in (W/O) emulsion.
It may be considered that these leakage features are due to a great lack of stability of water droplets in the microcapsules.
Figure 7 shows the color of microcapsules before and after immersion into the water phase. The color of microcapsule changes from blue to dark due to the leakage of the aqueous solution of Azur B.
From these results, it is found that there is a great lack of protection and isolation of core material for the microcapsules prepared under the conditions as presented above (especially CS = 2.0 wt%).
3.2. Effect of Concentration of Oil Soluble Surfactant
To improve the defects of microcapsules as stated above, it was tried to investigate the effect of the concentration of oil soluble surfactant in the (W/O) emulsion on the stability of water droplet in the (W/O) droplets.
Figure 8 shows the photographs of microcapsules prepared at the concentrations of oil soluble surfactant of Cs = 2.0 wt% and 10 wt%. Because the microcapsules show the uniform blue color, the aqueous solution of Azur B is microencapsulated satisfactorily.
Next, the leakage in the water phase was investigate.
Figure 9 shows the transient features of the leakage ratio of Azur B from the microcapsules prepared by changing the concentration of oil soluble surfactant in the (W/O) emulsion. The leakage ratios are found to considerably decrease with the concentration of oil soluble surfactant. Especially, the leakage ratio becomes very low at the concentration of oil soluble surfactant of Cs = 10 wt% because of extreme improvement of stability of waterdroplets in the (W/O) droplets. As the leakage ratio is kept almost constant after t = 20 min, the observed leakage ratio may be due to the initial burst of microcapsules.
Figure 10 shows the dependence of the content of core material on the concentration of oil soluble surfactant in the (W/O) emulsion. From this result, it is found that the content slightly increases with the concentration and is almost equal to the content (4.3) calculated on the basis of feed. The leakage of aqueous solution of Azur B has to be perfectly prevented in the water phase before destruction of the microcapsules at Cs = 10 wt%. Accordingly, it is confirmed that the droplets of aqueous solution of Azur B are able to be stably microencapsulated.
3.3. Thermal Responsibility of Microcapsules
Figure 11 shows the results obtained by DSC for the microcapsules prepared with the various concentrations of oil soluble surfactant.
From these results, it is found that all the microcapsules have the same melting point of ca. T = 74˚C and the oil soluble surfactant dissolved in the shell material does not affect the thermal responsibility of microcapsules.
Figure 12 shows the results of observing the thermal responsibility of microcapsules. The release of the aqueous solution of Azur B was observed by heating the microcapsules in the water phase. Only the microcapsules heated to 85˚C were destroyed down and released the aqueous solution of Azur B. Thus, the microcapsules are found to reveal the sharp thermal responsibility.
Figure 13 shows the quantitative results of measuring the thermal responsibility of microcapsules. Namely, the concentration of Azur B in the water phase was measured before and after heating at each temperature. From these results, it is found that the microcapsules are broken at 85˚C and release the aqueous solution of Azur B as the core material in an instant. The scanty leakage ratios at 45˚C and 65˚C may be due to the initial burst as stated above. No leakage ratio was observed after immersion in the water phase at temperature lower than 85˚C.
3.4. Application to DNA Amplification Detector
It was tried to apply the microcapsules to the DNA-Amplification Detector as follows.
The microcapsules of the same weight were added into the eight test tubes in which the DNA aqueous solution of different concentration was poured beforehand.
When each test tube was heated to 85˚C, the microcapsules were destroyed to release the aqueous solution of Azur B.
DNA in the water phase reacts with Azur B and results in change in color of the DNA aqueous solution.
Figure 14 shows the color of DNA aqueous solution before (T = 65˚C) and after (T = 85˚C) heating the microcapsules. After heating to 85˚C, the color of the DNA aqueous solution at the lower concentration region of each test tube becomes blue due to releasing of Azur B.

Figure 7. Photographs of microcapsules before and after immersion into water phase.
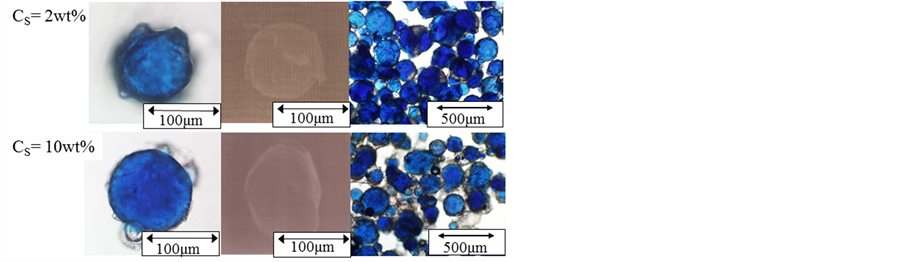
Figure 8. Photographs of microcapsules prepared at Cs = 2.0 and 10 wt% (φ = 0.39).

Figure 9. Transient features of the leakage ratio of Azur B from the microcapsules prepared by changing the concentration of oil solublesurfactant (φ = 0.39).
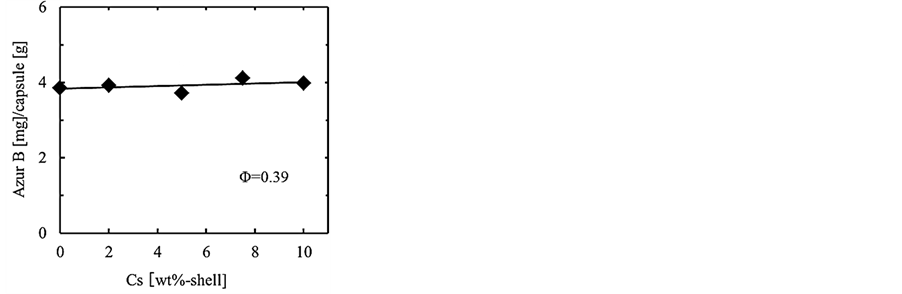
Figure 10. Dependence of content of core material on concentration of oil soluble surfactant.
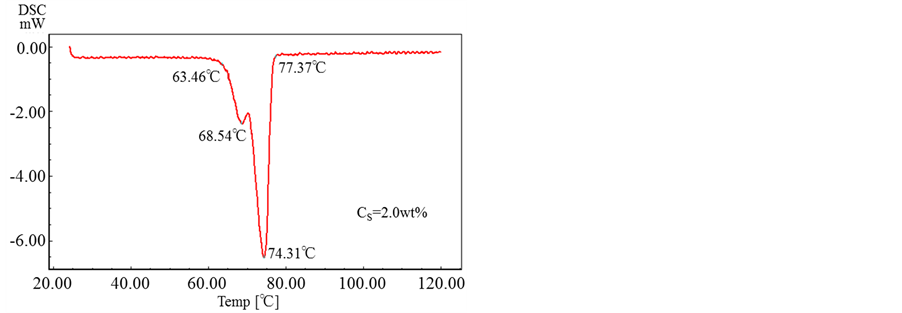
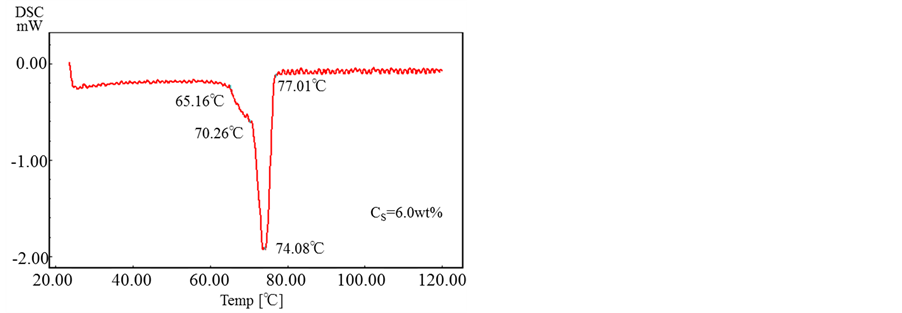
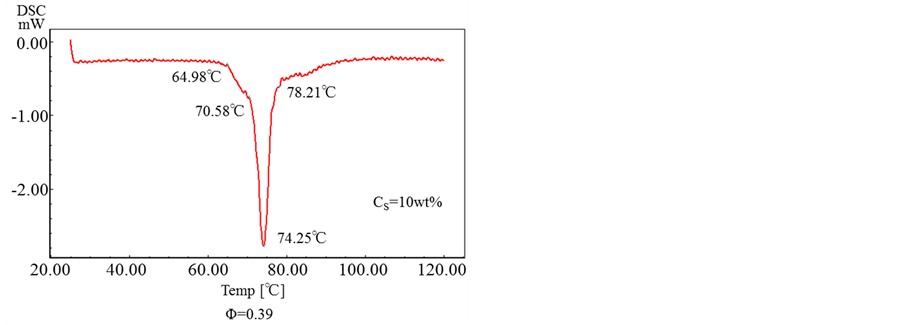
Figure 11. DSC of microcapsules prepared with various concentrations of oil soluble surfactant..

Figure 12. Thermal responsibility of microcapsules prepared with Cs = 10 wt% (φ = 0.39).
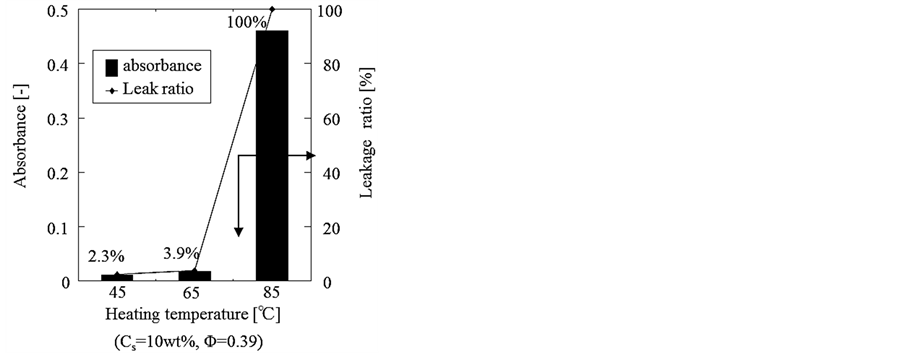
Figure 13. Estimation of thermal responsibility.
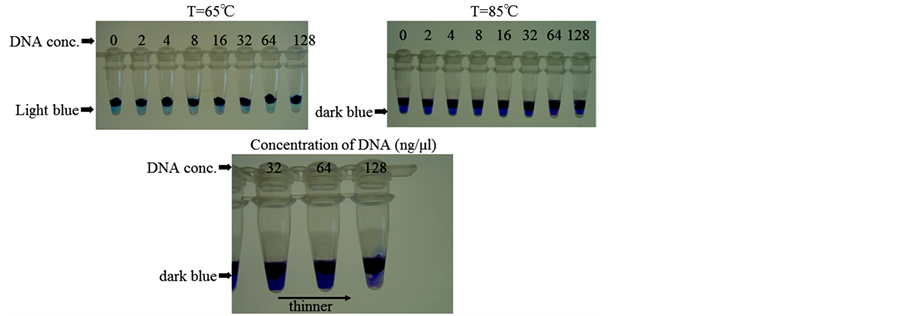
Figure 14. Application of microcapsules to DNAAmplification Detector.
However, the higher the concentration of DNA, the thinner the color of the DNA aqueous solution due to the reaction of DNA with Azur B becomes (as shown in the lower photographs in Figure 14).
This result must reveal that the microcapsules containing the aqueous solution of Azur B are able to be applied to the DNA amplification detector.
4. Conclusion
The thermal responsible microcapsules containing the aqueous solution of Azur B were able to be prepared with the melting dispersion cooling method by use of paraffin wax as the shell material. The content of aqueous solution of Azur B was close to the maximum value calculated on the basis of feed under the conditions such as the hold up of 0.39 and the concentration of 10 wt% of oil soluble surfactant in the (W/O) emulsion. The microcapsules revealed the sharp thermal responsibility and were found to be able to be applied to the DNA amplification detector.
References
- Kondo, T. (1967) Microencapsulation Technique. ETS, Tokyo.
- Tanaka, M. (2008) Key Point of Preparation of Nano/Microcapsules. Techno System Publishing Co. Ltd., Tokyo.
- Shirokawa, K., Taguchi, Y., Yokoyama, H., Ono, F. and Tanaka, M. (2013) Preparation of Temperature and Water Responsive Microcapsules Containing Hydroquinone with Spray Drying Method. Journal of Cosmetics, Dermatological Sciences and Applications, 3, 49-54. http://dx.doi.org/10.4236/jcdsa.2013.33A2012
- Yuan, L., Gu, A., Nutt, S., Wu, J., Lin, C., Chen, F. and Liang, G. (2013) Novel Polyphenylene Oxide Microcapsules Filled with Epoxy Resins. Polymers for Advanced Technologies, 24, 81-89. http://dx.doi.org/10.1002/pat.3053
- Zhu, D.Y., Rong, M.Z. and Zhang, M.Q. (2013) Preparation and Characterization of Multiayered Microcapsule-Like Microreacter for Self-Healing Polymers. Polymer, 54, 4227-4236. http://dx.doi.org/10.1016/j.polymer.2013.06.014
- Sakai, K., Fuchigami, K., Taguchi, Y. and Tanaka, M. (2007) Microencapsulation of Aqueous Solution of Hardening Agent for Adhesive Based on Starch. Nippon Setchaku Gakkaishi, 43, 13-19.
- Taguchi, Y. and Tanaka, M. Microencapsulation of Ascorbic Acid as Redox Initiator with Tripalmitin. International Journal of Material Science, In Press.
- Fuchigami, K., Taguchi, Y. and Tanaka, M. (2006) Preparation of Microcapsules Containing Reactive Compound by the Drying-in-Liquid Method Using Calcium Carbonate as Stabilizer. Journal of Chemical Engineering of Japan, 39, 994-999. http://dx.doi.org/10.1252/jcej.39.994
- Sawatari, N., Fukuda, M., Taguchi, Y. and Tanaka, M. (2005) Composite Polymer Particles with a Gradated ResinComposition by Suspension Polymerization. Journal of Applied Polymer Science, 97, 682-690. http://dx.doi.org/10.1002/app.21823
- Xiao, J.X., Huang, G.Q., Wang, S.Q. and Sun, Y.T. (2014) Microencapsulation of Capsanthin by Soybean Protein Isolate-Chitosan Coacervation and Microcapsule Stability Evaluation. Journal of Applied Polymer Science, 131, 39671- 39677. http://dx.doi.org/10.1002/app.39671
- Chen, H. and Zhong, Q. (2014) Processes Improving the Dispersibility of Spray-Dried Zein Nanoparticles Using Sodium Caseinate. Food Hydrocolloids, 35, 358-366. http://dx.doi.org/10.1016/j.foodhyd.2013.06.012
- Guo, L., Yuan, W., Lu Z. and Li, C.M. (2013) Polymer/Nanosilver Composite Coatings for Antibacterial Applications. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 439, 69-83.
- Takahashi, T., Taguchi, Y. and Tanaka, M. (2005) Preparation of Polyurea Microcapsules Containing Pyrethroid Insecticide with Hexamethylene Diisocyanate Uretidione and Isocyanurate. Journal of Chemical Engineering of Japan, 38, 929-936. http://dx.doi.org/10.1252/jcej.38.929
- Sophocleous, A.M., Desai, K.G., Mazzara, J.M., Tong, L., Cheng, J.X., Olsen K.F. and Schwendeman, S.P. (2013) The Nature of Peptide Interactions with Acid End-Group PLGAs and Facile Aqueous-Based Microencapsulation of Therapeutic Peptides. Journal of Controlled Release, 172, 662-670. http://dx.doi.org/10.1016/j.jconrel.2013.08.295
- Xia, Y., Ribeiro, P.F. and Pack, D.W. (2013) Controlled Protein Release from Monodisperse Biodegradable DoubleWall Microspheres of Controllable Shell Thickness. Journal of Controlled Release, 172, 707-714. http://dx.doi.org/10.1016/j.jconrel.2013.08.009
- Yokoyama, H., Taguchi, Y. and Tanaka, M. (2008) Effect of Viscosity of Shell Solution on the Content of Solid Powder as Core Material in Microencapsulation by the Drying-in-Liquid Method. Journal of Applied Polymer Science, 109, 1585-1593. http://dx.doi.org/10.1002/app.24765
- Gui, R., Wang, Y. and Sun, J. (2014) Encapsulating Magnetic and Fluorescent Mesoporous Silica into Thermosensitive Chitosan Microspheres for Cell Imaging and Controlled Drug Release in Vitro. Colloids and Surfaces B: Biointerfaces, 113, 1-9. http://dx.doi.org/10.1016/j.colsurfb.2013.08.015
- Vivek, R., Babu, V.N., Thangam, R., Subramanian, K.S. and Kannan, S. (2013) pH-Responsive Drug Delivery of Chitosan Nanoparticles as Tamoxifen Carriers for Effective Anti-Tumor Activity in Breast Cancer Cells. Colloids and Surfaces B: Biointerfaces, 111, 117-123. http://dx.doi.org/10.1016/j.colsurfb.2013.05.018
- Lay, C.L., Kumar, J.N., Liu, C.K., Lu X. and Liu, Y. (2013) A Rocket-Like Encapsulation and Delivery System with Two-Stage Booster Layers: pH-Responsive Poly(Methacrylic Acid)/Poly(Ethylene Glycol) Complex-Coated Hollow Silica Vesicles. Macromolecular Rapid Communications, 34, 1563-1568. http://dx.doi.org/10.1002/marc.201300529
- Chan, G. and Mooney, D.J. (2013) Ca2+ Released from Calcium Alginate Gels Can Promote Inflammatory Responses in Vitro and in Vivo. Acta Biomaterialia, 9, 9281-9291. http://dx.doi.org/10.1016/j.actbio.2013.08.002
- Notomi, T., Okayama, H., Masubuchi, H., Yonekawa, T., Watanabe, K., et al. (2000) Loop-Mediated Isothermal Amplification of DNA. Nucleic Acids Research, 28, 63-71. http://dx.doi.org/10.1093/nar/28.12.e63
- Okuda, M., Matsumoto, M. and Tanaka, Y. (2005) Characterization of the tufB-secE-nusG-rp/KAJL-rpoB Gene Cluster of the Citrus Greening Organism and Detection by Loop-Mediated Isothermal Amplification. Plant Disease, 89, 705-711.
NOTES

*Corresponding author.


