Journal of Cosmetics, Dermatological Sciences and Applications
Vol. 3 No. 2 (2013) , Article ID: 32977 , 6 pages DOI:10.4236/jcdsa.2013.32023
Evaluation of the Efficacy and Safety for Atopic Dry Skin of a Medicinal Moisturizing Lotion (Mentholatum AP Soft Medicinal Moisture Lotion) Prepared by a High-Pressure Emulsification Method
![]()
1Department of Dermatology, Queen’s Square Medical Center, Yokohama, Japan; 2Ochiai Yuriko Dermatology Clinic, Sendai, Japan.
Email: *t.omi@queens-sq.or.jp
Copyright © 2013 Tokuya Omi, Yuriko Ochiai. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received March 27th, 2013; revised April 28th, 2013; accepted May 6th, 2013
Keywords: atopic dermatitis; nanoparticle; TEWL; vaseline; moisturizer
ABSTRACT
In order to evaluate the efficacy and safety of Mentholatum®AP Soft Lotion (APA), which is a Vaseline-based preparation that has been converted to nanoparticles by high-pressure emulsification technology, we conducted a study of 4-week continuous use on the atopic dry skin of patients with minor to moderate atopic dermatitis (AD). The results showed that improvements in the skin findings (dryness and scaling) and itching were observed beginning one week after starting to apply APA and that the symptoms had almost completely resolved at 3 to 4 weeks after the start of application. In addition, a significant increase in the degree of high-frequency conductivity, which is an indicator of the amount of moisture in the stratum corneum, and significant decrease in transepidermal water loss (TEWL) were observed, and improvements in skin functions were observed. The results for overall degree of improvement showed “improvement” or better in 100% of the subjects, and no side effects were observed in any of the cases. Based on the above results, it was concluded that APA is a preparation that has excellent efficacy and safety as skin care for atopic dry skin, in which reduced stratum corneum function is observed in the skin, and as a supplemental method of treatment of AD or treatment method to maintain remissions.
1. Introduction
Symptoms of xerosis of the skin, so-called atopic dry skin, are often seen as a predisposing condition in atopic dermatitis (AD). Moreover, because the barrier function of the stratum corneum of skin placed in dry conditions is reduced [1], it is a state in which allergens, various irritating substances, etc., easily invade the skin. Pruritus develops as a result of these irritants, scratching the skin in turn causes inflammation, and that leads to the development or the aggravation of dermatitis. Thus, treating atopic dry skin with humectants, which can protect the stratum corneum from dryness and hydrate it, will prevent the development of AD, and doing so is considered necessary to increase the therapeutic efficacy of treating AD with adrenocorticosteroid hormones (steroids), etc.
More specifically, this means performing skin care with urea ointments and preparations containing heparinnoids, hyaluronic acid, amino acids, glycerin, etc., as a means of keeping the stratum corneum moist, and with Vaseline and preparations containing squalene, camellia oil, olive oil, synthetic ceramides, etc., to maintain the barrier function of the stratum corneum, but Vaseline, preparations containing heparinoids, urea preparations, etc., are used in medical institutions, in particular, as humectants for AD. One of them, Vaseline, is widely used because it is not irritating and has high sealing performance, but it has a sticky feel when used, and it is difficult to apply over rather wide areas. A Vaselinebased preparation that has been converted into nanoparticles by means of high-pressure emulsification technology (Figure 1) is an improved preparation. Although the nanoparticle preparation is Vaseline-based, it has been reported not to be very sticky, and not only to be a milky, smooth preparation that spreads easily, but to have a
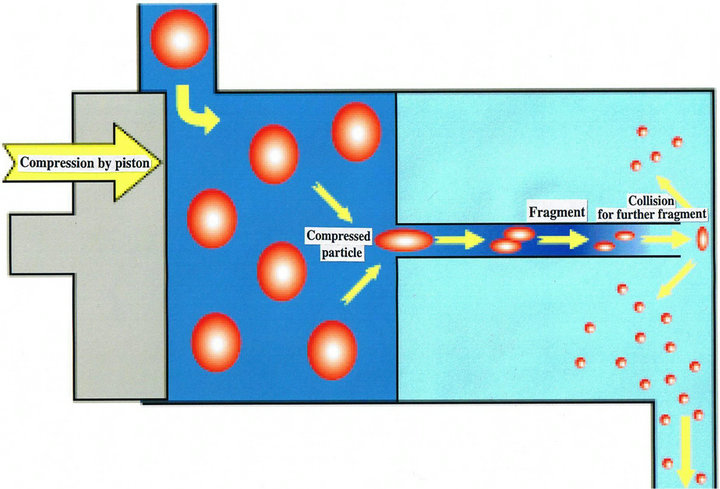
Figure 1. High-pressure emulsification technology.
strong hydrating effect in addition to the protective action that the Vaseline originally possessed [2].
In the present study we conducted a 4-week continuous use study of a quasi-drug lotion (Mentholatum®AP Soft Moisture Lotion [APA]) created by the same technology for the purpose of daily skin care as a treatment for the atopic dry skin of patients with up to minor to moderate AD, and we assessed the efficacy and safety of APA, including an objective evaluation of the stratum corneum function by measuring skin functions [3,4].
2. Methods
2.1. Period of the Study and Study Sites
This study was conducted over a 4-month period from February 2012 to May 2012 at two sites, the Department of Dermatology of Queen’s Square Medical Center (Yokohama city) and the Ochiai Yuriko Dermatology Clinic (Sendai city).
2.2. Study Product
The test product we used was the nanoparticle preparation APA (Rohto Pharmaceutical Co. Ltd.), a white quasi-drug lotion that contains the active ingredients dicalcium glycyrrhizinate and allantoin, and is based on Vaseline, glycerin, etc.
2.3. Subjects
The subjects of this study were 20 patients, male and female, between the ages of 3 and 60 years old, who had been diagnosed with minor AD or mild to moderate AD (10 at each site) in the remission phase, and the study was conducted after obtaining the informed consent of the subjects or their proxies. We excluded patients whose disease was considered to be severe judged on the basis of the definition or diagnostic criteria for AD, patients with poor disease control, patients who were taking adrenocorticosteroid hormones, immunosuppressive drugs, etc., patients who were taking drugs that might affect the evaluation of the study, and patients who were pregnant or breast-feeding.
In principle, newly treating with antihistaminic drugs or anti-allergy drugs during the study period was prohibited. Use of other humectants (including quasi-drugs and cosmetics) and topical drugs at the application sites was also prohibited. This study was approved by the ethics committee of Queen’s Square Medical Center, and it was conducted in accordance with ethical principles based on the Declaration of Helsinki.
2.4. Method of Application and Application Period
The application sites were determined according to the patient, and, in principle, APA was applied to the site 2 or 3 times daily for 4 weeks. The application site was in the upper limb in 8 patients, the lower limb in 9 patients, and the trunk in 3 patients.
The application sites were evaluated in relation to the following parameters at the start of the study, after 1 week, after 2 weeks, after 3 weeks, and after 4 weeks.
2.4.1. Skin Findings
The severity of dryness and scaling was evaluated by using the following 5-grade scale: +++: severe (4), ++: moderate (3), +: mild (2), ±: minor (1), −: absent (0).
2.4.2. The Symptom Itching
The degree of itching was evaluated on a 0-to-10 visual analogue scale (VAS) before the start of the study and at 1 week, 2 weeks, 3 weeks, and 4 weeks after the start of the study.
2.4.3. Evaluation of Stratum Corneum Functions by Making Measurements with Instruments
Stratum corneum functions at the application site of the 10 patients who were the subjects of the study at the Department of Dermatology of Queen’s Square Medical Center were evaluated by making measurements with instruments. Throughout the period of the study the room temperature and humidity of the examination room were maintained at a temperature of around 23.5˚C and relative humidity of around 49% by the building’s airconditioning system. High-frequency conductivity, which is an indicator of the water content of the stratum corneum, and transepidermal water loss (TEWL), which is an indicator of the barrier function of the stratum corneum, were measured with a SKICON®200 EX hygrometer (IBS Co., Ltd.) and a VapoMeter®(Delfin Technologies, Ltd.), respectively.
2.5. Statistics
Efficacy in regard to each of the symptoms was evaluated by statistically analyzing the symptom scores for the two skin findings at 1 week, 2 weeks, 3 weeks, and 4 weeks after the start of the study in comparison with their scores at the start of the study, by the Wilcoxon rank-sum test.
Efficacy in alleviating the symptom itching was evaluated by using the t-test to statistically analyze the changes in the VAS scores for itching between the start of the study and at 1 week, 2 weeks, 3 weeks, and 4 weeks after the start of the study.
Hydrating effect on the stratum corneum and restorative effect on the barrier function of the stratum corneum were evaluated by using the t-test to statistically analyze the changes in high-frequency conductivity and TEWL values between the start of the study and at 2 weeks and 4 weeks after the start of the study.
2.6. Degree of Overall Improvement
The clinical findings and the symptom itching at 1 week, 2 weeks, 3 weeks, and 4 weeks after the start of the study were evaluated in comparison with their values at the start of the study on the following 5-grade scale: 1) great improvement, 2) improvement, 3) slight improvement, 4) no change, 5) worse.
2.7. Safety
Safety was evaluated on the following 4-grade scale in view of the side effects and their severity on the day the study was completed: 1) safe, 2) almost completely safe, 3) there is a safety problem, 4) unsafe.
2.8. Patients’ Impression
At the conclusion of the study the patients’ impression in regard to the product was surveyed based on an interview and their journals, and their impression was evaluated on the following 5-grade scale: 1) very good, 2) good, 3) somewhat good, 4) no change, 5) not good.
3. Results
The study was conducted on 20 patients, and their background factors are shown in Table 1.
3.1. Skin Findings According to Symptom
The changes in mean scores for the skin findings according to symptom are shown in Table 2 and Figure 2.
The scores for the symptom dryness were 3.1 ± 0.1 at the start of the study, 1.7 ± 0.1 after 1 week, 0.9 ± 0.2 after 2 weeks, 0.4 ± 0.1 after 3 weeks, and 0.5 ± 0.1 after 4 weeks, and a significant improvement in the score in
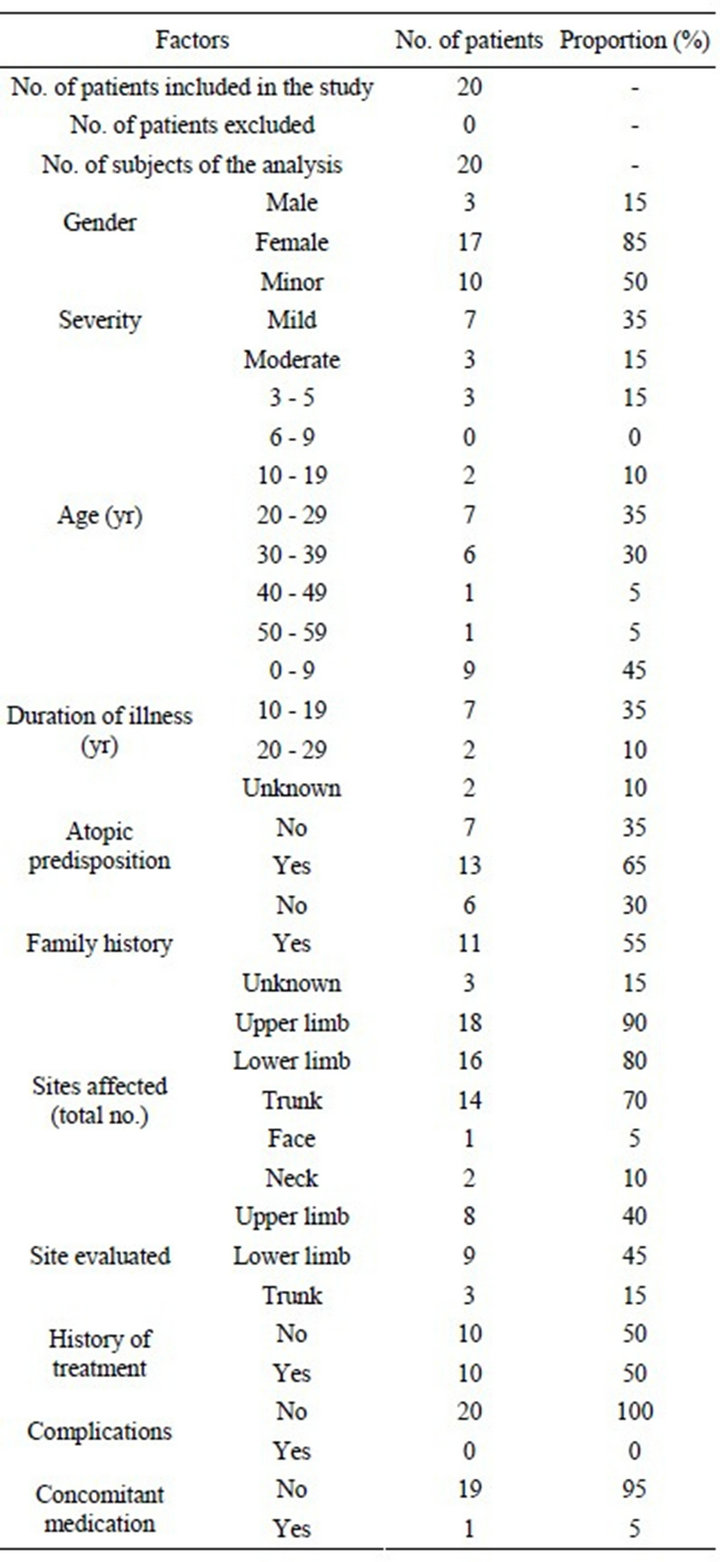
Table 1. Patient background factors.
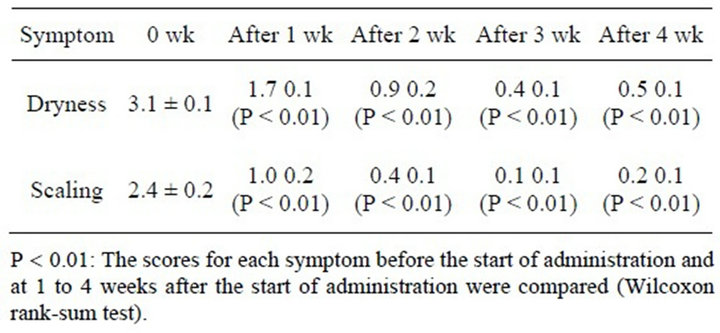
Table 2. Mean skin finding scores according to symptom.
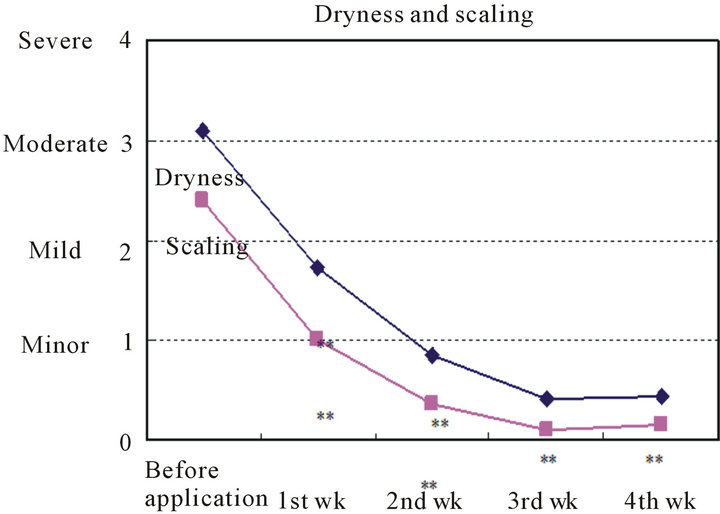
Comparisons between the scores for each of the symptoms before treatment and after 1, 2, 3, and 4 weeks of treatment.
Figure 2. Changes in skin finding scores according to symptom.
comparison with the score at the start of treatment observed was observed at every time point (P < 0.01). The improvements in scores as of 1 week and 2 weeks after the start of treatment were particularly marked.
The scores for the symptom scaling were 2.4 ± 0.2 at the start of the study, 1.0 ± 0.2 after 1 week, 0.4 ± 0.1 after 2 weeks, 0.1 ± 0.1 after 3 weeks, and 0.2 ± 0.1 after 4 weeks, and a significant improvement in the score for scaling in comparison with the score at the start of the study was also observed at every time point (P < 0.01), and the improvements in score as of 1 week and 2 weeks after the start of treatment were particularly marked.
3.2. The Symptom Itching
The changes in mean VAS scores for the symptom itching are shown in Table 3 and Figure 3.
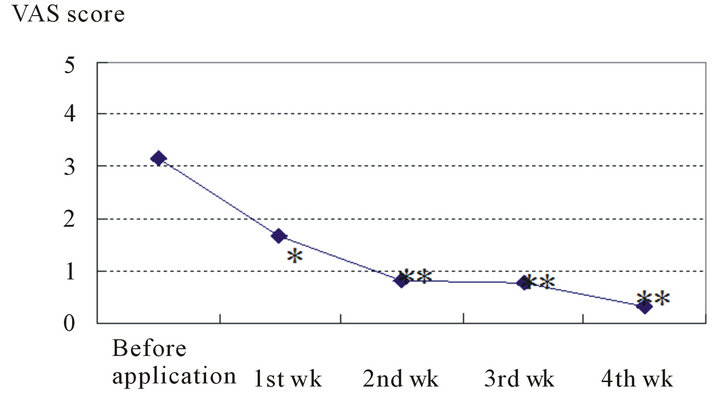
The VAS scores were 3.2 ± 0.5 at the start of the study, 1.7 ± 0.4 after 1 week, 0.8 ± 0.3 after 2 weeks, 0.8 ± 0.2 after 3 weeks, and 0.3 ± 0.1 after 4 weeks, and they had significantly decreased (after 1 week: P < 0.05; after 2 weeks: P < 0.01).
3.3. Stratum Corneum Functions
3.3.1. Stratum Corneum Water Content
High-frequency conductivity was 39.1 ± 18.4 mS at the start of the study, and it had increased significantly to 87.6 ± 22.8 mS after 2 weeks and to 150.9 ± 44.8 mS after 4 weeks (P < 0.01) (Table 4 and Figure 4).
3.3.2. Stratum Corneum Barrier Function
TEWL was 12.9 ± 5.8 g/m2·h at the start of the study, and it had decreased to 9.7 ± 1.6 g/m2·h after 2 weeks and to 8.4 ±2.6 g/m2·h after 4 weeks. The difference after 4 weeks was significant (P < 0.05) (Table 5 and Figure 5).
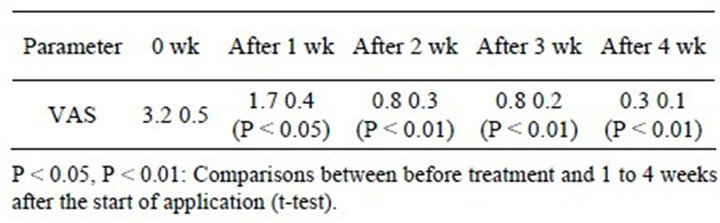
Table 3. Mean VAS scores.
Comparisons between before treatment and 1 to 4 weeks after the start of treatment (t-test).*P < 0.05, **P < 0.01.
Figure 3. Changes in itching score (VAS score).
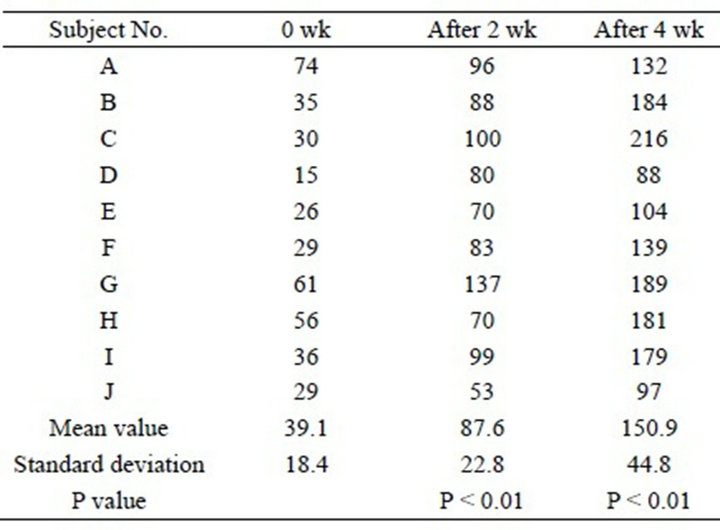
Table 4. Changes in high-frequency conductivity (units: mS).
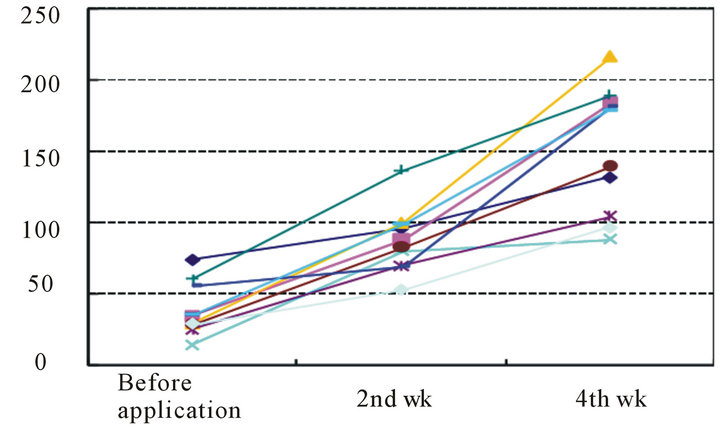
Figure 4. Changes in high-frequency conductivity after the start of application (units: mS).
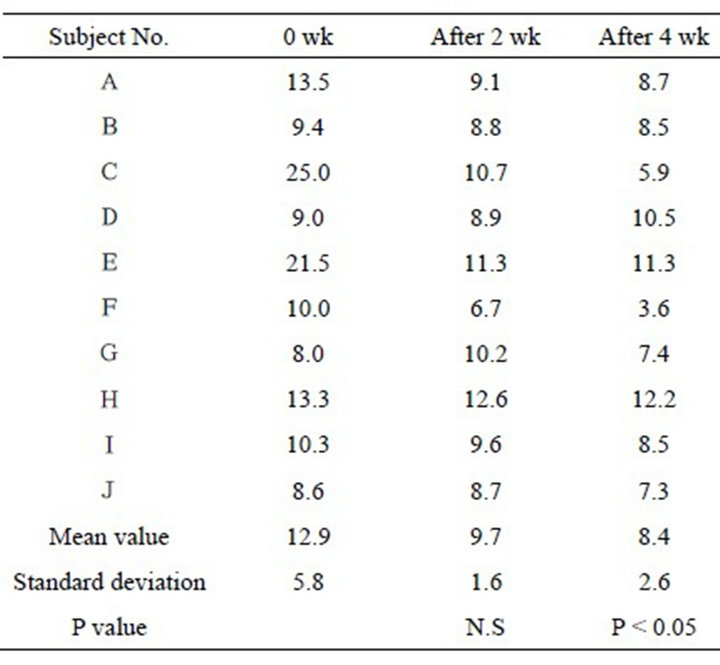
Table 5. Transepithelial water loss (units: g/m2·h).
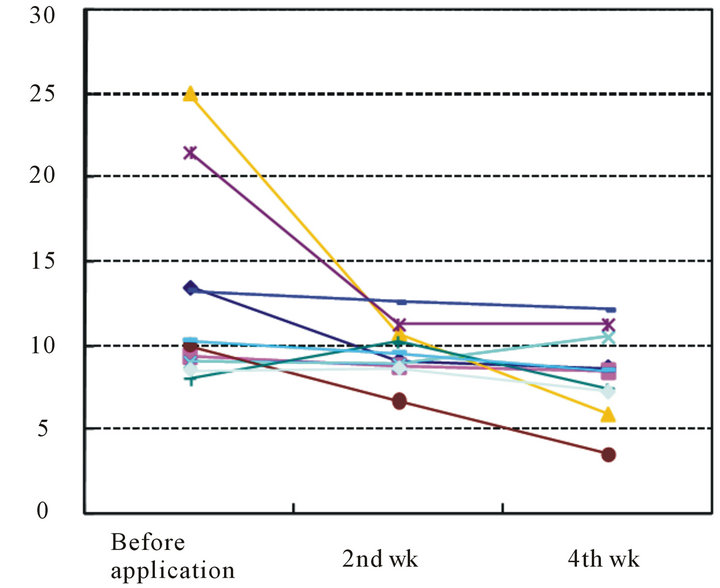
Figure 5. Changes in transepithelial water loss after the start of treatment (units: g/m2/h).
3.4. Degree of Overall Improvement, Safety, Patients’ Impression
The results for overall degree of improvement after 4 weeks in comparison with before the start of the study were: “great improvement” in 30% (6/20) and “improvement” or better in 100% (20/20) (Table 6). No adverse effects were observed in any of the patients.
The results for patients’ impression were “very good” in 30% (6/20) and “good” or better in 100% (20/20) (Table 6).
A case in which an affected site in the abdomen improved (Case 1), a case in which an affected site in a lower limb improved (Case 2), and a case in which an affected site on the fingers and back of the hand improved (Case 3) are shown in Figure 6 as representative
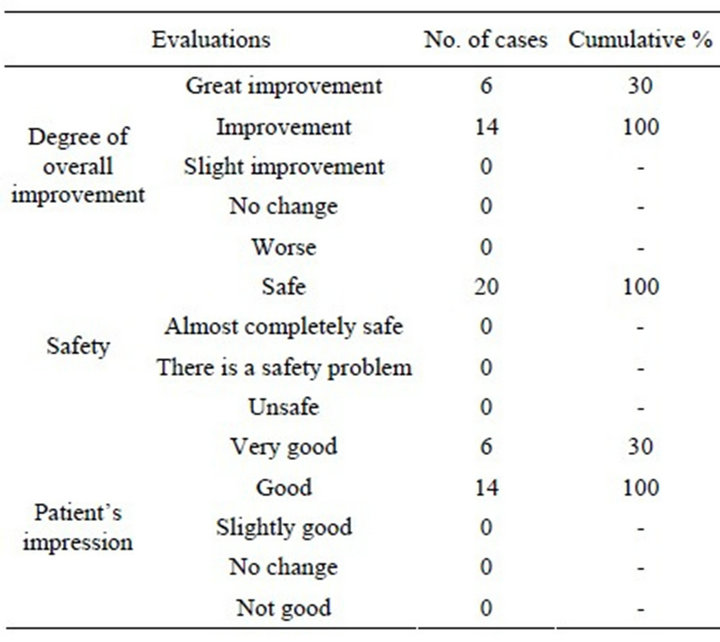
Table 6. Degree of overall improvement, safety, and patients’ impression.
cases.
4. Discussion
When APA was used continuously to treat patients with from minor to moderate AD over a 4-week period in this study, improvement in the skin findings (dryness and scaling) was observed beginning one week after the start of APA application, and at 2 weeks the symptoms had abated even more. By 3 weeks to 4 weeks the symptoms had almost completely resolved. Similar abatement of the symptom itching was also observed. Based on these findings it was concluded that therapeutic efficacy of APA is seen immediately after application, and that after using it for approximately 2 weeks improvement is seen in most of the symptoms.
Moreover, the evaluation of stratum corneum functions [5] by means of instruments showed that high-frequency conductivity, an indicator of the water content of the stratum corneum, was low (39.1 mS) before treatment but more than doubled by 2 weeks after the start of treatment, and more than tripled by 4 weeks. TEWL was 12.9 g/m2∙h before treatment and had been significantly reduced to 9.7 g/m2∙h after 2 weeks of treatment and to 8.4 g/m2∙h after 4 weeks. Based on these findings, the water content and the barrier function of the stratum corneum were found to have clearly improved in response to APA application.
The degree of overall improvement was “great improvement” in 30% and “improvement” or better in 100%, and no adverse effects developed in any of the patients.
In addition, the impressions of all of the patients were “very good” or “good”. In particular, since 75% replied
Case 1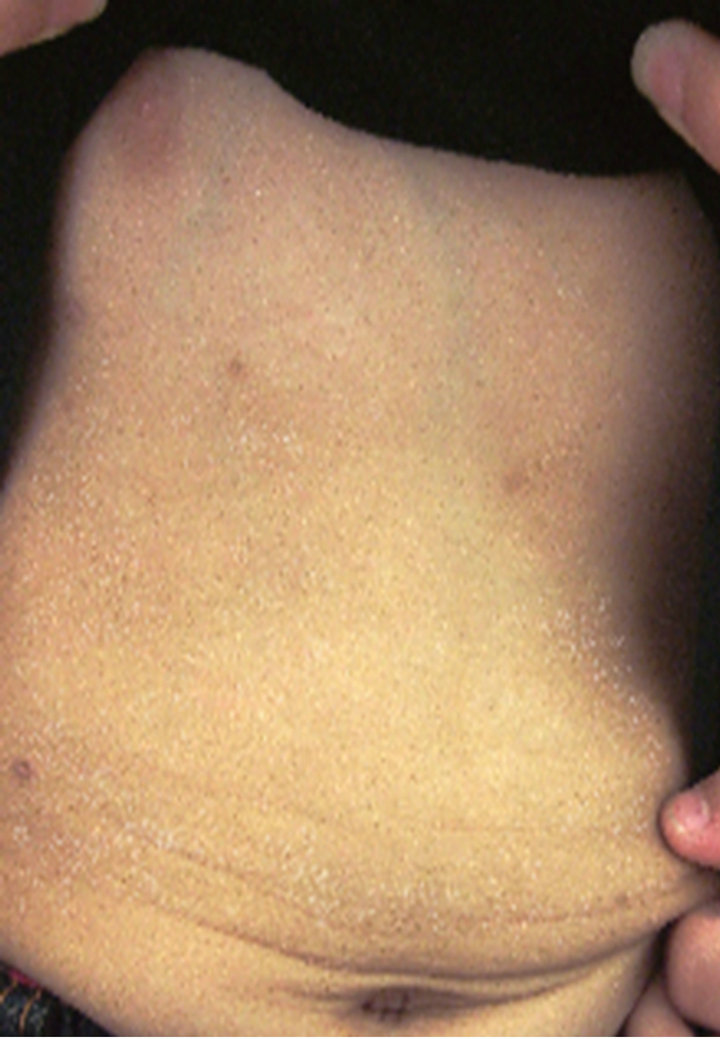
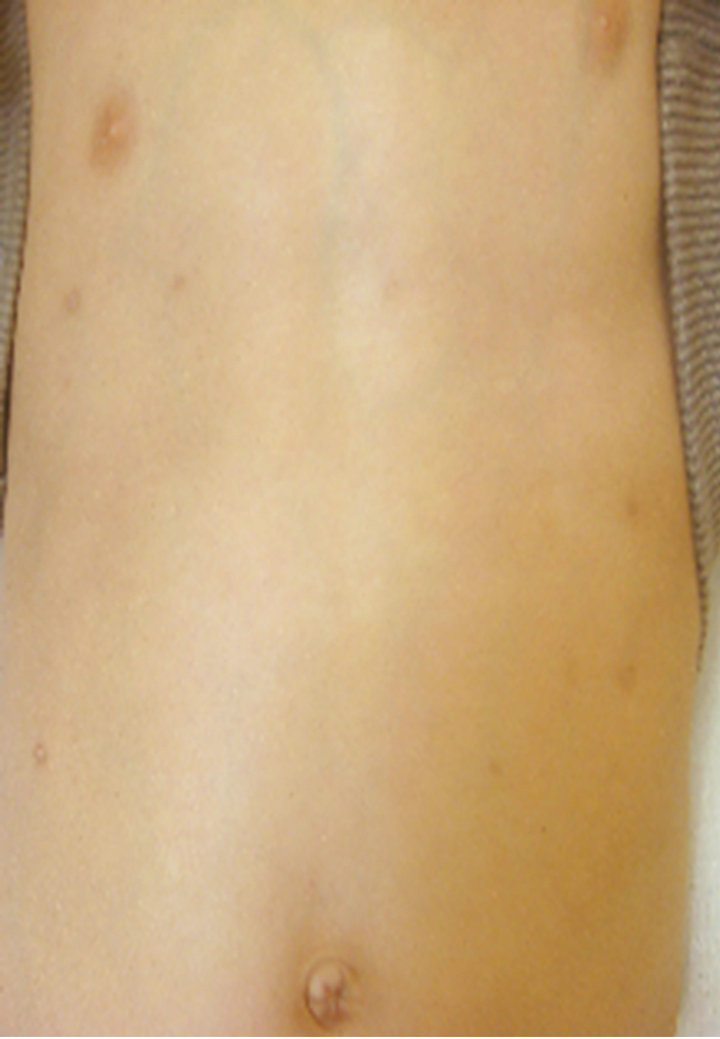
0 weeks 4th weeks
Case 1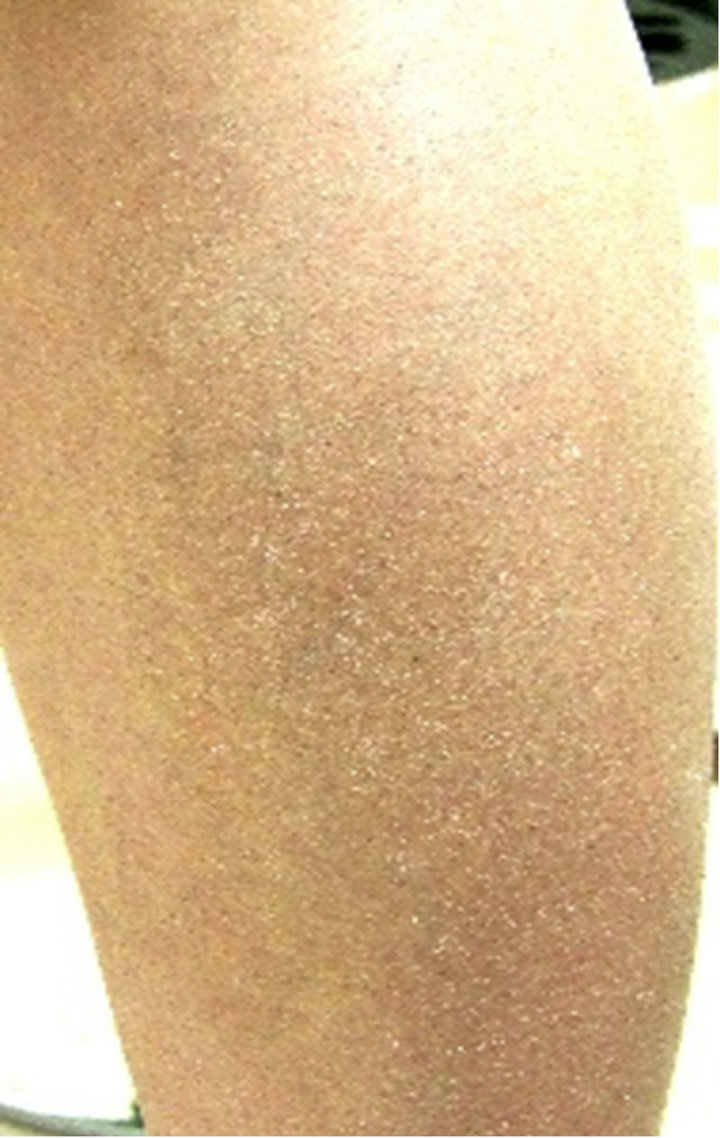
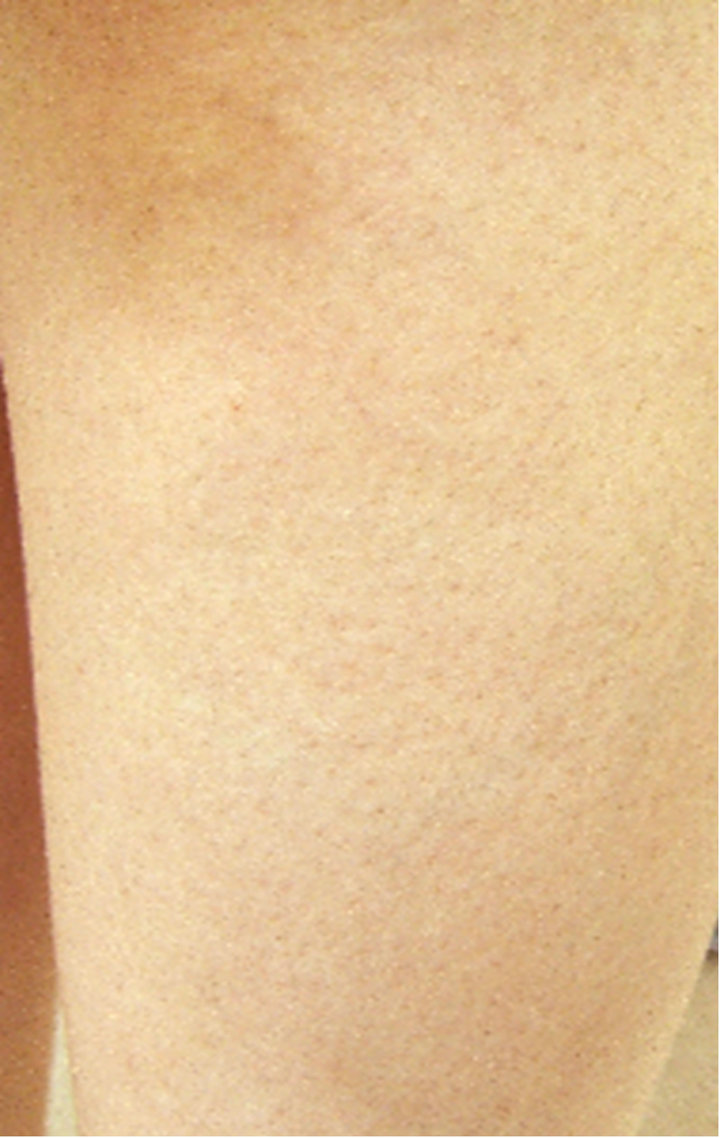
0 weeks 4th weeks
Case 1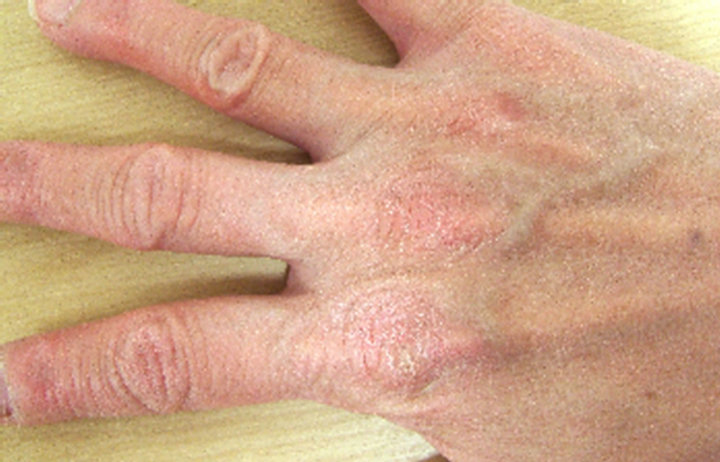
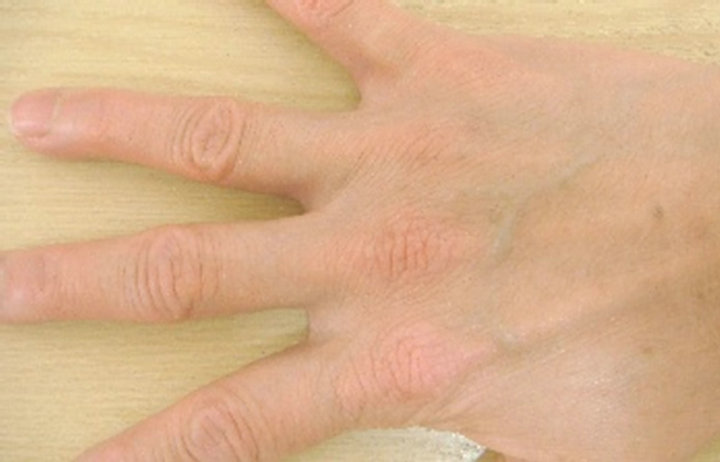
0 weeks 4th weeks
Figure 6. Representative cases.
“yes, there was” in regard to “moist feeling”, and 100% replied “yes, a little” or more, and 65% replied “hardly any” etc. in regard to “stickiness”, the patients’ evaluations of how it felt to use APA were high, and some made such comments as: “because it’s a lotion, its easy to spread” and “the longer you continue to use it, the moister your skin begins to feel”. None of the patients noted any sense of irritation, etc.
APA contains dicalcium glycyrrhizinate, which has an anti-inflammatory action, and allantoin, which has an astringent action, and APA is expected to have an action that prevents rough skin, but, in addition to that, as a result of high-pressure emulsification technology it is in a form in which bound water is present around miniatureized emulsion-type Vaseline particles. Its sealing action as a result of the minute Vaseline particles covering the skin and its humectant action as a result of the bound water around the particles hydrating the skin appear to have made it possible to realize a preparation that both has excellent moisturizing ability and is pleasant to use.
Based on all of the above taken together, APA appears to be an excellent preparation in terms of efficacy and safety as skin care for atopic dry skin, in which a decrease in functions of the stratum corneum is seen in the skin, and as an adjuvant treatment or a remission-maintaining treatment method for AD.
Lastly, we are very grateful to Rohto Pharmaceutical Co. Ltd. for providing us with APA.
REFERENCES
- M. Watanabe, et al., “Functional Analyses of the Superficial Stratum Corneum in Atopic Xerosis,” Archives of Dermatology, Vol. 127, No. 11, 1991, pp. 1689-1692.
- K. Kikuchi, et al., “Evaluation of the Skin Surface Hydration for 0.15% Prednisolone Butylacetate ‘Mentholatum®AP Soft Lotion’ by High Pressure Emulsification Manufacturing Method and Effects and Safety in Patients with Atopic Dermatitis,” Japanese Journal of Medicine and Pharmaceutical Science, Vol. 58, No. 2, 2007, pp. 279- 287.
- I. Angelova-Fischer, et al., “The Objective Severity Assessment of Atopic Dermatitis (OSAAD) Score: Validity, Reliability and Sensitivity in Adult Patients with Atopic Dermatitis,” British Journal of Dermatology, Vol. 153, No. 4, 2005, pp. 767-773.
- J. L. Sugarman, et al., “The Objective Severity Assessment of Atopic Dermatitis Score: An Objective Measure Using Permeability Barrier Function and Stratum Corneum Hydration with Computer-Assisted Estimates for Extent of Disease,” Archives of Dermatology, Vol. 139, No. 11, 2003, pp. 1417-1422. doi:10.1001/archderm.139.11.1417
- H. Tagami, et al., “Evaluation of the Skin Surface Hydration in Vivo by Electrical Measurement,” Journal of Investigative Dermatology, Vol. 75, No. 6, 1980, pp. 500- 507. doi:10.1111/1523-1747.ep12524316
NOTES
*Corresponding author.

