New Journal of Glass and Ceramics
Vol. 2 No. 4 (2012) , Article ID: 23633 , 6 pages DOI:10.4236/njgc.2012.24021
Influence of Composite Non Magnetic Ions (Cd-Ti) Doping on Structural and Electrical Properties of Li-Mn Ferrite
![]()
1Ramkrishna Paramhansa Mahavidyalaya, Osmanabad College, Osmanabad, India; 2Physics Department Rajaram College, Kolhapur, India; 3Physics Department, Solapur University, Solapur, India; 4Physics Department, Shivaji University, Kolhapur, India.
Email: *ketakiketan@yahoo.com
Received May 6th, 2012; revised July 23rd, 2012; accepted August 16th, 2012
Keywords: Electronic Materials; Magnetic Ceramics; Electrical Characterization; Powder Diffraction
ABSTRACT
The Li-Mn ferrites with composite divalent and tetravalent non-magnetic ions doping were prepared by ceramic method and studied for the first time to investigate their structural and electrical properties. It has been confirmed from the studies that these materials result in properties suitable for microwave applications. The structural properties have confirmed the formation of cubic spinel ferrite and the substitutions of non magnetic ions have resulted in increase of unit cell dimensions and hence the grain size with increase in dopant content. An IR study asserts the same. Electrical Properties show increase in dc resistivity and decrease in dielectric loss tangent with increase in dopant concentration.
1. Introduction
Li ferrite is becoming increasingly attractive for microwave applications replacing garnets and other spinel ferrites [1-3]. Microwave devices such as circulators, isolators, magnetostatic resonators, filters, switches, limiters and tunable electroptic modulators are the microwave applications of Li ferrites [4]. Recent exponential growth in microwave communication through mobile and satellite communications has further stressed the worldwide need for extremely low-loss and economical microwave devices using ferrite materials. In preparation of microwave ferrite materials, particular attention should be given to the purity of the raw materials, stoichiometry of the composition and porosity as well as grain characteristics of the final product. Characteristics of various microwave ferrites have been minutely reported by Voronkov et al. [5]. The emergence of Li ferrite as a competent material in microwave devices has resulted from some appropriate chemical substitutions made in it, which in turn result in low dielectric loss tangent, a low magnetic loss tangent at the operating bias field, a low coercive force and a large remanence ratio [6-8]. Low dielectric and magnetic losses are the essential requisites for microwave applications Small amounts of Mn3+ is added to microwave Li ferrites to ensure an acceptably low dielectric loss tangent [9]. Moreover, manganese addition also alter the hysteresis property, reduces magnetocrystalline anisotropy and magnetostriction in ferrites [9].
Non magnetic ions like Cd2+ and Ti4+ substitutions have been found to be most suitable to obtain high resistivity [10,11]. The site occupancies of the various cations known from earlier works are given as follows. Li1+ has strong preference for B-Site [12], Cd2+ has strong preference for A site [1], Ti4+ also has strong preference for B-site [13], Fe2+ has strong preference for B-site [1] and Mn3+ has strong preference for B-site [14]. From the above survey, it can be envisaged that investigations on the electrical properties of composite non-magnetic ions doping in Li-Mn ferrite may lead to more interesting results as the studies on their independent doping in Lithium ferrites have already resulted in properties suitable for microwave applications [10,11].
In this view, the present paper aims to communicate structural and electric properties in Li0.35CdxTixMn0.1 Fe2.55–2xO4 where x varies from 0 to 0.5.
2. Experimental
Six samples of different compositions were prepared by standard ceramic technique using pure metal oxides in the form of a series Li0.35CdxTixMn0.1Fe2.55–2xO4 with x = 0.0, 0.1, 0.2, 0.3, 0.4 and 0.5. AR grade chemicals of Li2CO3, CdO, TiO2, Mn2O3 and Fe2O3 were used for the preparation of various compositions in the above ferrite series. These oxides were weighed in the required mole proportions using a single pan balance having least count 0.001 gm and mixed thoroughly in the agate-mortar in acetone for about 2 hrs. The mixture was sieved using a sieve of mesh size 200 micron. The mixture of each composition was preheated in platinum crucible and were presintered at 300˚C for 2 hours and followed by 600 Influence of Composite Non Magnetic ions (Cd-Ti) Doping on Structural and Electrical Properties of Li-Mn Ferrite for 4 hrs and finally sintered at 1000˚C for 8 hours.
X-ray Diffractograms of various compositions were obtained using X ray diffractometer Model PW 3710. The various parameters used for X ray diffraction were Target—Cu Kα; Wavelengths λ1 = 1.54056 Å and λ2 = 1.54439 Å; Rate of Scanning—2˚/min and scanning angle range 2θ—20˚ to 90˚. Micrographs of various samples were obtained using the scanning electron microscope SEM (model JSM-6360A). IR absorption peaks of various compositions were studied using PerkinElmer IR spectrometer (Model 783) with KBr as a solvent. DC resistivities of various prepared samples were studied using two probe set up. Dielectric constant and loss tangent in the frequency range 100 Hz - 1 MHz were also measured using HP LCR meter 4284A model.
3. Results and Discussion
The X-Ray diffraction patterns for Li0.35CdxTixMn0.1Fe2.55–2xO4 system show sharp peaks indicating formation of single phase spinel ferrite for all compositions. However trace amount of α-Fe2O3 phase is found for x = 0.3 sample. Hence the XRD of x = 0.3 composition is given in the Figure 1. The α-Fe2O3 phase is formed because at higher sintering temperature (>500˚C) there is possibility for a fraction of ferric oxide to get converted into α-Fe2O3. The presence of such a phase in different ferrite is already reported by earlier workers [15].
The lattice parameter increases with increasing the content of Cd2+and Ti4+ ions and is shown in Figure 2. This is in accordance with Vegard’s law. The Cd2+ ions have larger ionic radius (0.97 Å) as compared to Fe3+
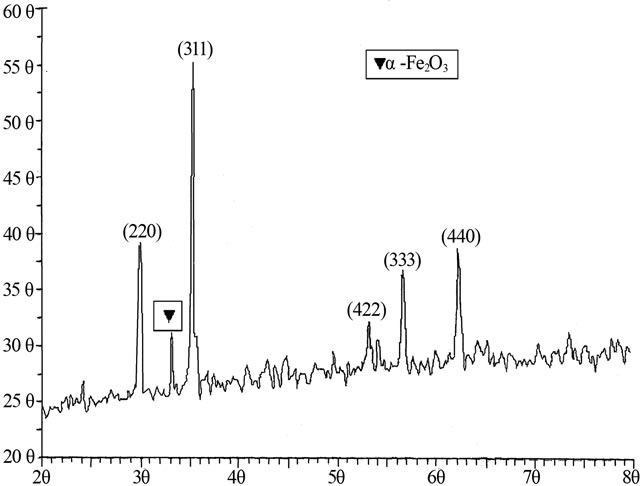
Figure 1. Fe2O3 pattern XRD of Li0.35Cd0.3Ti0.3Mn0.1Fe1.95O4.

Figure 2. Variation of lattice parameter with Cd and Ti content [x] in Li0.35 CdxTixMn0.1Fe2.55–2xO4 series.
(0.65 Å), Ni2+ (0.74 Å) and Li1+ (0.71 Å) ions. The Cd2+ ions successively replace Fe3+ ions on A-site this results in an increase of lattice parameter with Cd content. Same is true for Ti ions at B-sites. Similar results were obtained when Cd and Ti were separately doped in Li ferrite [10,11]. The compositional variations however suggest that the lattice parameters for composite non-magnetic ions doping is increased to larger extent in comparison to their separate doping in ferrites.
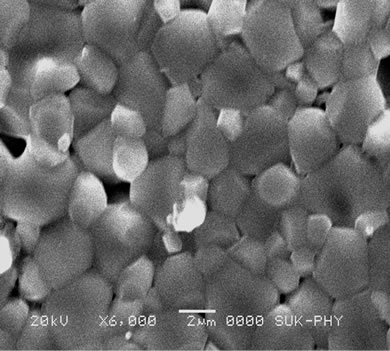
The SEM technique was studied to understand the surface morphology of the samples. All compositions have grains with sharp boundaries indicating that grains are fully developed, well packed, crack free with clear grain boundaries. The grain boundaries are district and grains are closely packed in some cases which suggest that compositions exhibit high density values. The SEM images denoted by a, b, c, d, e, and f shows micrographs for compositions x = 0.0, 0.1, 0.2, 0.3, 0.4, and 0.5. With the addition of Cd-Ti ions the average grain size increases as shown in the Figures 3(a)-(f). The increase in grain diameter with Cd and Ti content is attributed to the smaller solid solubility of lithium in the samples. It is obvious from the generic formula.
The IR spectra of one representative member of ferrite series i.e. x = 0.3 is shown in Figure 4. The IR absorption bands were observed in the range 600 cm–1 to 400 cm–1. The absorption bands obtained in the present investigation are found to be in the range reported for many other lithium containing ferrites [16,17]. The difference in band position n1 and n2 is expected because the Fe3+-O2– distance for B site (0.199 nm) is different from that of A site (0.189 nm). The tetrahedral vibrations are of bond stretching type while octahedral vibrations are of bond bending type. These types of vibrations also affect the absorption frequency. The octrahedral complex band is found to be suppressed with increase in composite nonmagnetic ions content in the ferrite. This can be attributed to increase in the lattice parameter and average grain size with increase in Cd-Ti content.
 (a)
(a) (b)
(b) (c)
(c) (d)
(d)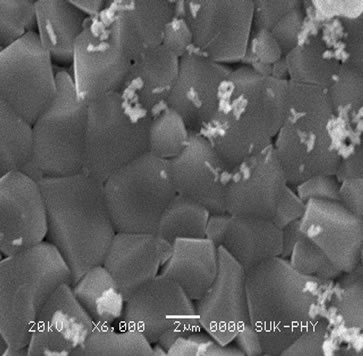 (e)
(e)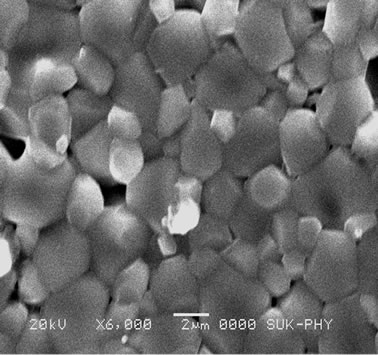 (f)
(f)
Figure 3. SEM of series Li0.35CdxTixMn0.1Fe2.55–2xO4 with x = 0 to 0.5.
Variation of logρ vs 103/T for the samples Li0.5CdxTix Mn0.1Fe2.55–2xO4 and Li0.35CdxTixMn0.1Fe2.55–2x O4 with x = 0, 0.1, 0.2, 0.3, 0.4, and 0.5 are shown in Figure 5. All the plots exhibit a linear relationship suggesting that the resistivity obeys a relation ρ = ρ0 exp(ΔE/kT) and change in slope is observed in case of all the compositions. From figure, it is seen that the variation is almost linear up to Curie temperature where a break occurs indicating a change of magnetic ordering from ferrimagnetic to paramagnetic. The temperatures where breaks occur coincide with the Curie temperature determined by permeability measurements which is not reported here. The exponential increase in resistivity on decrease in temperature is due to decrease in thermally activated mobility of the charge carrier. The change in slope is attributed to the change in the activation energy due to phase transition of the material from ferromagnetic to paramagnetic state. This anomaly strongly supports the influence of magnetic ordering upon the conductivity process in-ferrites. Many workers have studied logρ vs (1/T) and observed similar behaviour suggesting that resistivity obeys the Arrhenius relation [11,18]. Resistivity studies show that resistivity (ρdc) of the samples in the compositions increase as the Cd2+, Ti4+ concentration increases similar to that reported in 10 and 11 D.C. resistivity of Li-Cd-Ti ferrite can be explained on the basis of a model based on phonon assisted electron hopping. Trace amount of Fe2+ ions are present in the present ferrites. The electrons are known to participate in the exchange process by the following reaction Fe2+ « Fe3+ + e. Hence these electrons are strongly coupled to the lattice and tunnel from one site to the other due to phonon induced transfer mechanism. Ti4+ ions, being tetravalent, localize Fe2+ ions in the system and tunneling of electrons by transfer mechanism is retarded due to the reduction of Fe3+ ions which enhances the resistivity. The activation energy is estimated to be 0.21 eV, which in turn confirms the conduction in present ferrites are due to small polarons [18].
The dielectric constant decreases with increase in frequency showing dielectric dispersion as depicted in Figure 6. The decrease in ε with frequency is natural because of the fact that any species contributing to polarizability is found to show lagging behind the applied Li-Cd-Ti ferrite can be explained on the basis of a model based on phonon assisted electron hopping. Traceamount of Fe2+ ions is present in the present ferrites. The electrons are known to participate in the exchange process by the following reaction Fe2+ « Fe3+ + e. Hence these electrons are strongly coupled to the lattice and tunnel from one site to the other due to phonon induced transfer mechanism. Ti4+ ions, being tetravalent, localize Fe2+ ions in the system & tunneling of electrons by transfer mechanism is retarded due to the reduction of Fe3+ ions which enhances the resistivity. The activation energy is estimated to be 0.21 eV, which in turn confirms the conduction in present ferrites are due to small polarons [18].
4. Conclusion
The Li0.35CdxTixMn0.1Fe2.55–2xO4 system has been successfully prepared with standard ceramic technique. The most intense (311) peak in XRD pattern confirms the formation of cubic spinel ferrite. The SEM micrograph shows the agglomerated grain structure with sharp grain boundaries due to high sintering temperature. The IR studies show the absorption bands which are in good agreement to the studied literature. The electrical properties study shows the Li-ferrites are the n-type semiconductors. The dielectric constant shows usual dispersion behaviour.
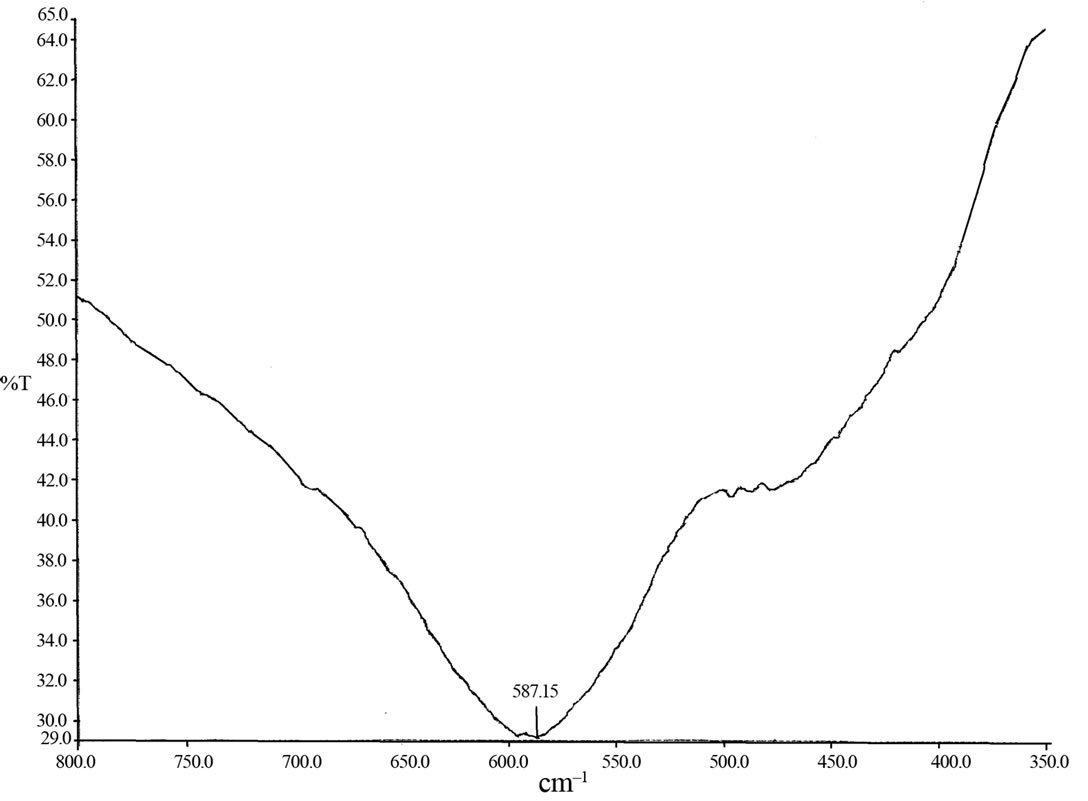
Figure 4. IR spectra of Li0.35CdxTixMn0.1Fe2.55–2xO4 with x = 0.3.

Figure 5. Variation of logρ vs 103/T for Li0.35CdxTixMn0.1Fe2.55–2xO4.
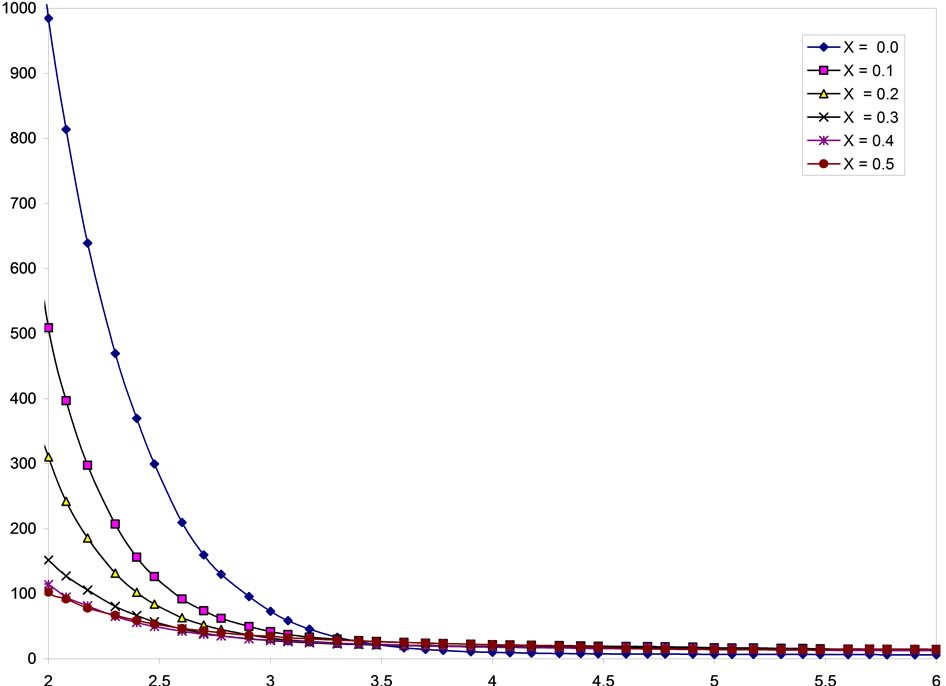
Figure 6. Variation of dielectric constant Є vs logf for ferrite system Li0.35CdxTixMn0.1Fe2.55–2xO4.

Figure 7. The plot of dielectric loss tangent (tanδ) vs logf for ferrite system Li0.35CdxTixMn0.1Fe2.55–2xO4.
5. Acknowledgements
Author K. K. Patankar is thankful to UGC, New Delhi for the financial assistance in the form of major research project extended to her.
REFERENCES
- R. G. Kharabe, R. S. Devan, C. M. Kanamadi and B. K. Chougule, “Dielectric Properties of Mixed Li-Ni-Cd Ferrites,” Smart Materials and Structures, Vol. 15, No. 2, 2006, pp. 229-334. doi:10.1088/0964-1726/15/2/N02
- S. R. Sawant, D. N. Bhosale, N. D. Chaudhari and P. P. Bakare, “Electric Properties of NiCuZn Ferrites Synthesized by Oxalate Precursor Method,” Journal of Materials Science, Vol. 3, 2002, pp. 617-622.
- B. P. Ladgaonkar, P. N. Vasambekar and A. S. Vaingankar, “Structural and DC Electrical Resistivity Study of Nd3+ Substituted Zn-Mg Ferrites,” Journal of Materials Science Letters, Vol. 19, No. 5, 2000, pp. 1375-1377. doi:10.1023/A:1006713518433
- M. Pardavi-Horvath, “Microwave Applications of Soft Ferrites,” Journal of Magnetism and Magnetic Materials, Vol. 215-216, 2000, pp. 171-183. doi:10.1016/S0304-8853(00)00106-2
- V. Voronkov, “Microwave Ferrites: The Present and Future,” Journal of Physics IV (Paris), Vol. 7, No. 1, 1997, pp. 35-38.
- D. Ravinder, “Dielectric Behaviour of Lithium-Cadmium Ferrites,” Physica Status Solidi (A), Vol. 129, No. 2, 1992, pp. 549-554. doi:10.1002/pssa.2211290225
- S. S. Bellad, S. C. Watawe and B. K. Chougule, “Some Ac Electrical Properties of Li-Mg Ferrites,” Materials Research Bulletin, Vol. 34, No. 7, 1999, pp. 1099-1106. doi:10.1016/S0025-5408(99)00107-5
- V. P. Reddy and D. V. Reddy, “Far-Infrared Spectral Studies of Some Lithium-Nickel Mixed Ferrites,” Journal of Magnetism and Magnetic Materials, Vol. 136, No. 3, 1994, pp. 279-283. doi:10.1016/0304-8853(94)00321-1
- P. P. Hankare, R. P. Patil, U. B. Sankpal, S. D. Jadhav, I. S. Mulla, K. M. Jadhav and B. K. Chougule, “Magnetic and Dielectric Properties of Nanophase Manganese-Substituted Lithium Ferrite,” Journal of Magnetism and Magnetic Materials, Vol. 321, No. 19, 2009, pp. 2977-3372. doi:10.1016/j.jmmm.2009.05.074
- R. G. Kharabe, R. S. Devan and B. K. Chougale, “Structural and Electrical Properties of Cd-Substituted Li-Ni Ferrites,” Journal of Alloys and Compounds, Vol. 463, No. 1-2, 2008, pp. 67-72.
- D. Kothari, S. Phanjoubam and J. S. Baijal, “Electrical Conduction and Dielectric Behaviour of the Oxidic Spinel Li0.5+0.5xCr0.3TixFe2.2−1.5XO4,” Journal of Materials Science, Vol. 25, No. 12, 1990, pp. 5142-5146. doi:10.1007/BF00580142
- S. Chander, M. P. Sharma, A. Krishnamurthy and B. K. Srivastava, “Mössbauer Study of Nano-Particles of Spinel Ferrites LixFe3−xO4,” Indian Journal of Pure and Applied Physics, Vol. 45, No. 10, 2007, pp. 816-821.
- K. P. Chaea, J. G. Lee, H. S. Kweona and Y. B. Lee, “The Crystallographic, Magnetic Properties of Al, Ti Doped CoFe2O4 Powders Grown by Sol-Gel Method,” Journal of Magnetism and Magnetic Materials, Vol. 283, No. 1, 2004, pp. 103-108. doi:10.1016/j.jmmm.2004.05.010
- K. K. Patankar, “Synthesis and Characterization of Magnetoelectric Composites,” Ph.D. Thesis, Shivaji University, Kolhapur, 2000.
- A. F. Junior, E. C. de O. Lima, M. A. Novak and P. R. Wells, “Synthesis of Nanoparticles of CoxFe(3−x)O4 by Combustion Reaction Method,” Journal of Magnetism and Magnetic Materials, Vol. 308, No. 2, 2007, pp. 198-202. doi:10.1016/j.jmmm.2006.05.022
- B. K. Bammannavar, L. R. Naik, R. B. Pujar and B. K. Chougule, “Preparation, Characterization and Physical Properties of Mg-Zn Ferrites,” Indian Journal of Pure and Applied Physics, Vol. 14, No. 5, 1998, pp. 381-385.
- P. V. Redy and V. D. Reddy, “Far-Infrared Spectral Studies of Some Lithium-Nickel Mixed Ferrites,” Journal of Magnetism and Magnetic Materials, Vol. 136, No. 3, 1994, pp. 279-283. doi:10.1016/0304-8853(94)00321-1
- R. S. Patil, S. V. Kakatkar, S. A. Patil, P. K. Maskar and S. R. Sawant, “Electrical Properties of Ferrites,” Indian Journal of Pure and Applied Physics, Vol. 29, 1991, pp. 131-135.
- V. P. Reddy and D. V. Reddy. “Far-Infrared Spectral Studies of Some Lithium-Nickel Mixed Ferrites,” Journal of Magnetism and Magnetic Materials, Vol. 136, No. 3, 1994, pp. 279-283. doi:10.1016/0304-8853(94)00321-1
- S. A. Rahman, “Temperature, Frequency and Composition Dependence of Dielectric Properties of Nb Substituted Li Ferrite,” Egyptian Journal of Solids, Vol. 29, No. 1, 2006, pp. 131-141.
NOTES
*Corresponding author.

