International Journal of Organic Chemistry
Vol.05 No.01(2015), Article ID:54478,13 pages
10.4236/ijoc.2015.51003
Synthesis, Spectroscopic Characterization and Antimicrobial Activity of Some New 2-Substituted Imidazole Derivatives
Asmaa S. Salman, Anhar Abdel-Aziem, Marwa J.S. Alkubbat
Department of Chemistry, Faculty of Science, Al-Azhar University, Girls’ Branch, Nasr City, Cairo, Egypt
Email:salman_2007_ok@yahoo.com
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 15 January 2015; accepted 1 March 2015; published 9 March 2015
ABSTRACT
The reaction of imidazole-2-thione derivative 1 with 2-chloro-N-p-tolylacetamide afforded the corresponding 2-(1HI-imidazol-2-ylthio)-N-p-tolylacetamide 2.Reaction compound 2 with different reagents such as p-chlorobenzaldehyde and p-chlorophenyldiazonium chloride afforded the corresponding arylidene derivative 3 and hydrazone derivative 6. Reactions of 2 with carbon disulfide in dimethylformamide (DMF) in one equivalent potassium hydroxide afforded intermediate potassium sulphide salt 8, which treatment with dilute hydrochloric acid and phenacyl bromide afforded the corresponding 2-[p-tolylcarbamoyl]ethanedithioic acid 9 and 3-[benzo-ylmethylth- io]-N-p-tolyl-3-thioxo-propaneamide 10. While the reaction 2 with carbon disulphide in the presence of two equivalent potassium hydroxide in DMF gave non-isolated potassium salt 11, which was allowed to react with halogenated compounds namely ethyl chloroacetate and methyl iodide afforded the corresponding 3, 3-bis[(ethoxycarbonyl)methylthio]-N-p-tolylacrylamide 12 and 3,3-bis-(methylthio)-N-p-tolylacrylamide 13 respectively. Reaction 2 with phenyl isothiocyanate in ba- sic DMF yielded the intermediate potassium sulphide salt 18. Acidification 18 with dilute hydro- chloric acid afforded the corresponding thiocarbamoyl derivative 19.Treatment of intermediate 18 with methyl iodide, phenacyl bromide and ethylchloroacetate afforded the 3-anilino-3-(methyl- thio)-N-p-tolylacrylamide 20, 2-(1,3-thiazol-2(3H)-ylidene)-N-p-tolylacetamide 21 and 2-(4-oxo-3-phenyl-1,3-thiazolidin-2-ylidene)-N-p-tolylacetamide 22 respectively.The structure of the newly synthesized compounds has been confirmed by elemental analysis and spectra data.Synthesized compounds 2, 3, 6, 13, 15a, 15b, 17, 20, 21, 22 and 23 were screened for their antibacterial activities in vitro against Gram-positive (Staphylococcus aureus and Bacillus subtilis), Gram-negative(Pseudomonas aeuroginosa and Escherichia coli )and antifungal activities against (Aspergillus fumigates, Syncephalastrumracemosum, Geotrichumcandidum and Candida albicans).
Keywords:
Imidazole-2-Thione, α-OxoketenDithioacetals, Thiocarbamyl, Thiazole, Antibacterial, Antifungal

1. Introduction
Aromatic heterocycles are valuable synthetic templates for the preparation of new compounds with specific biological, pharmaceutical and material properties. The pursuit of these properties requires efficient synthetic routes that allow rapid construction of diverse aromatic heterocycles with defined substitution patterns. Therefore,α-oxoketendithioacetals and thiocarbamyl as the organic synthetic intermediates have been widely used in the for- mation of alicyclic, aromatic and heterocyclic compounds[1] -[4] .In view of the above and in continuation of our studies on the synthesis of heterocyclic compounds exhibiting biological activity, we report here the synthesis of some novel heterocycles compounds incorporating imidazole moiety fromα-oxoketendithioacetals and thiocarbamyl.
2. Material and Methods
2.1. Experiment
All melting points were determined in open glass capillaries on a Gallenkamp apparatus and are uncorrected. IR spectra (cm−1) were recorded on a Pye-Unicam spectrophotometer type 1200 using KBr discs. 1H-NMR spectra were recorded on a Varian EM-390 (90MHz) spectrometer using TMS as an internal standard and dimethyl sulphoxide (DMSO-d6) as a solvent. Chemical shifts were expressed inδ (ppm) values and mass spectra were determined on FinniganIncos 500 (70 ev). Elemental analyses were determined using a Parkin-Elmer 240CMicroanalyzer.The microanalyses were performed at the Microanalytical Unit, Faculty of Science, Cairo University.
2.1.1. 2-[1-(4-Chlorophenyl)-4,5-diphenyl-1H-imidazol-2-ylthio]-N-p-tolylacetamide 2
A mixture of 1 (0.01 mol) and2-chloro-N-p-tolylacetamide (0.01 mol) in DMF (25 ml) contain few drop oftriethylamine was heated under reflux for 6 h.The reaction mixture was left to cool and then poured to ice cooledwater (100 ml).The solid product that formed was filtered off, dried well and recrystallized from ethanol to give 2 as pall yellow crystals. Yield: 70%.M.p.:156˚C -158˚C;IR (K Br) cm−1:3246(NH), 1683 (C=O), 3041, 2931(CH), 1600(C=N); 1H-NMR (DMSO-d6) δ ppm:4.11(s, 2H, CH2), 2.24 (s, 3H, CH3), 10.36(s, 1H, NH), 7.09-7.47 (m, 18H, Ar-H); MSm/z (%):510 (M+, 26.25), 404(17.60), 375(27.06), 362(8.612), 193 (100), 147 (10.5); Anal.Calcd.for C30 H24 ClN3OS (510.05): C, 70.64; H, 4.74; Cl, 6.95; N, 8.24; S, 6.29.Found: C, 70.44; H, 4.54; Cl, 6.65; N, 8.04; S, 6.09.
2.1.2. 2-[1-(4-Chlorophenyl)-4,5-diphenyl-1H-imidazol-2-ylthio]-3-(4-chlorophenyl)-N-p- tolylacrylamide3
A mixture of 2(0.01 mol) and p-chlorobenzaldehyde (0.01 mol) in ethanol (30 ml) containing few drop ofpiperidine (0.5 ml) was refluxed for 3h.The reaction mixture was left to cool then poured onto ice water contain few drops of HCl and the obtained solid was recrystallized from ethanol to give 3 as white crystals.Yield:60%. M.p.:170˚C -171˚C;IR (KBr) cm−1: 3247(NH), 1687 (C=O), 3046, 2951 (CH); 1H-NMR (DMSO-d6) δ ppm: 2.24 (s, 3H, CH3), 10.31(s, 1H, NH), 7.09-7.47(m, 23H, Ar-H and =CH); MSm/z (%):632(M+, 11.24), 548(23.22), 520 (398.33), 430 (23.22), 386(27.34), 309 (24.34), 80 (100); Anal.Calcd.for C37 H27 Cl2 N3OS (632.60): C, 70.25; H, 4.30; Cl, 11.21; N, 6.64; S, 5.07. Found:C, 70.00; H, 4.00; Cl, 11.00; N, 6.44; S, 5.00.
2.1.3. 5-(4-Chlorophenyl)-4-[1-(4-chlorophenyl)-4,5-diphenyl-1H-imidazol-2-ylthio]-N-p-tolyl- 1H-pyrazole-3-amine 4
A mixture of 3 (0.01 mol) and hydrazine hydrate (0.01 mol) in 30 ml absolute ethanol was added few drops of glacial acetic and refluxed for 8-10 h. After completion of the reaction, excess of solvent was distilled off; the separated solid was filtered, washed with water, and recrystallized from methanol to give 4 as yellow crystals.Yield:55%. M.p.:138˚C-140˚C;IR (KBr) cm−1: 2922, 3054(CH), 3437 (NH); 1H-NMR (DMSO-d6) δ ppm:1.74(s, 3H, CH3), 7.17-7.49 (m, 23H, Ar-H and NH), 9.40(s, 1H, NH); MSm/z (%):644(M+, 11.02), 630(12.44), 558 (9.13), 537 (5.98), 511(8.03), 483(6.61), 464(12.76), 405(17.64), 333 (9.29), 388 (9.49), 233(100); Anal. Calcd.for C37 H27 Cl2N5S (644.61): C, 68.94; H, 4.22; Cl, 11.00; N, 10.86; S, 4.97.Found:C, 68.64; H, 4.00; Cl, 10.99; N, 10.66; S, 4.67.
2.1.4. 5-(4-Chlorophenyl)-4-[1-(4-chlorophenyl)-4,5-diphenyl-1H-imidazol-2-ythio]-1-phenyl-N- p-tolyl-1H-pyrazol-3-amine 5
A mixture of 3 (0.01 mol), phenyl hydrazine (0.01 mol), glacial acetic acid (20 ml)and few drop of HCl wasrefluxed for 8-10 h.The reaction mixture was then left to cool at room temperature, and poured onto ice cold water (100 ml).The solid product was collected by filtration and recrystallized from acetic acid to give 5 as grey crystals. Yield: 60%.M.p.:290˚C-292˚C; IR (KBr)cm−1:3426 (NH), 3048, 2926 (CH), 1594(C=N);1H-NMR (DMSO- d6) δ ppm:1.90(s, 3H, CH3), 7.13-7.48 (m, 27H, Ar-H), 13.03 (s, 1H, NH); MSm/z (%): 720 (M+, 8.26), 628(7.05), 554 (6.68), 538 (7.90), 615(8.51), 512 (8.26), 423 (6.93), 266(11.42); Anal. Calcd.for C43 H31Cl2 N5 S (720.71):C, 71.66; H, 4.34; Cl, 9.84; N, 9.72; S, 4.45.Found:C, 71.36; H, 4.04; Cl, 9.54; N, 9.42; S, 4.25.
2.1.5. 2-[2-(4-Chlorophenyl)hydrazono]-2-[1-(4-chlorophenyl)-4,5-diphenyl-1H-imidazol-2- ylthio]-N-p-tolylacetamide 6
To a cold solution of 2 (0.01 mol) in pyridine (30 ml) was added with continuous stirring 4-chloro phenyl-diazo- nium salt (0.01 mol) [prepared by adding sodium nitrite(0.02 mol) in water (8 ml) to a cold solution ofthe p-chloroanilinein the appropriate amount of hydrochloric acid].The reaction mixture was stirred at room temperature for 2 h, and the solid products, so formed, were collected by filtration and recrystallized from benzene to give 6 as red crystals. Yield: 67%.M.p.:118˚C-120˚C; IR (KBr, cm−1): 3259, 3136 ( NH ), 1604(C=N), 1686 (C=O); 1H-NMR ((DMSO-d6)δ ppm:2.24(s, 3H, CH3), 7.09-7.47 (m, 22H, Ar-H), 8.58(s, 1H, NH), 10.32(s, 1H, NH); MSm/z (%):648 (M+,40.12), 543( 43.83), 471(45.68), 442 (33.33), 362(33.95), 367 (33.95), 287(55.02), 211(35.80), 196(54.94), 77(100); Anal.Calcd.for: C36 H27Cl2N5OS(648.60):C, 66.66; H, 4.20; Cl, 10.93; N, 10.80; S, 4.94. Found:C, 66.36; H, 4.0 0; Cl, 10.73; N 10.50; S, 4.74.
2.1.6. 2-[1-(4-Chlorophenyl)-4,5-diphenyl-1H-imidazol-2-ylthio]-2-[p-tolylcarbamoyl] ethanedithioicacid 9
To suspension of finely powdered potassium hydroxide (0.01mol) in dry DMF (20 ml) at 0˚C, the acetamide derivative 2 (0.01mol) was added, the resulted mixture was cooled at 10˚C in an ice bath, then carbon disulfide (0.01 mol) was added slowly over the course of 10 min. After complete addition, stirring of the reaction mixture was continued for 6 h. Then hydrochloric acid (2 M, 20ml) was added drop wise and stirring continued for additional 1h.Then, the reaction mixture was poured into ice water.The solid product that formed was filtered off, dried, and recrystallized from the ethanol to give 9 as yellow crystals.Yield:75%.M.p.:222˚C-224˚C; IR (KBr, cm−1: 3231 (NH), 1682(CO), 3043, 2855(CH), 1271(C=S); MS m/z (%): 587(M+ +1, 4.08), 586 (M+, 4.53), 553 (3.18), 520(5.47), 480(3.35), 415(3.46), 233(100), 221(3.80), 77 (96.65); Anal.Calcd.for C31H24ClN3OS3 (586.19):C, 63.52; H, 4.13; Cl, 6.05; N, 7.17; S, 16.41.Found:C, 63.32; H, 4.00; Cl, 6.00; N, 7.00; S, 16.21.
2.1.7. 3-[Benzoylmethylthio]-2-[1-(4-chlorophenyl)-4,5-diphenyl-1H-imidazol-2-ylthio]-N-p- tolyl-3-thioxo-propaneamide 10
To suspension of finely powdered potassium hydroxide (0.01mol) in dry DMF (20 ml) at 0˚C, the acetamide derivative 2 (0.01mol) was added, the resulted mixture was cooled at 10˚C in an ice bath, then carbon disulfide (0.01 mol) was added slowly over the course of 10 min. After complete addition, stirring of the reaction mixture was continued for 6 h. Then cooled again to 0˚C, phenacyl bromide (0.01 mol) was added slowly over the course of 10 min. After complete addition, stirring of the reaction mixture was continued for 6 h.Then poured into crushed ice, the resulting precipitate was filtrated off, dried and recrystallized from ethanol to give 10 as yellow crystals.Yield:80%.M.p.:146˚C-148˚C; IR (KBr, cm−1):3416 (NH), 1762, 1686 (CO), 1280(C=S), 1600 (C=N), 3052, 2967, 2913(CH); 1H-NMR (DMSO-d6) 𝛿 ppm: 2.25 (s, 3H, CH3), 4.39 (s, 2H, CH2), 4.57(s, H, CH), 7.10- 8.02 (m, 23H, Ar-H), 10.31 (s, 1H, NH); Anal. Calcd. For C39H30ClN3O2S3 (704.32): C, 66.51; H, 4.29; Cl, 5.03; N, 5.97; S, 13.66.Found: C, 66.31; H, 4.09; Cl, 5.00; N, 5.77; S, 13.46.
2.1.8. 3,3-Bis[(ethoxycarbonyl)methylthio]-2-[1-(4-chlorophenyl)-4,5-diphenyl-1H-imidazol-2- ylthio]-N-p-tolylacrylamide12
To suspension of finely powdered of potassium hydroxide (0.02mol) in dry DMF(20 ml) at 0˚C the acetamide derivative 2 (0.01mol) was added, the resulted mixture was cooled at 10˚C in an ice bath, then carbon disulfide (0.01 mol) was added slowly over the course of 10 min. After complete addition, stirring of the reaction mixture was continued for 6 h. Then cooled again to 0˚C, ethyl chloroacetate(0.02 mol) was added slowly over the course of 10 min. After complete addition, stirring of the reaction mixture was continued for 6 h.Then poured into crushed ice, the resulting precipitate was filtrated off, dried and recrystallized from benzene to give 12 as yellow crystals. Yield: 65%.M.p.:120˚C -122˚C; IR (KBr, cm−1): 3252 (NH), 1736, 1685(CO), 3049, 2980, 2925(CH); 1H-NMR (DMSO-d6)𝛿 ppm: 1.13-1.23(m, 6H, 2-CH2 ?CH3), 4.33(s, 2H, CH2), 4.37(s, 2H, CH2), 4.04- 4.153 (m, 4H, 2CH2-CH3 ), 2.24(s, 3H, CH3), 7.09-7.51 (m, 18H, Ar-H), 10.32 (s, 1H, NH); Anal. Calcd.for C39H36ClN3O5S3 (758.37): C, 61.77; H, 4.78; Cl, 4.67; N, 5.54; S, 12.68.Found:C, 61.67; H, 4.58; Cl, 4.47; N, 5.34; S, 12.38.
2.1.9. 3,3-Bis(methylthio)-2-[1-(4-chlorophenyl)-4,5-diphenyl-1H-imidazol-2-ylthio]-N-p- tolylacrylamide13
Compound 13 was synthesized as mentioned above in synthesis of 12 but using methyl iodide (0.02 mol) ins- tead of ethyl chloroacetate, the resulting product was recrystallized from ethanol to give 13 as yellow crystals. Yield:60%.M.p.:166˚C-168˚C; IR (KBr, cm−1):3438(NH), 1674(CO), 3050, 2919 (CH); 1H-NMR (DMSO-d6)𝛿 ppm2.21(s, 3H, CH3), 7.18-7.68 (m, 19H, Ar-H and NH ), 2.28 (s, 6H, 2SCH3); MS m/z (%):614 ( M+, 20.64), 611(16.71), 568(13.154), 506(19.41), 415 (13.27), 315 (14.74), 252 (15.48), 206(17.44), 237(15.97), 75(100); Anal. Calcd.for C33H28ClN3OS3(614.24):C, 64.53; H, 4.59; Cl, 5.77; N, 6.84; S, 15.66. Found:C, 64.23; H, 4.39; Cl, 5.57; N, 6.64; S, 15.36.
2.1.10. 2-[1-(4-Chlorophenyl)-4,5-diphenyl-1H-imidazol-2-ylthio]-3,3-dihydrazino-N-p- tolylacrylamide 14
A mixture of compound 13 (0.01mol) and hydrazine hydrate (80%, 0.02 mol) was heated under reflux for 4 h, then left to cool. The obtained solid product was triturated with ethanol (10 ml), filtered off, washed with ethanol, dried and recrystallized from butanol afford compound 14 as white crystals.Yield:60%.M.p.:210˚C-213˚C; IR(KBr, cm−1):3297, 3269, 3196, 3126 (NH2, NH), 1682(CO), 3061, 2959 (CH); 1H-NMR(DMSO-d6) 𝛿 ppm: 2.24 (s, 3H, CH3), 7.09-7.56(m, 19H, Ar-H and NH), 10.33(s, 1H, NH ), 10.51 (s.1H, NH), 4.84(s, 2H, NH2), 4.89 (s, 2H, NH2); MS m/z (%):582(M+, 66.04), 517(13.21), 470 (50.94), 444(61.32), 397(100), 381 (58.49), 321 (24.53); Anal.Calcd.for C31H28ClN7OS(582.11): C, 63.96; H, 4.85; Cl, 6.09; N, 16.84; S, 5.51. Found: C, 63.66; H, 4.65; Cl, 6.00; N, 16.64; S, 5.41.
2.1.11. Reaction of 3,3-Bis(methylthio)-2-[1-(4-chlorophenyl)-4,5-diphenyl-1H-imidazol-2- ylthio]-N-p-tolylacrylamide 13 with Amines
A mixture of 13 (0.01 mol) suitable amine such as o-phenylenediamine, o-aminophenol and/or p-chloroaniline(0.01 mol) in DMF(25 ml) was heated under reflux for 6h.The reaction mixture was left to cool and then poured to ice cooled water (100 ml). The solid product that formed was filtered off, dried well and recrystallized from appropriate solvent.
2-(1H-Benzimidazol-2-yl)-2-[1-(4-chlorophenyl)-4,5-diphenyl-1H-imidazol-2-ylthio]-N-p-tolylacetamide 15a
White powder.Yield: 65%.M.p.:238˚C-240˚C (ethanol and DMF); IR(KBr, cm−1):3245, 3188 (NH); 3055, 2924, 2865(CH), 1659(CO), 1607(C=N); 1H-NMR (DMSO-d6)𝛿 ppm:2.25(s, 3H, CH3), 7.09-7.53(m, 22H, Ar-H); 7.95 (s, 1H, NH), 10.30 (s, 1H, NH), 4.61(s, 1H, CH); MS m/z (%):626 (M+, 29.10), 491 (212.31), 424 (29.10), 391 (31.97), 379(35.25), 328(26.64), 75(100); Anal. Calcd.for C37H28ClN5OS(626.17):C, 70.97; H, 4.51; Cl, 5.66; N, 11.18; S, 5.12.Found:C, 70.67; H, 4.21; Cl, 5.46; N, 11.08; S, 5.02.
2-(1,3-Benzoxazol-2-yl)-2-[1-(4-chlorophenyl)-4,5-diphenyl-1H-imidazol-2-ylthio]-N-p-tolylacetamide15b
Pale grey crystals.Yield: 55%.M.p.:176˚C-178˚C (ethanol); IR (KBr, cm−1) 3246 (NH); 1687 (CO), 1600 (C=N), 3048, 2922, 2864(CH); 1H-NMR(DMSO-d6) 𝛿 ppm: 2.24(s, 3H, CH3), 7.09-7.47(m, 22H, Ar-H); 10.43(s, 1H, NH), 4.16(s, 1H, CH); MSm/z (%): 627 (M+, 11.96), 611(11.52), 516 (10.49), 492(8.57), 405(8.86), 330 (9.16), 264 (10.34), 173(10.49), 77(100); Anal. Calcd.for C37H27Cl N4O2S( 627.15):C, 70.86; H, 4.34; Cl, 5.65; N, 8.93; S, 5.11. Found: C, 70.66; H, 4.04; Cl, 5.45; N, 8.73; S, 5.00.
2-[1-(4-Chlorophenyl)-4,5-diphenyl-1H-imidazol-2-ylthio]-3-(4-chlorophenylamino]-3-(methylthio)- N-p-tolylacrylamide 16
White powder.Yield: 65%.M.p.:190˚C-192˚C(ethanol); IR (KBr, cm−1) 3246, 3191 (NH); 1687(CO), 1599 (C=N); 1H-NMR (DMSO-d6)𝛿 ppm:2.25(s, 3H, CH3), 2.39(s, 3H, SCH3), 7.09-7.62 (m, 23H, Ar-H and NH); 10.29 (brs, 1H, NH); MSm/z (%):693(M+, 53.29), 631(43.13)622(40.72), 607(40.72), 471(35.93), 330(53.89), 234(35.93), 257(51.51), 272 (34.13), 77(100); Anal.Calcd.for C38H30Cl2N4OS2(693.70):C, 65.79; H, 4.36; Cl, 10.22; N, 8.08; S, 9.24.Found:C, 65.59; H, 4.26; Cl, 10.02; N, 8.00; S, 9.04.
2.1.12.4-[1-(4-Chlorophenyl)-4,5-diphenyl-1H-imidazol-2-ylthio]-3-(p-tolylamino)-5- (methylthio)-1H-pyrrole-2-carboxylic Acid 17
A mixture of compound 13 (0.01mol) and glycine (0.01 mol) in ethanol (30 ml) containing triethylamine (5drops) was heated under reflux for 8 h. The formed solid product was filtered off, dried and recrystallized from ethanol to give 17 as a yellow powder. Yield: 62%.M.p.:180˚C-182˚C; IR(KBr, cm−1):3436(OH), 3247, 3184 (NH); 1773(CO), 1607(C=N); 1HNMR((DMSO-d6)𝛿 ppm:2.25(s, 3H, CH3), 2.29(s, 3H, SCH3), 7.09-7.47(m, 20H, Ar-H and 2NH); 13.29(s, 1H, OH); MSm/z(%):623(M+, 7.89), 579(13.92), 575(21.87), 516(10.93), 480(19.28), 470(14.12), 390 (12.33), 293 (15.52), 261(13.12), 214(24.25), 77(100); Anal.Calcd.for C34H27ClN4O2S2(623.18):C, 65.53; H, 4.37; Cl, 5.69; N, 8.99; S, 10.29.Found:C, 65.23; H, 4.17; Cl, 5.49; N, 8.79; S, 10.09.
2.1.13. 3-Anilino-2-[1-(4-chlorophenyl)-4,5-diphenyl-1H-imidazol-2-ylthio]-N-p-tolyl-3- thioxopropan 19
To a stirred solution of powdered potassium hydroxide (0.02mol) in DMF (20 ml), compound 2 (0.02 mol)was added. After stirring for 30 min, phenyl isothiocyanate(0.02 mol) was added to the resulting mixture, stirring was continued for 6 h, and then poured over crushed ice containing hydrochloric acid.The solid product that formed was filtered off, washed with water, dried and recrystallized from ethanol to give19 as yellow crystals. Yield: 64%.M.p.:186˚C-188˚C; IR(KBr, cm−1):3246, 3191(NH), 3084, 2925(CH), 1656(C=O), 1606(C=N), 1238 (C=S); 1H-NMR(DMSO-d6)𝛿ppm:2.24(s, 3H, CH3), 10.40(s, 1H, NH), 10.20(s, 1H, NH), 7.05-7.59(m, 23H, Ar-H), 4.12(s, 1H, CH); MS m/z(%):645 (M+, 23), 629(22.03), 612(23), 554 (18.32), 521(20.91), 512(23), 526(18.12), 316 (22.65), 300 (187.12), 224(29.21), 192(20.21), 80(100); Anal. Calcd.for C37H29ClN4OS2(645.23): C, 68.87; H, 4.53; Cl, 5.49; N, 8.68; S, 9.94. Found:C, 68.67; H, 4.23; Cl, 5.29 N, 8.48; S, 9.64.
2.1.14. 3-Anilino-2-[1-(4-chlorophenyl)-4,5-diphenyl-1H-imidazol-2-ylthio]-3-(methylthio)-N-p- tolylacrylamide 20
To a stirred solution of potassium hydroxide (0.01 mol) in DMF (20 ml) was added compound 2(0.01mol).After the mixture was stirred for 30 min, phenyl isothiocyanate(0.01 mol) was added to the resulting mixture. Stirring was continued for 6 h, and then methyl iodide (0.01 mol) was added and stirring was continued for 6 h. The reaction mixture was poured onto ice-cold water. The solid product that formed was collected by filtration, dried and recrystallized from ethanol to give compound 20 as yellow crystals.Yield: 62%.M.p.:234˚C-236˚C; IR (KBr, cm−1): 3246, 3191 (NH), 1656 (C=O), 1604 (C=N), 3086, 2874(CH); 1H-NMR (DMSO-d6) 𝛿 ppm:2.25(s, 3H, CH3), 2.54 (s, 3H, SCH3), 10.30 (s, 1H, NH), 10.38(s, 1H, NH), 7.03-7.70 (m, 23H, Ar-H); MS m/z (%): 659 (M+, 44.26), 597 (63.93), 476 (27.87), 433(56.84), 366(51.64), 328(63.93), 296(42.62), 73(100); Anal. Calcd.for C38 H31ClN4OS2( 659.26):C, 69.23; H, 4.74; Cl, 5.38; N, 8.50; S, 9.73.Found:C, 69.03; H, 4.54; Cl, 5.08; N, 8.30; S, 9.53.
2.1.15.2-[1-(4-Chlorophenyl)-4,5-diphenyl-1H-imidazol-2-ylthio]-2-(3,4-diphenyl-thiazol- 2(3H)-ylidene)-N-p-tolylacetamide 21 and 2-[1-(4-Chlorophenyl)-4,5-diphenyl-1H- imidazol-2-ylthio]-2-(4-oxo-3-phenyl-1,3-thiazolidin-2-ylidene)-N-p-tolylacetamide 22
To a cold suspension of powdered potassium hydroxide (0.01mol) in DMF (20 ml) was added compound 2(0.01 mol) and phenyl isothiocyanate (0.01 mol).The reaction mixture was stirred at room temperature for 6 h, and then treated with phenacyl bromide and/or ethyl chloroacetate(0.01 mol) and the stirring was continued atroom temperature for further 10 h. The reaction mixture was poured into 50 ml of cold water. The result solid products were collected by filtration and recrystallized from a mixture of ethanol/DMF (1:1) to give compounds 21 and 22.
21:Yellow powder.Yield: 65%.M.p.:240˚C -242˚C; IR (KBr, cm−1): 3248(NH), 1659(C=O), 3056, 2937, 2871 (CH), 1604(C=N); 1H-NMR(DMSO-d6) 𝛿 ppm:2.24(s, 3H, CH3), 10.40(s, 1H, NH), 7.05-7.59 (m, 29 H, Ar-H andH-5 thiazoline); MS m/z (%):745(M+, 43.29), 695(40.24), 577(41.40), 450 (39.63), 421 (36.59), 313 (43.29), 217 (50.00), 172(46.34), 111(100).Anal.Calcd.for C45H33Cl N4OS2 (745.35):C, 72.51; H, 4.46; Cl, 4.76; N, 7.52; S, 8.60.Found: C, 72.31; H, 4.26; Cl, 4.46; N, 7.32; S, 8.50.
22:Pale grey crystals.Yield: 62%.M.p.:228˚C-230˚C; IR (KBr, cm−1):3245(NH), 3084, 2929, 2873 (CH), 1726, 1657(C=O); 1H-NMR (DMSO-d6) 𝛿 ppm:2.25(s, 3H, CH3), 10.41(s, 1H, NH), 7.06 - 7.60 (m, 23H, Ar-H), 4.13 (s, 2H, CH2); MS m/z (%):670(M+-CH3, 12.66), 654(10.97), 594(19.83), 503(14.77), 475 (12.24), 348 (10.97), 139 (14.98), 125(18.35), 55(100).Anal.Calcd.for C39H29ClN4O2S2(685.25):C, 68.36; H, 4.27; Cl, 5.17; N, 8.18; S, 9.36. Found: C, 68.06; H, 4.07; Cl, 5.00; N, 8.00; S, 9.16.
2.1.16. 2-[5-Benzylidene-4-oxo-3-phenyl-1,3-thiazolidin-2-ylidene]-2-[1-(4-chlorophenyl)-4,5- diphenyl-1H-imidazol-2-ylthio]-N-p-tolylacetamide 23
To a well-stirred solution of compound 22 (0.01mol) in DMF (20 ml), piperidine(0.2 ml) and benzaldehyde (0.01mol) were added. The reaction mixture was stirred at 80˚C for 3 h.The separated crystals was filtered, dried and recrystallized from ethanol to give 23 as white crystals. Yield: 60%.M.p.:252˚C-254˚C; IR (KBr, cm−1): 3249 (NH), 3090, 2997(CH), 1657, 1734(C=O); 1H-NMR(DMSO-d6)𝛿 ppm:2.25(s, 3H, CH3), 10.53(s, 1H, NH), 7.03-7.70 (m, 29H, Ar-H and =CH); MS m/z (%):773 (M+, 6.72), 723 (77.3), 707(57.14), 570(50.42), 479(54), 388(100), 372 (58.82)Anal.Calcd.forC46H33ClN4O2S2(773.36):C, 71.44; H, 4.30; Cl, 4.58; N, 7.24; S, 8.29.Found:C, 71.24; H, 4.00; Cl, 4.28; N, 7.04; S, 8.09.
2.2. Antimicrobial Assays
Synthesized compounds 2, 3, 6, 13, 15a, 15b, 17, 20, 21, 22 and 23 were screened for their antimicrobial activities in vitro against two species of Gram-positive bacteria, namely Staphylococcus aureus(RCMB 0100010) and Bacillus subtilis (RCMB 010067), two Gram-negative bacteria, namely Pseudomonas aeuroginosa(RCMB010043) and Escherichia coli (RCMB 010052) and against four species of fungi, namely Aspergillusfumigatus (RCMB 02568), Syncephalastrumracemosum(RCMB 05922), Geotrichumcandidum(RCMB05097) and Candida albicans (RCMB 05036).The antibacterial and antifungal activities were determined by means of inhibition% ± standard deviation at a concentration of 100 μg/ ml of tested samples [5] - [7] . Optical densities of antimicrobial were measured after 24 hours at 37˚C to bacteria and measured after 48 hours at 28˚C to fungal using a multidetection microplate reader at the Regional Center for Mycology and Biotechnology (Sun Rise-Tecan, USA at 600 nm) Al-Azhar University. Ampicillin, gentamicin and amphotericin B were used as references to evaluate the potency of the tested compounds under the same conditions.
3. Results and Discussion
3.1. Chemistry
The synthetic procedures adopted to obtain the target compounds are depicted inSchemes 1-4.S-Alkylation of 1-(4-chlorophenyl)-4,5-diphenyl-1H-imiazole-2-thione 1 with 2-chloro-N-p-tolylacetamide afforded the corre- sponding 2-(1H-imidazol-2-ylthio)-N-p-tolylacetamide derivative 2. The assignment of structure 2 was based on both elemental analysis and spectral data.1H-NMR spectrum of 2 in (DMSO-d6) revealed signals at 2.24 ppm corresponding to CH3 group and a single at 4.11 ppm for CH2 group. Moreover, mass spectrum showed a molecular ion peak at m/z 510 corresponding to a molecular formula C30H24ClN3OS.Further evidence for the structure of compound 2 was obtained through studying their chemical reactivity via some chemical reactions. Thus, interaction of compound 2 with p-chlorobenzaldehyde yielded the arylidene derivative 3(Scheme 1).1H-NMR spectrum in (DMSO-d6) of 3 show the disappearance of CH2 protons observed with the respective starting precursors 2 at δ 4.11 ppm, and the appearance multiple signals in the region at δ 6.41-7.27 ppm corresponding to
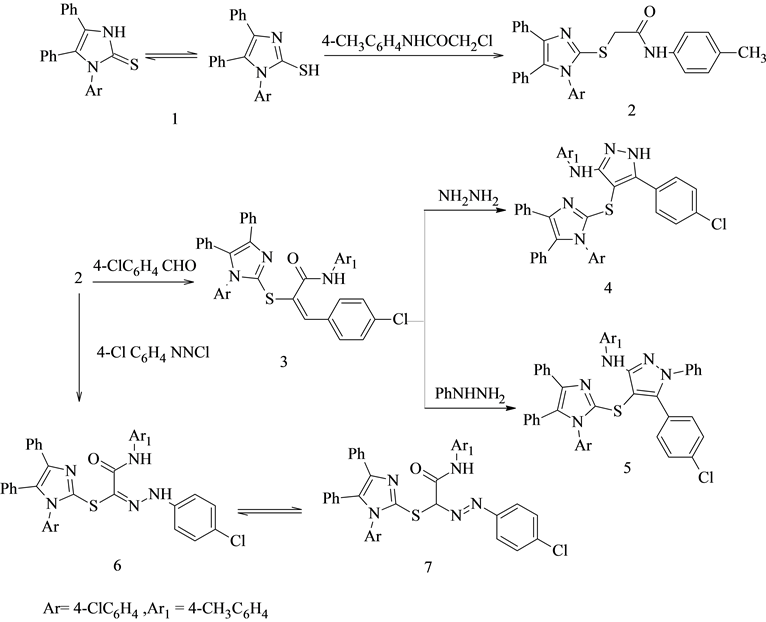
Scheme 1.Formation of compounds 2-6.

Scheme 2.Formation of compounds 8-13.

Scheme 3.Formation of compounds 14-17.

Scheme 4.Formation of compounds 18-22.
the aromatic protons together with 1Hbenzylidene C=CH proton. Mass spectrum of 3 showed a molecular ion peak at m/z 632 corresponding to the molecular formula C37H27Cl2N3OS (see experimental section).
Reaction of arylidene derivatives 3 withhydrazine hydrate in ethanol [8] and few drop of acetic acid gave the corresponding 1H-pyrazol-3-amine derivative 4.While, reaction 3 with phenyl hydrazine in the presence of acetic acid and few drops of hydrochloric acid [9] afforded the corresponding 1-phenyl-1H-pyrazol-3-amine 5.The structure of the pyrazole derivatives 4 and 5 were established on the basis of analytical and spectral data. The IR spectrum of 4 showed the disappearance of absorption band of C=O group and appearance of new absorption band of NH at 3238 cm−1.Mass spectrum of 4 showed a molecular ion peak at m/z 644 corresponding to the molecular formula C37H27Cl2N5S.
Diazotization of p-chloroaniline followed by coupling with active methylene group in compound 2 in pyridine yielded the hydrazone form 6 rather than the azo form 7 based on spectral data [10] .The 1H-NMR spectrum of compound 6 recorded in (DMSO-d6) revealed a signal at δ8.58 ppm, which could be attributed to hydrazone NH group(Scheme 1).
Reaction of compound 2 with carbon disulphide and one equivalent potassium hydroxide in dimethylformamide(DMF) gave non-isolated intermediate potassium salt 8.Treatment of the non-isolable potassium salts 8 with dilute hydrochloric acid [11] afforded the corresponding 2-(1H-imidazol-2-ylthio)-2-(p-tolylcarbamoyl)ethanedithioic acid 9.The assignment of structure 9 was based on both elemental analysis and spectral data.The IR spectrum displayed absorptions band at 3231cm−1(NH) and 1271 cm−1 (C=S).The mass spectrum showed the molecular ion peak at m/z586 corresponding to the molecular formula C31H24ClN3OS3.On the other hand, treatment of intermediate salt 8 with phenacyl bromide [12] to give the corresponding 3-[benzoylmethyl- thio]-2-[1H-imidazol-2-ylthio]-N-p-tolyl-3-thioxo-propaneamide derivatives 10.The 1H-NMR spectra of 10 in (DMSO-d6)revealed a single at 𝛿 4.39 ppm and δ 4.57ppm assigned to the CH2 and CH protons respectively(Scheme 2).
While the reaction 2 with carbon disulphide in the presence of two equivalent potassium hydroxide in DMF to give non-isolated potassium salt 11, which was allowed to react with halogenated compounds namely ethylchloroacetate[13] and methyl iodide [14] afforded the corresponding 3,3-bis[(ethoxycarbonyl)methylthio]-N-p-toly- lacrylamide 12 and 3,3-bis-(methylthio)-N-p-tolylacrylamide 13 respectively.We suggest a mechanism forthe for- mation of 12 in which the intermediate I is obtained first, then elimination of potassium chloride (Figure 1).
The structure of synthesis compound 12 and 13 ware elucidated on the basis of the elemental analysis and spectral data. For example, 1H-NMR spectra in (DMSO-d6) of 12 displayed two multiple at 1.13-1.23 and 4.04-4.13 for ethoxy protons of two carboethoxy group and two single at 4.33 and 4.37 ppm for two methylene protons. On the other hand, 1H-NMR spectrum (DMSO-d6) of 13 showed single signal at δ 2.28 ppm for 6 protons of two similar methyl protons. The mass spectrum of compound 13 showed a molecular ion peak at m/z 614 corresponding to a molecular formula C33H28ClN3OS3(Scheme 2).
Moreover, condensation of 13 with hydrazine hydrate afforded the corresponding 3, 3-dihydrazino-N-p-toly- lacrylamide derivative 14.The structure of 14 was identified as the reaction product on the basis of its elemental analysis and spectral data.The 1H-NMR spectrum of 14 showed a multiple signals in the region at δ 7.09-7.56 ppm corresponding to the aromatic protons together with the NH proton, two single signals at δ 4.84 ppm and 4.89 corresponding to the two NH2 protons, and another two single signals at δ 10.33 and δ 10.51 ppm assignable to two NH protons. Mass spectrum of 14 showed a molecular ion peak at m/z 582 corresponding to a molecular formula C31H28ClN7OS. In addition, the condensation of 13 with suitable amine namely o-phenylenedi- amine, and o-aminophenol [15] in refluxing absolute ethanol to afford the corresponding 2-(1H-benzimidazol- 2-yl)- and 2-(1,3-benzoxazol-2-yl)-N-p-tolylacetamide15a, b respectively(Scheme 3).The structures of compounds 15a, b were established and confirmed by their elemental analysis and spectral data (see experimental section). The formation of 15 a, b were assumed to proceed through nucleophilic attack of the two -NH2 group in o-phenylenediamine,orNH,OH groups ino-aminophenol to the ethylenic double bond in the compound 13 followed by elimination of two moles of methyl mercaptan(Figure 2).
S,S-acetals13 was converted to corresponding S,N-acetals by reacting with appropriate primary.Thus, reaction of 13 with p-chloroaniline afforded ketene N,S-acetals16(Scheme 3).The assignment of the structure of 16 was basedon spectral data. The IR spectrum of 16 showed absorption bands at 3246, 3191 cm−1 for two NH. Its 1H-NMR spectrum (DMSO-d6) of 16 showed single signal at δ2.39 ppm for SCH3 protons, δ 7.09-7.62 ppm corresponding to the aromatic protons together with the NH proton and single signals at δ 10.29 ppm assignable to NH. The mass spectrum of 16 showed a molecular ion peak at m/z 693 corresponding to the molecular formula C38 H30Cl2N4OS2.
Furthermore, the reaction of 13 with Glycine in ethanol containing triethylamine [16] afforded the corresponding 1H-pyrrole-2-carboxylic acidderivatives 17(Scheme 3).The assignment of the structure of 17was
Figure 1. Proposed mechanism formation of 3,3-bis[(ethoxycarbonyl)methylthio]-N-p-tolylacrylamide derivatives 12.
Figure 2. The proposed mechanism formation 15a, b.
based on spectral data.The IR spectrum of 17 showed absorption bands at 3436 cm−1 (OH) and 3247, 3184 cm−1 (NH). Its 1H-NMRspectrum (DMSO-d6) single signal at δ2.29 ppm for SCH3 protons, δ7.09-7.47 ppm corresponding to the aromatic protons together with the 2NH protons.The mass spectrum of 17 showed a molecular ion peak at m/z623corresponding to the molecular formula C34 H27Cl N4O2S2.
2-(1H-imidazol-2-ylthio)-N-p-tolylacetamide2 was utilized as a key intermediate for the synthesis of thiocarbamoyl derivative 19 via its reaction with phenyl isothiocyanate.Thus, reaction 2 with phenyl isothiocyanate in DMF in the presence of an equimolar amount of potassium hydroxide yielded the non-isolable intermediate potassium sulphide salt 18. Acidification of the potassium salt 18 with dilute hydrochloric acid afforded the corresponding thiocarbamoyl derivative 19, which can exist in two tautomericthione-thiol forms A and B (Scheme4). Assignment of the product 19 was based on elemental analysis and spectral data.1H-NMR spectrumdisplayed multiple signals at δ 7.05 - 7.59 ppm for aromatic protons and two single signals at δ 10.40 and 10.20 ppm assignable to two NH protons. Mass spectrum showed a molecular ion peak at m/z 645 corresponding to a molecular formula C37H29ClN4OS2.
Treatment of the non-isolable potassium sulfide salt 18 with methyl iodide [17] afforded the ketene N, S- acetal20. The structure of 20 was established on the basis of its elemental analysis and spectral data. Its IR spectrum showed absorption bands at 3246, 3191cm−1 due to two NH groups.On addition 1H NMR spectrum (DMSO-d6) displayed single signal at δ2.54 ppm for SCH3.The mass spectrum showed a molecular ion peak at m/z 659corresponding to a molecular formula C38H31ClN4OS2(Scheme 4).
On the other hand, reaction of 18 with phenacyl bromide and ethyl chloroacetate [18] afforded 2-(3, 4-di- phenyl-1,3-thiazol-2(3H)-ylidene)-and 2-(4-oxo-3-phenyl-1,3-thiazolidin-2-ylidene)-N-p-tolylacetamide 21 and 22 respectively.The structures of compounds 21 and 22 were established and confirmed by their elemental ana- lysis and spectral data.The 1H-NMR spectrum of 21 showed a multiple signals in the region at δ7.05-7.59 ppm corresponding to the aromatic protons together with the H-5 protons of the thiazole ring and a single signal at δ 10.40 ppm for NH proton. Mass spectrum of 21 revealed a molecular ion peak at m/z 745 corresponding to a molecular formula C45H33ClN4OS2. The IR spectrum of 22 showed absorption bands at 1726 cm−1 due to CO ofthiazolidinone ring. The 1H-NMR spectrum of 22 showed a single signal equivalent to two protons at 𝛿 4.13ppm which represent the CH2protons of the thiazolidinone ring.The Claisene Schmidt condensation of thiazolidin-5-one 22 with benzaldehyde [19] in DMF and in the presence of a catalytic amount of piperidine afforded arylidene derivatives 23(Scheme 4).The structures of latter products were confirmed based on elemental analysis and spectral data (see experimental section).
3.2. Antimicrobial Activity
Synthesized compounds 2, 3, 6, 13, 15a, 15b, 17, 20, 21, 22 and 23 were evaluated for antibacterial and antifungal activities.
3.2.1.Antibacterial Activity
Synthesized compounds 2, 3, 6, 13, 15a, 15b, 17, 20, 21, 22 and 23 were screened for their antibacterialactivities in vitro against Gram-positive namely Staphylococcus aureus(RCMB0100010) and Bacillus subtilis (RCMB 010067) andGram- negative Pseudomonas aeuroginosa (RCMB010043) and Escherichia coli (RCMB 010052).Ampicillin and gentamicin were used as references to evaluate the potency of the tested compounds.The inhibitory effects of the synthetic compounds against these organisms are given inTable 1,Figure 3.
In general, most of the tested compounds revealed better activity against the Gram-positive bacteria rather than the Gram-negative bacteria.Compounds 3, 13 and 15a exhibited excellent antibacterial activity against the tested organisms while compounds15b, 17, 20, 21 and 23 showed moderate antibacterial activity against the tested organisms and compound 2, 6 and 22 showed weak antibacterial activity against the tested organisms. In addition, all test compounds were found to be inactive against Pseudomonas aeuroginosa (RCMB010043).
3.2.2.Antifungal Activity
The newly synthesized compounds 2, 3, 6, 13, 15a, 15b, 17, 20, 21, 22 and 23 were screened for their antifungal activities in vitro against, Aspergillusfumigatus(RCMB02568),Syncephalastrumracemosum(RCMB 05922), Geotrichumcandidum(RCMB05097) and Candida albicans (RCMB05036).Amphotericin B was used as standards to evaluate the potency of the tested compounds.The inhibitory effects of the synthetic compounds against these organisms are given inTable 2,Figure 4.
Figure 3.Graphical representation of the antibacterial activity of tested compounds compared to ampicillin and gentamicin.
Figure 4.Graphical representation of the antifungal activity of tested compounds compared to amphotericin B.
Table 1.Antibacterial evaluation of the some synthesized compounds.
Table 2.Antifungal evaluation of the some synthesized compounds.
Compounds 13, 15a exhibited excellent antifunger activity, which is better than the amphotericin B against Syncephalastrumracemosum (RCMB05922), while its strong antifunger activity againstAspergillusfumigatus(RCMB 02568) and Geotrichumcandidum(RCMB05097)is comparable to amphotericin B. The compounds 3, 15b, 17, 20, 21, 22 and 23 showed strong moderate activity againstAspergillusfumigatus(RCMB02568),Syncephalastrumracemosum(RCMB05922) and Geotrichumcandidum(RCMB05097)compared to amphotericin B against. While the compounds 6 weak antifungal activity against Aspergillusfumigatus (RCMB02568), Syncephalastrumracemosum(RCMB05922), Geotrichumcandidum(RCMB05097).On the other hand, compound 2 inactive againstall organism. Furthermore, all test compounds were found to be inactive against Candida albicans(RCMB 05036).
4. Conclusion
In this paper, we report the synthesis of 2-(1H-imidazol-2-ylthio)-N-p-tolylacetamide2.The active methylene moiety of compound 2 was allowed to react with CS2and/or phenyl isothiocyanate in dimethylformamide in the presence of potassium hydroxide and yielded the non-isolable intermediate potassium sulphide salt 8, 11and 18, which is used as intermediate to synthesis series of novel substituted imidazole derivatives in good yield. Synthesized compounds 2, 3, 6, 13, 15a, 15b, 17, 20, 21, 22 and 23 were evaluated for antibacterial and antifungal activities.Most of the tested compounds revealed better activity against the Gram-positive rather than the Gram-negative bacteria. Compound 13 exhibited excellent antibacterial activity against Staphylococcus aureus(RCMB0100010),Bacillus subtilis (RCMB 010067) and Escherichia coli (RCMB 010052).Compounds 13, 15a exhibited excellent antifunger activity, which is better than the amphotericin B against Syncephalastrumracemosum(RCMB05922).
References
- Dieter, R.K. (1986) α-Oxo Ketene Dithioacetals and Related Compounds: Versatile Three-Carbon Synthons.Tetrahedron, 42, 3029-3096.http://dx.doi.org/10.1016/S0040-4020(01)87376-2
- Junjappa, H.,Ila, H. and Asokan, C.V.(1990) α-Oxoketene-S,S-, N,S- and N,N-acetals: Versatile Intermediates in Organic Synthesis.Tetrahedron, 46, 5423-5506.http://dx.doi.org/10.1016/S0040-4020(01)87748-6
- Khali, M.A.,Sayed, S.M. and Raslan, M.A. (2012)Reactivity of 2-Cyano-N-(4-(1-methyl-1H-benzo[d]imidazol-2-yl)-3-(methylthio)-1-phenyl-1H-pyrazol-5-yl)acetamide:A Facile Synthesis of Pyrazole, Thiazole, 1,3,4-Thiadiazole and PolysubstitutedThiophene Derivatives. American Journal ofOrganic Chemistry, 2, 161-170. http://dx.doi.org/10.5923/j.ajoc.20120206.06
- Abdelhamid, A.O and Afifi, M.A.(2010) Synthesis of Some New Thiazoles and Pyrazolo[1,5-a]pyrimidines Containing an Antipyrine Moiety.Synthetic Communications, 40, 1539-1550.http://dx.doi.org/10.1080/00397910903100726
- Kaya, E.G.,Ozbilge, H.and Albayrak, S.(2009)Determination of Effect of Gentamicin against Staphylococcus aureus by Using Microbroth Kinetic System.AnkemDerg, 23, 110-114.
- Sofy, A.R.,Hmed, A.A.,Sharaf, A.M. and El-Dougdoug, K.A.(2014)Structural Changes of Pathogenic Multiple Drug Resistance Bacteria Treated with T. vulgarisAqueous Extract.Nature and Science,12, 83-88.
- Caldeira, E.M., Osório, A.,Oberosler, E.L.,Vaitsman, D.S.,Alviano, D.S. and Nojima, M.D.G.(2013) Antimicrobial and Fluoride Release Capacity of Orthodontic Bonding Materials.Journal of Applied Oral Science, 21, 327-334. http://dx.doi.org/10.1590/1678-775720130010
- Shabaan, M.,Taher, A. and Osman, E.O.(2011) Synthesis of Novel 3,4-Dihydroquinoxalin-2(1H)-one Derivatives.European Journal of Chemistry, 3, 365-371.
- Voskiene, A.,Mickevicius, V. and Mikulskiene, G.(2007) Synthesis and Structural Characerization of Products Condensation4-Carboxy-1-(4-styrylcarbonylphenyl)-2-pyrrolidinones with Hydrazines.ARKIVOC:Archive for Organic Chemistry,2007,303-314.http://dx.doi.org/10.3998/ark.5550190.0008.f29
- Mohare, R.M.,Fleit, D.H.and Sakka, O.K. (2011) Novel Synthesis of Hydrazide-Hydrazone Derivatives and Their Utilization in the Synthesis of Coumarin, Pyridine, Thiazole and Thiophene Derivatives with Antitumor Activity.Molecules,16, 16-27.http://dx.doi.org/10.3390/molecules16010016
- El-Bayouki, K.A., Basyounia, W.M., Mohamed, Y.A., Aly, M.M. and Abbas, S.Y.(2011) Novel 4(3H)-Quinazolinones Containing Biologically ActiveThiazole, Pyridinone and Chromene of Expected Antitumor and Antifungal Activities.European Journal of Chemistry,2, 455-462. http://dx.doi.org/10.5155/eurjchem.2.4.455-462.171
- Elgemeie, G.H., Elghandour, A.H.,Ali, H.A. and Hussein, A.M.(2002) Novel 2-Thioxohydantoin Ketene Dithioacetals:Versatile Intermediates for Synthesis of Methylsulfanylimidazo[4,5-c]pyrazole and Methylsulfanylpyrrolo[1, 2-c]imi- dazoles.Synthetic Communications, 32, 2245-2253.http://dx.doi.org/10.1081/SCC-120005435
- Wang, Y., Dong, D., Yang, Y., Huang, J., Ouyang, Y.and Liu, Q. (2007) A Facile and Convenient One-Pot Synthesis of PolysubstitutedThiophenes from 1, 3-Dicarbonyl Compounds in Water.Tetrahedron,63, 2724-2728. http://dx.doi.org/10.1016/j.tet.2006.12.090
- Khalil, A.M., Berghot, M.A. andGoudal, M.A.(2009) Synthesis and Antibacterial Activity of Some New Heterocycles Incorporating Phthalazine. European Journal of Medicinal Chemistry, 44, 4448-4454. http://dx.doi.org/10.1016/j.ejmech.2009.06.003
- Elgemeie, G.H., Elghandour, A.H. and AbdElaziz, G.W.(2003)Novel Synthesis of Heterocyclic Ketene N,N-, N,O-, and N,S-Acetals Using CyanoketeneDithioacetals. Synthetic Communications, 33, 1659-1664. http://dx.doi.org/10.1081/SCC-120018927
- Sommena, G.L.,Comelb, A.and Kirsch, G. (2005)An Easy Access to Variously Substituted Pyrroles Starting from Ketene Dithioacetals. Synthetic Communications, 35, 693-699.http://dx.doi.org/10.1081/SCC-200050365
- Bondock, S., Rabie, R., Etman, H.A.and Fadda, A.(2008) Synthesis and Antimicrobial Activity of Some New Heterocycles Incorporating Antipyrine Moiety. European Journal of MedicinalChemistry, 43, 2122-2129. http://dx.doi.org/10.1016/j.ejmech.2007.12.009
- Bondock, S., Fadaly, W.and Metwally, M.A.(2010) Synthesis and Antimicrobial Activity of Some New Thiazole, Thiophene and Pyrazole Derivatives Containing Benzothiazole Moiety. European Journal of Medicinal Chemistry, 45, 3692-3701.http://dx.doi.org/10.1016/j.ejmech.2010.05.018
- Rao, R.M., Reddy, G.N. and Sreeramulu, J.(2011) Synthesis of Some New Pyrazolo-Pyrazole Derivatives Containing Indoles with Antimicrobial Activity. Der Pharma Chemica, 3, 301-309.






