International Journal of Organic Chemistry
Vol.3 No.2(2013), Article ID:32999,6 pages DOI:10.4236/ijoc.2013.32013
A Facile Synthesis of 9,10-Dimethoxybenzo[6,7]- oxepino[3,4-b]quinolin-13(6H)-one and Its Derivatives
Key Laboratory of Theoretical Chemistry of Environment, Ministry of Education, School of Chemistry and Environment, South China Normal University, Guangzhou, China
Email: *yangdq@scnu.edu.cn, *longyh@scnu.edu.cn
Copyright © 2013 Dingqiao Yang et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received March 28, 2013; revised May 5, 2013; accepted May 17, 2013
Keywords: The Intramolecular Friedel-Crafts Acylation Reaction: 9,10-Dimethoxybenzo[6,7]oxepino[3,4-b]quinolin-13(6H)-one and Its Derivatives: 6,7-Dimethoxy-2-(phenoxymethyl)quinoline-3-carboxylic Acid: Ethyl 6,7-dimethoxy-2-(phenoxymethyl)quinoline-3-carboxylate: PPA
ABSTRACT
A concise and efficient method for the synthesis of novel 9,10-dimethoxybenzo[6,7]oxepino[3,4-b]quinolin13(6H)-one and its derivatives 7a-p has been developed via the intramolecular Friedel-Crafts acylation reactions of 6,7-dimethoxy-2-(phenoxymethyl)quinoline-3-carboxylic acids 6a-p with polyphosphoric acid (PPA) as catalyst and solvent under mild conditions. The key intermediates 6a-p were prepared through the in situ formation of ethyl 6,7-dimethoxy-2-(phenoxymethyl)quinoline-3-carboxylates 5a-p followed by hydrolysis with aqueous ethanolic sodium hydroxide solution. The novel synthetic method has the advantages of good yields, easy work-up, and environmentally friendly character, which may provide a novel highly efficient process for making quinoline and related azaheterocycle libraries.
1. Introduction
Quinoline-fused ring systems are a back-bone of many natural products and pharmacologically significant compounds and display a broad range of biological activities [1-3], including antiasthmatic [4-6], antibacterial [7,8], anti-inflammatory [9] and antihypertensive [10,11] properties. In addition to the medicinal applications, quinolines have been employed in the study of bioorganic and bioorganometallic processes [12]. In addition, quinoline derivatives have been widely utilized as ligands for preparing metal complexes [13-15] and as useful materials for organic synthesis [16-19]. As a result, the synthesis of novel quinoline-fused compounds remains as an attractive topic in area of organic synthesis. For example, Liu and Gao recently reported the synthesis of quinolinefused tetracyclic benzoxepinoquinolinones, which showed excellent anti-inflammatory activities [20,21]. In literature, there were a wide variety of methods available for the construction of fused ring compounds, including Friedländer condensation reactions [22], radical cyclization reactions [23], and intramolecular Friedel-Crafts acylation reactions [21,24], conventionally, intramolecular Friedel-Crafts acylation reaction could be achieved by treating aryl acids with a variety of Lewis acid including HF (liquid), H2SO4, TiCl4, AlCl3, and AlCl3/ NaCl [25]. Polyphosphoric acid (PPA) was also used as catalyst and solvent for the intramolecular Friedel-Crafts acylation reaction which was once used in the synthesis of quinoline-fused tetracyclic benzoxepinoquinolinones [26]. However, there were several shortcomings in terms of operational simplicity, cost of the reagent, harsh reaction conditions, and relatively low yield. Therefore, a convenient and efficient procedure is highly demanded. In this paper, we reported an efficient and environment friendly method for the synthesis of new quinoline derivatives 5a-p, 6a-p and tetraheterocyclic compounds 7a-p with good to excellent yields.
2. Results and Discussion
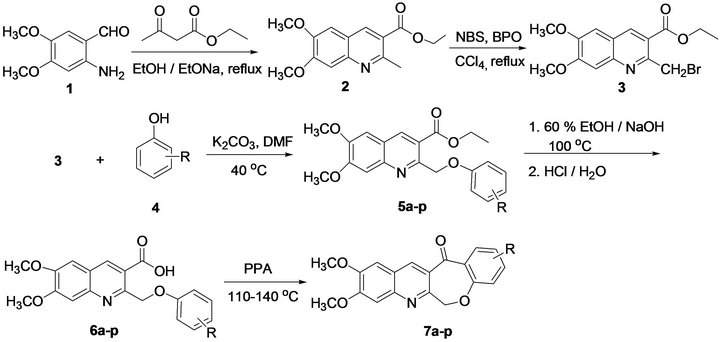
The starting material 2-amino-4,5-dimethoxybenzaldehyde 1 was obtained by reduction of 4,5-dimethoxy-2- itrobenzaldehyde according to our previous procedure [6]. Our approach to the synthesis of 2 were based on the Friedländer condensation strategy. The condensation reaction of the 2-amino-4,5-dimethoxybenzaldehyde 1 with ethyl acetoacetate in absolutely anhydrous ethanol, and in the presence of catalytic amount of sodium ethoxide, yielded the expected ethyl 6,7-dimethoxy-2-ethylquinoline-3-carboxylate 2 in high yields (86%). At the beginning, NaOH was used as a catalyst for the Friedländer reactions. However, it was found that the yield of the target compound was low. In seeking for a better catalyst, we found sodium ethoxide was better than sodium hydroxide for the Friedländer reactions. When it was used at low molar ratio (10 mol %) for the Friedländer reactions. Compound 3 was synthesized by Wohleigler bromination of compounds 2 using N-bromosuccinamide (NBS) in the presence of dibenzyl peroxide (BPO, 6 mol %) (Scheme 1). N-Bromosuccinamide (NBS) has been known for long time as a superior brominating agent to bromine in term of convenience of the handling. However, the preparation of monobrominated compounds from unsubstituted ethyl 6,7-dimethoxy-2-ethylquinoline-3-carboxylate 2 was found to be quite difficult as the bromo compounds were prone to underling disproportionation to dibrominated products. In the present case, the reaction of 2 with NBS using BPO as chain initiation in CCl4 as the aprotic solvent afforded the corresponding monobromo product 3 in an yield of 68% and in short reaction time (0.5 - 1.0 h) at reflux. Only trace of dibrominated product could be found in the reaction as the by-products.
The ethyl 6,7-dimethoxy-2-(phenoxymethyl)quinolinecarboxylates 5a-p were prepared by the Willamson reaction of ethyl 2-bromomethyl-6,7-dimethoxyquinoline- 3-carboxylate 3 with different substituted group phenols 4. The reaction was performed in DMF with an excess of K2CO3 as catalyst. After stirring at 40˚C for about 30 min, water was added and solid materials were gradually precipitated from solution, which were collected by suction filtration and washed by cold water. The crude products were dissolved in minimum amount of dichloromethane, and purified by flash chromatography to afford pure products 5a-p in high yields (up to 98%). It was also worth pointing out that electron-withdrawing groups on the phenyl ring of phenols were found to have favorable
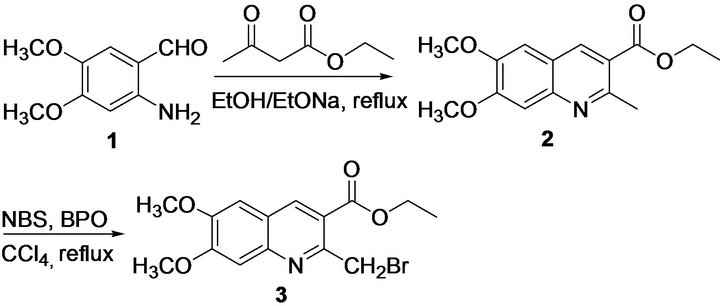
Scheme 1. Synthesis of ethyl 2-(bromomethyl)-6,7-dimethoxyquinoline-3-carboxylate 3.
impacts on the reaction in terms of reaction time and product yields (Table 1).
Compound 6,7-dimethoxy-2-(phenoxymethyl)quinoline-3-carboxylic acids 6a-p were prepared via in situ formation of compounds 5a-p followed by hydrolysis with aqueous ethanolic sodium hydroxide solution. The hydrolysis reaction was monitored by thin layer chromatography. After the reaction was confirmed to be completed, 1 M HCl was added slowly and the solid materials were gradually precipitated from the solution, which were collected by suction filtration and washed with cold water. The crude product was further purified by recrystallization from 95% ethanol, which afforded pure product 6a-p in very high yield (up to 96%) (Table 2).
PPA has been widely used for effecting intramolecular cyclizations under mild conditions. In connection with our studies, we envisioned that this reagent would be potentially useful to prepare compounds 7 through the intramolecular cyclization of 6,7-dimethoxy-2-(phenoxymethyl)quinoline-3-carboxylic acids 6a-p. It was found that 6a after simply dissolving in PPA and heating to only 110˚C afforded 7a in 54% yield within 45 min. PPA was quenched by pouring the reaction mixture slowly with stirring into saturated sodium carbonate icewater solution. The expected product was precipitated in the solution and was collected by suction filtration. Encouraged by the successful synthesis of compound 7a, we used this protocol for the intramolecular cyclization of
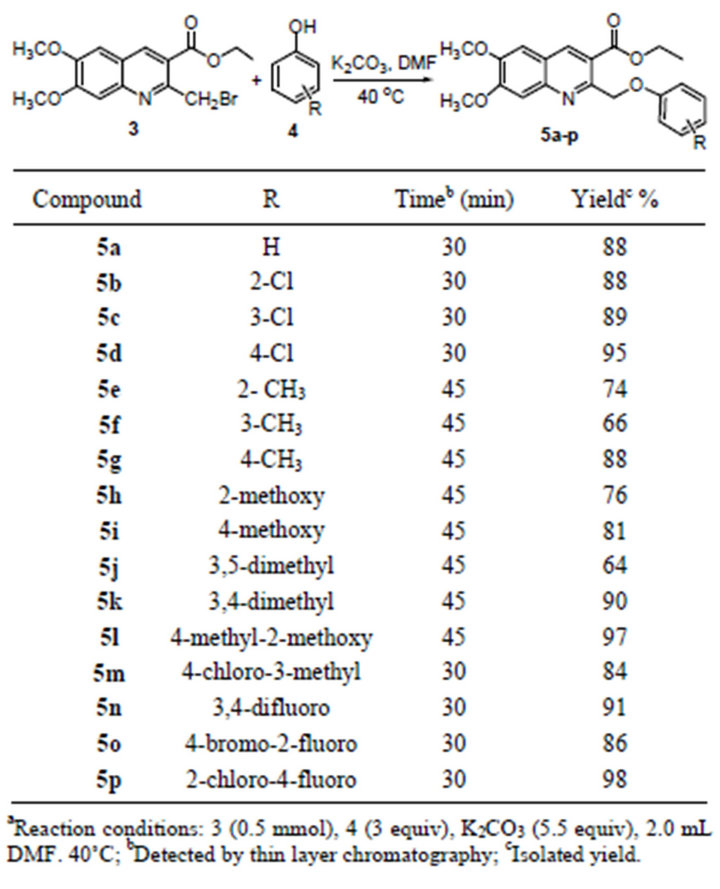
Table 1. Synthesis of ethyl 6,7-dimethoxy-2-(phenoxymethyl)- quinoline-3-carboxylates 5a-p.
other substrates 6b-p. It was found that the method worked well, and all the 6,7-dimethoxy-2-(phenoxymethyl)quinoline-3-carboxylic acids 6a-p were smoothly converted to the corresponding cyclic products with moderate yields (Table 3).
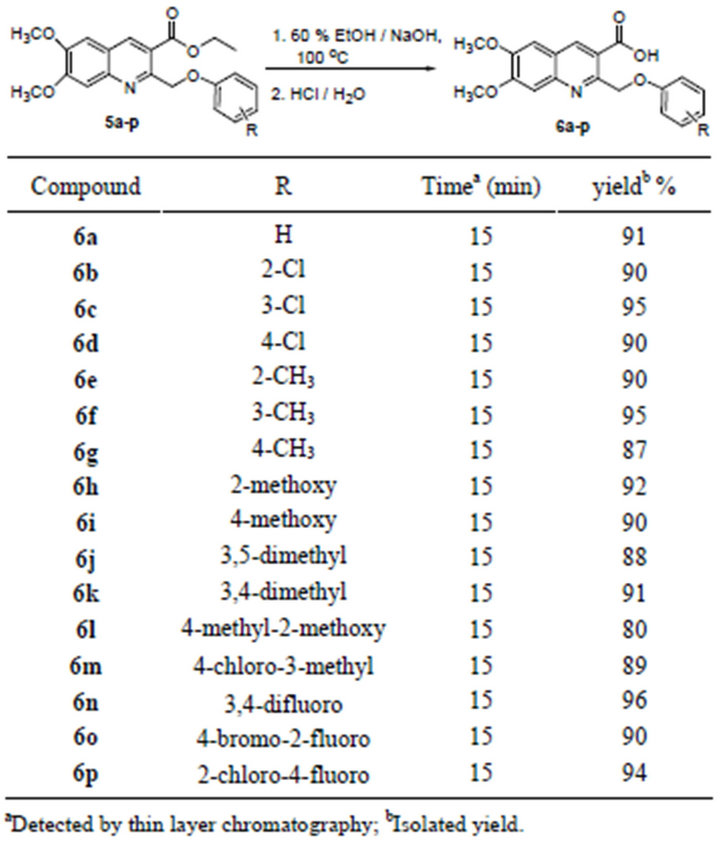
Table 2. Synthesis of 6,7-dimethoxy-2-(phenoxymethyl)- quinoline-3-carboxylic acids 6a-p.
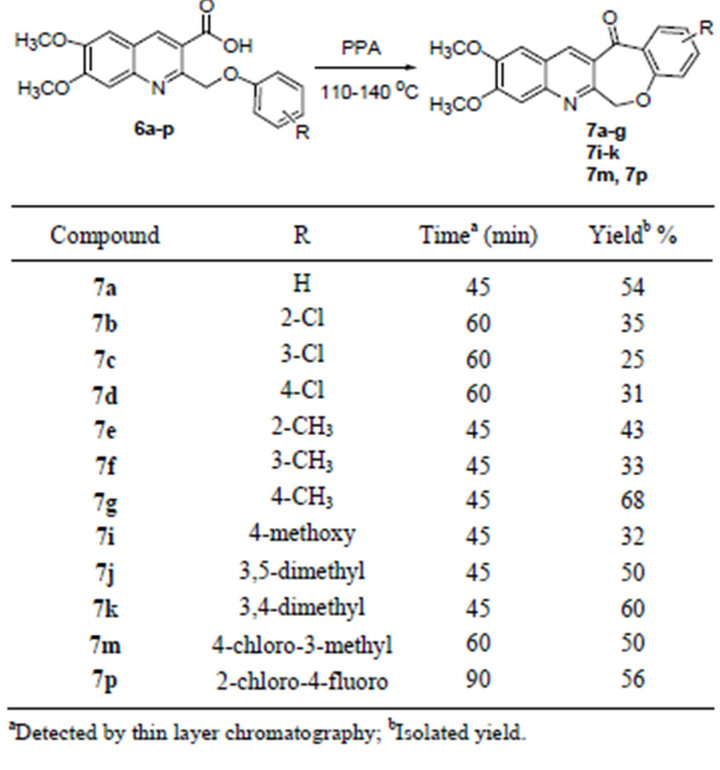
Table 3. Synthesis of 9,10-dimethoxybenzo[6,7]oxepino- [3,4-b]quinolin-13(6H)-one and its derivatives 7a-p.
A plausible intramolecular Friedel-Crafts acylation mechanism was proposed as outlined in Scheme 2. The substrate 6 was treated with polyphosphoric acid to form the phosphorate-carboxylate anhydride A, which further underwent the intramolecular Friedel-Crafts acylation to afford cyclic ketone 7 (Scheme 2).
3. Experimental Section
3.1. Synthesis of Ethyl 6,7-dimethoxy-2- methylquinoline-3-carboxylate 2
To a solution of 2-amino-4,5-dimethoxybenzaldehyde 1 (181 mg, 1 mmol) and ethyl acetoacetate (0.2 mL) in anhydrous ethanol (5 mL) was added sodium ethoxide (6.8 mg, 0.10 mmol). The solution was stirred at reflux for 0.5 h. The mixture was cooled down to room temperature. The solvent was removed by an half of its volume under vacuum. 5 mL water was added slowly and a solid was gradually precipitated from solution and was filtered, the crude product was recrystallized from 95% ethanol and obtained a white solid 2 (236 mg, 86%), mp 110˚C - 112˚C. Spectral data is identical to that reported in the literature [27].
3.2. Synthesis of Ethyl 2-(bromomethyl)-6,7- dimethoxyquinoline-3-carboxylate 3
N-Bromosuccinamide (NBS, 1.3 mmol, 231 mg) and benzoyl peroxide (BPO, 0.03 mmol, 7.3 mg) was added to a solution of ethyl 6,7-dimethoxy-2-methylquinoline- 3-carboxylate 2 (275 mg, 1 mmol) in CCl4 (10 mL ). The yellow reaction mixture was heated to reflux. The reaction was completed as judged by thin layer chromatography. The mixture was cooled to room temperature and then filtered to remove insoluble materials. The filtrate was washed with cold saturated aqueous NaHCO3 and then H2O. The combined organic layer dried over anhy-
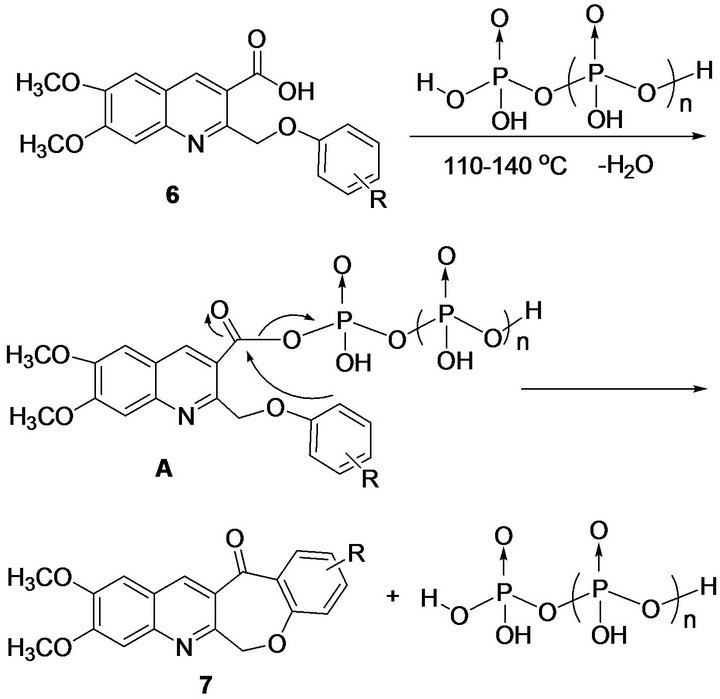
Scheme 2. Proposed mechanism for the formation of 9,10- dimethoxybenzo[6,7]oxepino[3,4-b]quinolin-13(6H)-ones 7a-p.
drous Na2SO4 and then filtrated. After removing the solvent under reduced pressure, the residue was purified by column chromatography to get 3 (68%) as a white solid, mp 158˚C - 160˚C. 1H NMR (400 MHz, CDCl3) δ 8.69 (s, 1H), 7.42 (s, 1H), 7.12 (s, 1H), 5.19 (s, 2H), 4.48 (q, J = 7.2 Hz, 2H), 4.06 (s, 3H),4.04 (s, 3H), 1.48 (t, J = 7.2 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 165.8, 154.7, 154.2, 150.8, 146.0, 138.9, 122.4, 120.9, 107.7, 105.4, 61.6, 56.4, 56.2, 33.9, 14.3. MS (ESI) calcd for C15H16BrNO4 (M+): 353.03; Found: 354.32 (M + H)+. Anal. Calcd. for C15H16BrNO4: C, 50.86; H, 4.55; N, 3.95. Found: C, 50.74; H, 4.57; N, 3.96.
3.3. General Procedure (I) for the Synthesis of 6,7-Dimethoxy-2-(phenoxymethyl) quinoline-3-carboxylates 5a-p
K2CO3 (2.8 mmol) was added to a solution of phenols (1.5 mmol) in anhydrous DMF (9 mL), and the mixture was stirred at room temperature for 20 min. Then ethyl 2-bromomethyl-6,7-dimethoxyquinoline-3-carboxylate 3 (0.5 mmol) was added and the mixture was slowly heated to 40˚C. The reaction was completed as judged by thin layer chromatography. Some water was added and a solid gradually precipitated from solution and was filtered and washed with water. The crude products were purified by column chromatography to get pure products 5a-p.
3.4. General Procedure (II) for the Synthesis of 6,7-Dimethoxy-2-(phenoxymethyl)quinoline-3-carboxylic Acids 6a-p
6,7-Dimethoxy-2-(phenoxymethyl)quinoline-3-carboxylates 5a-p (0.35 mmol) was added to a solution of sodium hydroxide (14 mmol) in 60% ethanol (8 mL) and the mixture was heated to reflux. The reaction was completed as judged by thin layer chromatography. The solution was cooled to room temperature and was reduced by half of its volume. 1 M HCl was added slowly and a solid gradually precipitated from solution and was filtered, washed with cold water. The crude products were recrystallized from 95% ethanol to get pure product 6a-p.
3.5. General Procedure (III) for the Synthesis of 9,10-Dimethoxybenzo[6,7]oxepino[3,4-b]- quinolin-13(6H)-ones 7a-p
6,7-Dimethoxy-2-(phenoxymethyl)quinoline-3-carboxylic acids 6a-p (0.25 mmol) and polyphosphoric acids (PPA) (5 g) were added to round flask (15 mL) and stirred at 110˚C - 140˚C for about 1 h. Then the reaction mixture was poured slowly with stirring into an icy saturated sodium carbonate solution. The mixture was extracted with ethyl acetate (3 × 10 mL). The combined organic layer was washed with saturated aqueous NaHCO3 (2 × 10 mL) and then H2O (2 × 10 mL), the combined organic layer dried over anhydrous CaCl2. After removing the solvent under reduced pressure, the residue was purified by column chromatography to get products 7a-p.
4. Conclusion
We described a very efficient and environmentally friendly method for the synthesis of novel 9,10-dimethoxybenzo[6,7]oxepino[3,4-b]quinolin-13(6H)-ones 7a-p through the intramolecular Friedel-Crafts acylation, the hydrolysis, the Willamson reaction, the Wohl-Zeigler bromination and Friedländer condensation. The experimental procedure was very simple. The advantages of these methods included high yield, low cost, and easy purification. The novel method may be potentially useful for the construction of large quinoline and related azaheterocycle libraries. The inhibition of acetylcholinesterase (AChE) of these new quinoline derivatives are currently under evaluation.
5. Acknowledgements
We are grateful to the National Natural Science Foundation of China (Nos. 21172081 and 20772036), Guangzhou Pearl River New Star Plan of Science and Technology Program (2012J2200015) and Produce and Learning and Research Project of Education Department of Guangdong Province (No. 2011A090200039) for financial support. We are also grateful to Dr. Qingqi Chen of MedKoo Biosciences for reviewing this manuscript.
REFERENCES
- R. D. Larsen, E. G. Corley, A. O. King, J. D. Carrol, P. Davis, T. R.Verhoeven, P. J. Reider, M. Labelle, J. Y. Gauthier, Y. B. Xiang and R. J. Zamboni, “Practical Route to a New Class of LTD4 Receptor Antagonists,” Journal of Organic Chemistry, Vol. 61, No. 10, 1996, pp. 3398-3405. doi:10.1021/jo952103j
- Y. L. Chen, K. C. Fang, J. Y. Sheu, S. L. Hsu and C. C. Tzeng, “Synthesis and Antibacterial Evaluation of Certain Quinolone Derivatives,” Journal of Medicinal Chemistry, Vol. 44, No. 14, 2001, pp. 2374-2377. doi:10.1021/jm0100335
- J. P. Michael, “Quinoline, quinazoline and acridone alkaloids,” Natural Product Reports, Vol. 15, No. 6, 1998, pp. 595-606. doi:10.1039/a815595y
- M. E. Zwaagstra, H. Timmerman, A. C. Van De Stolpe, F. J. De Kanter, M. Tamura, Y. Wada and M. Q. Zhang, “Synthesis and Structure-Activity relatIonships of Carboxyflavones as Structurally Rigid CysLT1 (LTD4) Receptor Antagonists,” Journal of Medicinal Chemistry, Vol. 41, No. 9, 1998, pp. 1428-1438. doi:10.1021/jm970179x
- A. Von Sprecher, M. Gerspacher, A. Beck, S. Kimmel, H. Wiestner, G. P. Anderson, U. Niederhauser, N. Subramanian and M. A. Bray, “Synthesis and SAR of a Novel, Potent and Structurally Simple LTD4 Antagonist of the Quinoline Class,” Bioorganic & Medicinal Chemistry Letters, Vol. 8, No. 8, 1998, pp. 965-970. doi:10.1016/S0960-894X(98)00137-1
- D. Doub`e, M. Blouin, C. Brideau. C. Chan, S. Desmarais, D. Ethier, J. P. Falgueyret, R. W. Friesen, M. Girard, Y. Girard, J. Guay, P. Tagari and R. N. Young, “Quinolines as Potent 5-Lipoxygenase Inhibitors: Synthesis and Biological Profile of L-746,530,” Bioorganic & Medicinal Chemistry Letters, Vol. 8, No. 10, 1998, pp. 1255- 1260. doi:10.1016/S0960-894X(98)00201-7
- B. Vaitilingam, A. Nayyar, P. B. Palde, V. Monga, R. Jain, S. Kaur and P. P. Singh, “Synthesis and Antimycobacterial Activities of Ring-Substituted Quinolinecarboxylic Acid/Ester Analogues. Part 1,” Bioorganic & Medicinal Chemistry, Vol. 12, No. 15, 2004, pp. 4179-4188. doi:10.1016/j.bmc.2004.05.018
- Y. L. Chen, K. C. Fang, J. Y. Sheu, S. L. Hsu and C. C. Tzeng, “Synthesis and Antibacterial Evaluation of Certain Quinolone Derivatives,” Journal of Medicinal Chemistry, Vol. 44, No. 14, 2001, pp. 2374-2377. doi:10.1021/jm0100335
- G. Roma, M. D. Braccio, G. Grossi, F. Mattioli and M. Ghia, “1,8-Naphthyridines IV. 9-Substituted N, N-Dialkyl -5-(Alkylamino or Cycloalkylamino) [1,2,4]Triazolo[4,3- a][1,8]naphthyridine-6-carboxamides, New Compounds with Anti-Aggressive and Potent Anti-Inflammatory Activities,” European Journal of Medicinal Chemistry, Vol. 35, No. 11, 2000, pp. 1021-1035. doi:10.1016/S0223-5234(00)01175-2
- Y. Morizawa, T. Okazoe, S.-Z. Wang, J. Sasaki, H. Ebisu, M. Nishikawa and H. Shinyama, “A Novel Trifluoromethanesulfonamidophenyl-Substituted Quinoline Derivative, GA 0113: Synthesis and Pharmacological Profiles,” Journal of Fluorine Chemistry, Vol. 109, No. 1, 2001, pp. 83-86. doi:10.1016/S0022-1139(01)00383-9
- P. L. Ferrarini, C. Mori, M. Badawneh, V. Calderonem, R. Greco, C. Manera, A. Martinelli, P. Nieri and G. Saccomanni, “Synthesis and β-Blocking Activity of (R,S)-(E)- Oximeethers of 2,3-Dihydro-1,8-naphthyridine and 2,3- Dihydrothiopyrano [2,3-b]Pyridine: Potential Antihypertensive Agents. Part IX,” European Journal of Medicinal Chemistry, Vol. 35, No. 9, 2000, pp. 815-826. doi:10.1016/S0223-5234(00)00173-2
- D.-Q. Yang, K.-L. Jiang, J.-N. Li and F. Xu, “Synthesis and Characterization of Quinoline Derivatives via the Friedländer Reaction,” Tetrahedron, Vol. 63, No. 32, 2007, pp. 7654-7658. doi:10.1016/j.tet.2007.05.037
- Y.-Z. Hu, G. Zhang and R. P. Thummel, “Friedländer Approach for the Incorporation of 6-Bromoquinoline into Novel Chelating Ligands,” Organic Letter, Vol. 5, No. 13, 2003, pp. 2251-2253. doi:10.1021/ol034559q
- A. Ohashi, A. Tsuguchi, H. Imura and K. Ohashi, “Synergistic Cloud Point Extraction Behavior of Aluminum(III) with 2-Methyl-8-quinolinol and 3,5-Dichlo-rophenol,” Analytical Sciences, Vol. 20, No. 7, 2004, pp. 1091-1093. doi:10.2116/analsci.20.1091
- H. Wang, Z. Liu, C. Liu, D. Zhang, Z. Lu, H. Geng, Z. Shuai and D. Zhu, “Coordination Complexes of 2-(4-Quinolyl)nitronyl Nitroxide with M(hfac)2 [M = Mn(II), Co(II), and Cu(II)]: Syntheses, Crystal Structures, and Magnetic Characterization,” Inorganic Chemistry, Vol. 43, No. 13, 2004, pp. 4091-4098. doi:10.1021/ic049946q
- C.-L. Chiang and C.-F. Shu, “Synthesis and Characterization of New Polyquinolines Containing 9,9‘-Spirobifluorene Units,” Chemistry of Materials, Vol. 14, No. 2, 2002, pp. 682-687. doi:10.1021/cm010665f
- K. Nakatani, S. Sando and I. Saito, “Improved Selectivity for the Binding of Naphthyridine Dimer to GuanineGuanine Mismatch,” Bioorganic & Medicinal Chemistry, Vol. 9, No. 9, 2001, pp. 2381-2385. doi:10.1016/S0968-0896(01)00160-2
- K. Nakatani, S. Sando and I. Saito, “Recognition of a Single Guanine Bulge by 2-Acylamino-1,8-naphthyridine,” American Chemical Society Publications, Vol. 122, No. 10, 2000, pp. 2172-2177. doi:10.1021/ja992956j
- C. H. Nguyen, C. Marchand, S. Delane, J.-S. Sun, H. Garestier and E. Bisagni, “Synthesis of 13H-Benzo[6,7]- and 13H-Benzo[4,5]indolo[3,2-c]-quinolines: A New Series of Potent Specific Ligands for Triplex DNA,” American Chemical Society Publications, Vol. 120, No. 11, 1998, pp. 2501-2507. doi:10.1021/ja971707x
- S.-X. Liu, Y.-J. Gao and G.-J. Jiang, “Synthesis of Benzoxepinoquinolinone,” Chemical Journal of Chinese Universities, Vol. 7, No. 12, 1986, pp. 1104-1108.
- Y.-J. Gao, C.-J. Li and R.-S. Jiang, “Synthesis of New Benzoxepinoquinolinones,” Chinese Chemical Letters, Vol. 5, No. 9, 1994, pp. 727-728.
- S. Chackal, R. Houssin and J. P. Henichart, “An Efficient Synthesis of the New Benzo[c]pyrido[2,3,4-kl]acridine Skeleton,” The Journal of Organic Chemistry, Vol. 67, No. 10, 2002, pp. 3502-3505. doi:10.1021/jo011057m
- W. R. Bowman, C. F. Bridge, P. Brookes, M. O. Cloonan and D. C. Leach, “Cascade Radical Synthesis of Heteroarenes via Iminyl Radicals,” Journal of the Chemical Society, Perkin Transactions 1, No. 1, 2002, pp. 58-68. doi:10.1039/b108323f
- D.-Q. Yang, Y.-J. Gao and G.-J. Jiang, “Synthesis of Ethyl 2-Phenoxy (Thio) Methyl-6,7-methylenedioxy- 3-quinolinecarboxylate and Its Derivatives,” Chinese Journal of Organic Chemistry, Vol. 14, 1994, pp. 626-629.
- K. Lan, S. Fen and Z. X. Shan, “Synthesis of Aromatic Cycloketones via Intramolecular Friedel-Crafts Acylation Catalyzed by Heteropoly Acids,” Australian Journal of Chemistry, Vol. 60, No. 1, 2007, pp. 80-82. doi:10.1071/CH06277
- Y. Li, “Synthesis and Characterization of Histidine Modified Porphyrin,” Chemical Research, Vol. 19, No. 4, 2008, pp. 44-47.
- C. Patteux, V. Levacher and G. Dupas, “A Novel Traceless Solid-Phase Friedländer Synthesis,” Organic Letters, Vol. 5, No. 17, 2003, pp. 3061-3063. doi:10.1021/ol035049z
Graphic for the Table of Content
NOTES
*Corresponding authors.

