International Journal of Organic Chemistry
Vol.3 No.1(2013), Article ID:28885,5 pages DOI:10.4236/ijoc.2013.31003
Synthesis and in Vitro Cytotoxic Activity of Novel Pyrazole-3,4-dicarboxylates
Laboratoire de Chimie Organique et Analytique, Faculté des Sciences et Techniques, Université Sultan Moulay Slimane, Béni-Mellal, Morocco
Email: *elmostapha1@ymail.com
Received January 4, 2013; revised February 5, 2013; accepted March 1, 2013
Keywords: Nitrile Imines; Cycloaddition; Pyrazoles; Cytotoxic Activity
ABSTRACT
N-Aryl-C-ethoxycarbonylnitrile imines (3a-g) react with ethyl cyanoacetate 1 in 1,3-dipolar cycloaddition to yield novel pyrazole-3,4-dicarboxylates (4a-g) in moderate yields. The reaction of pyrazole-3,4-dicarboxylates (4a, d) with hydrazine afforded pyrazolo[4,3-d]pyridazine-4,7-diones (5a, d) in good yields. All compounds were fully characterized by spectroscopic methods. Some of the newly synthesized compounds were evaluated for their cytotoxic activity against murine P815 mastocytoma cell line.
1. Introduction
Pyrazole and its derivatives, occupy an important position in medicinal chemistry with a wide range of bioactivities. They possess anti-obesity [1], receptor antagonists [2], HIV reverse transcriptase inhibitors [3], and anti-hyperglycemic activities [4]. They are also used as anti-inflammatory [5,6], antipyretic [7], antiarrhythmic [8], antitumor [9,10], monoamine oxidase inhibiting [11] and antibacterial agents [12]. Considering the important biological properties of pyrazole compounds, numerous methods toward pyrazoles syntheses have been developed over the past decades [13-17]. Despite numerous diverse approaches toward syntheses of pyrazoles developed so far, it is still challenging to prepare polysubstituted pyrazoles with various substituents from readily available building blocks. Herein we report a facile approach to provide polysubstituted pyrazoles via 1,3-dipolar cycloaddition of ethyl cyanoacetate with N-Aryl-Cethoxycarbonylnitrile imines. Evaluation of their cytotoxic activity toward cell line P815 is reported.
2. Results and Discussion
2.1. Chemistry
The ethyl hydrazono-α-bromoglyoxylates (2a-g) (Figure 1) selected to generate the corresponding N-aryl-C-ethoxycarbonylnitrile imines were prepared from the reaction of the adequate diazonium salts with ethyl acetoacetate, followed by bromination of the resulting azoacetoacetic esters [18]. The nitrile imines were generated in situ by basic treatment of the hydrazono-α-bromoglyoxylates [19,20].
We performed the cycloaddition reaction of ethyl cyanoacetate with nitrile imines obtained initially in situ from the treatment of hydrazonyl bromides with sodium ethoxide in dry ethanol at room temperature. The progress of the reaction was followed by TLC until all nitrilimines has been consumed. The overall reaction yielded a polysubstituted pyrazoles in moderate yields 40% - 56% (Scheme 1).
The structures of compounds (4a-g) were deduced from their IR, 1H NMR, 13C NMR and MS spectra. For example, the 1H NMR spectrum of (4a) exhibited two signals at 1.33, 1.39 for the methyl of ethoxy group and a broad signal at 5.40 ppm for NH2 group, along with multiplets for the aromatic protons at N-1 position of the pyrazolic cycle. In the IR spectrum of (4a), two absorption bands at 1725 and 1696 cm−1, which are related to

Figure 1. Structures of ethyl hydrazono-α-bromoglyoxylates used in this study as the precursors of N-aryl-C-ethoxycarbonylnitrile imines.
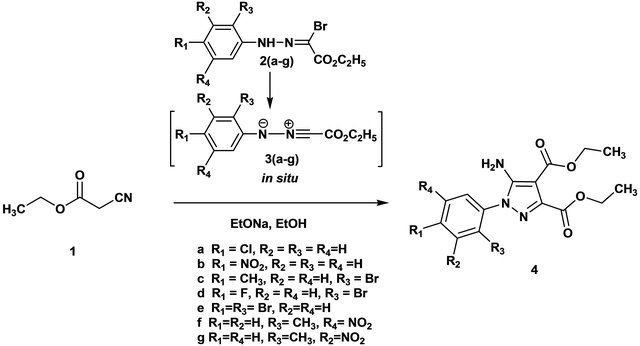
Scheme 1. Cycloaddition reaction of ethyl cyanoacetate with nitrile imines obtained.
two C=O stretching frequencies, clearly indicated the most significant functional groups of the product.
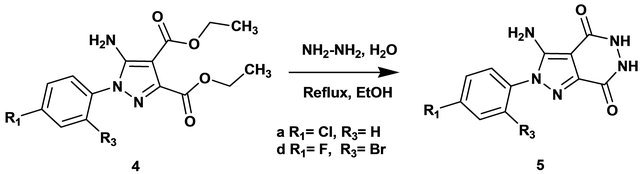
Pyrazoles (4a-g) are suitable intermediates for the preparation of new pyrazolopyridazinedione derivatives. In fact, the reaction between hydrazine hydrate and pyrazoles (4a, d) afforded pyrazolo[4,3-d]pyridazine-4,7- diones (5a, d) in good yields (Scheme 2).
The structures of the synthesized compounds (5a, d) were established on the basis of IR, 1H NMR, and 13C NMR spectral data. In the IR spectra of compound (5a) the absence of the absorption band at 1725 cm−1 and 1696 cm−1, for C=O ester confirms the formation of pyridazinedione cycle. The 1H NMR spectra of 5a exhibited two broad signals at δ = 10.05 and 12.11 ppm, respectively, due to the NH proton.
2.2. Cytotoxic Activity
The preliminary cytotoxic activities of some compounds against the murine P815 mastocytoma cell line were evaluated in vitro as shown in Table 1. The IC50 represents the drug concentration (µg/mL) required to inhibit cell growth by 50%. The polysubstituted pyrazoles (4a), (4c) and (4g) have shown slight cytotoxic activity against cell line P815. Compound (4a) (R1=Cl) showed significant activity against cell line P815 (IC50 = 32 µg/mL). It should be noted that the substituent at the position of benzene in compounds (4a-d), (4f), (4g) may also play an important role in determining relative activities.
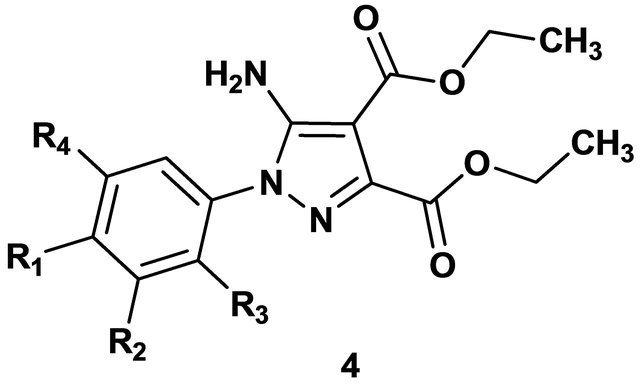
Table 1. In vitro cytotoxicity of some new substituted pyrazoles against the murine mastocytoma cell line P815
3. Conclusion
In summary, with a simple approach, a new series of pyrazole-3,4-dicarboxylates (4a-g) was synthesized by 1,3-dipolar cycloaddition of N-aryl-C-ethoxycarbonyl nitrile imines with ethyl cyanoacetate. Novel pyrazolo [4,3-d]pyridazine-4,7-diones were also prepared by condensation reaction of hydrazine with pyrazole-3,4-dicarboxylates. In vitro cytotoxicity of some compounds were assessed and showed a lower cytotoxicity on murine mastocytoma cell line P815. Pyrazole (4a) with chlorine atom substitution on pyrazole-3,4-carboxylate at para position of benzene group, showed significant activity against cell line P815 (IC50 = 32 µg/mL). Compound (4a) can be useful as a lead for the development of novel anticancer agents.
4. Experimental Section
Melting points were determined using a Büchi-Tottoli apparatus and are uncorrected. IR spectra were recorded on a Perkin-Elmer 577 spectrometer using KBr disks; only noteworthy IR absorptions are listed (cm−1). 1H and 13C NMR spectra were recorded in CDCl3, DMSO-d6 and
Scheme 2. The reaction between hydrazine hydrate and pyrazoles.
solution (unless otherwise specified) with TMS as an internal reference using a Bruker AC 300 (1H) or 75MHz (13C) instruments. Chemical shifts are given in d parts per million (ppm) downfield from TMS. Multiplicities of 13C NMR resources were assigned by distortionless enhancement by polarization transfer (DEPT) experiments. Low-resolution mass spectra (MS) were recorded on a Perkin-Elmer Sciex API 3000 spectrometer. Column chromatography was carried out on SiO2 (silica gel 60 Merck 0.063 - 0.200 mm). Thin-layer chromatography (TLC) was carried out on SiO2 (silica gel 60, F 254 Merck 0.063 - 0.200 mm), and the spots were located with UV light. Commercial reagents were used without further purification unless stated.
Synthesis of polysubstituted pyrazoles (4a-g). To a solution of ethyl cyanoacetate 1 (0.56 g, 0.005 mol) and hydrazonyl bromides 2a-g (0.005 mol) in dry ethanol, ethoxide sodium (0.68 g, 0.01 mol) was added. The mixture was stirred at room temperature for 12 h, during which the bromide dissolved and the crude pyrazole precipitated. The precipitate was filtered, washed with cold water, dried and purified by column chromatography (EtOAc/hexane 2/8).
Diethyl 5-amino-1-(4-chlorophenyl)-1H-pyrazole-3, 4-dicarboxylate (4a). Yield: 56%, mp: 95˚C - 97˚C; IR (KBr, cm−1): 3405, 3328 (NH2), 1725 (CO), 1696 (CO), 1622 (C=N); 1H NMR (CDCl3): δ 1.33 (t, 3H, CH3, J = 7.2 Hz), 1.39 (t, 3H, CH3, J = 7.2 Hz), 4.29 (q, 2H, OCH2, J = 7.2 Hz), 4.39 (q, 2H, OCH2, J = 7.2 Hz), 5.40 (s, 2H, NH2), 7.48 (d, 2H, J = 7.8 Hz), 7.51 (d, 2H, J = 7.8 Hz); 13C NMR (CDCl3): δ 14.2 (CH3), 14.3 (CH3), 60.3 (CH2O), 61.8 (CH2O), 125.6 (2CH), 129.0 (C), 129.2 (2CH), 134.6 (C), 135.3 (C), 144.6 (C), 150.0 (C), 162.8 (CO), 163.7 (CO); MS m/z = 338 [M + 1], 340 [M + 3].
Diethyl 5-amino-1-(4-nitrophenyl)-1H-pyrazole-3,4- dicarboxylate (4b). Yield: 46%, mp: 138˚C - 140˚C; IR (KBr, cm−1): 3397, 3317 (NH2), 1728 (CO), 1690 (CO), 1622 (C=N); 1H NMR (CDCl3): δ 1.34 (t, 3H, CH3, J = 7.2 Hz), 1.38 (t, 3H, CH3, J = 7.2 Hz), 4.30 (q, 2H, OCH2, J = 7.2 Hz), 4.40 (q, 2H, OCH2, J = 7.2 Hz), 5.61 (s, 2H, NH2), 7.81 (d, 2H, J = 8.0 Hz), 8.36 (d, 2H, J = 8.0 Hz); 13C NMR (CDCl3): δ 14.2 (CH3), 14.3 (CH3), 60.5 (CH2O), 62.1 (CH2O), 119.3 (C), 123.8 (2CH), 124.7 (C), 125.4 (2CH), 142.3 (C), 146.7 (C), 150.2 (C), 162.6 (CO), 163.5 (CO); MS m/z = 349 [M + 1].
Diethyl 5-amino-1-(2-bromo-4-methylphenyl)-1H-
pyrazole-3,4-dicarboxylate (4c). Yield: 45%, mp: 93˚C - 95˚C; IR (KBr, cm−1): 3445, 3335 (NH2), 1730 (CO), 1690 (CO), 1616 (C=N); 1H NMR (CDCl3): δ 1.34 (t, 3H, CH3, J = 7.2 Hz), 1.39 (t, 3H, CH3, J = 7.2 Hz), 2.40 (s, 3H, CH3), 4.30 (q, 2H, OCH2, J = 7.2 Hz), 4.39 (q, 2H, OCH2, J = 7.2 Hz), 5.01 (s, 2H, NH2), 7.28 (m, 2H), 7.55 (d, 1H, J = 1.8 Hz); 13C NMR (CDCl3): δ 14.2 (CH3), 14.4 (CH3), 60.2 (CH2O), 61.7 (CH2O), 93.9 (C), 121.8 (C), 129.6 (CH), 129.9 (CH), 132.7 (C), 134.2 (CH), 142.7 (C), 144.3 (C), 151.0 (C), 162.6 (CO), 163.7 (CO); MS m/z = 397 [M + 1], 399 [M + 3].
Diethyl 5-amino-1-(2-bromo-4-fluorophenyl)-1H-
pyrazole-3,4-dicarboxylate (4d). Yield: 51%, mp: 105˚C - 107˚C; IR (KBr, cm−1): 3430, 3354 (NH2), 1718 (CO), 1697 (CO), 1621 (C=N); 1H NMR (CDCl3): δ 1.35 (t, 3H, CH3, J = 7.2 Hz), 1.39 (t, 3H, CH3, J = 7.2 Hz), 4.30 (q, 2H, OCH2, J = 7.2 Hz), 4.39 (q, 2H, OCH2, J = 7.2 Hz), 5.18 (s, 2H, NH2), 7.19 (m, 1H), 7.46 (m, 2H); 13C NMR (CDCl3): δ 14.2 (CH3), 14.3 (CH3), 60.3 (CH2O), 61.8 (CH2O), 94.0 (C), 116.3 (CH), 121.4 (CH), 123.2 (C), 131.7 (CH), 144.6 (C), 151.1 (C), 161.1 (C), 162.2 (C), 163.6 (CO), 164.7 (CO); MS m/z = 401 [M + 1], 403 [M + 3].
Diethyl 5-amino-1-(2,4-dibromophenyl)-1H-pyrazole-
3,4-dicarboxylate (4e). Yield: 40%, mp: 91˚C - 93˚C; IR (KBr, cm−1): 3447, 3333 (NH2), 1737 (CO), 1693 (CO), 1610 (C=N); 1H NMR (CDCl3): δ 1.35 (t, 3H, CH3, J = 7.0 Hz), 1.39 (t, 3H, CH3, J = 7.0 Hz), 4.30 (q, 2H, OCH2, J = 7.0 Hz), 4.39 (q, 2H, OCH2, J = 7.0 Hz), 5.24 (s, 2H, NH2), 7.33 (d, 1H, J = 7.8 Hz), 7.61 (dd, 1H, J = 7.8 et 2.1 Hz), 7.91 (d, 1H, J = 2.1 Hz); 13C NMR (CDCl3): δ 14.2 (CH3), 14.3 (CH3), 60.3 (CH2O), 61.8 (CH2O), 94.1 (C), 123.1 (C), 125.2 (C), 131.3 (CH), 132.2 (CH), 134.6 (C), 136.4 (CH), 144.8 (C), 150.9 (C), 162.5 (CO), 163.6 (CO); MS m/z = 462 [M + 1], 464 [M + 3].
Diethyl 5-amino-1-(2-methyl-5-nitrophenyl)-1H-
pyrazole-3,4-dicarboxylate (4f). Yield: 49%, mp: 96˚C - 98˚C; IR (KBr, cm−1): 3443, 3331 (NH2), 1731 (CO), 1691 (CO), 1617 (C=N); 1H NMR (CDCl3): δ 1.35 (t, 3H, CH3, J = 7.2 Hz), 1.39 (t, 3H, CH3, J = 7.2 Hz), 2.31 (s, 3H, CH3), 4.32 (q, 2H, OCH2, J = 7.2 Hz), 4.40 (q, 2H, OCH2, J = 7.2 Hz), 5.20 (s, 2H, NH2), 7.55 (d, 1H, J = 7.1 Hz), 8.26 (m, 2H); 13C NMR (CDCl3): δ 14.2 (CH3), 14.3 (CH3), 18.2 (CH3), 60.4 (CH2O), 61.9 (CH2O), 94.2 (C), 120.5 (C), 123.5 (CH), 125.1 (CH), 132.6 (CH), 135.6 (C), 144.7 (C), 146.8 (C), 150.8 (C), 162.4 (CO), 163.6 (CO); MS m/z = 363 [M + 1].
Diethyl 5-amino-1-(2-methyl-3-nitrophenyl)-1H-
pyrazole-3,4-dicarboxylate (4g). Yield: 52%, mp: 120˚C - 122˚C; IR (KBr, cm−1): 3381, 3317 (NH2), 1716 (CO), 1695 (CO), 1626 (C=N); 1H NMR (CDCl3): δ 1.35 (t, 3H, CH3, J = 7.2 Hz), 1.39 (t, 3H, CH3, J = 7.2 Hz), 2.29 (s, 3H, CH3), 4.32 (q, 2H, OCH2, J = 7.2 Hz), 4.40 (q, 2H, OCH2, J = 7.2 Hz), 5.19 (s, 2H, NH2), 7.51 (t, 1H, J = 7.5), 7.62 (d, 1H, J = 7.5 Hz), 8.02 (d, 1H, J = 7.5); 13C NMR (CDCl3): δ 14.2 (CH3), 14.3 (CH3), 18.3 (CH3), 60.4 (CH2O), 61.8 (CH2O), 94.1 (C), 126.2 (CH), 127.7 (CH), 132.4 (C), 132.7 (CH),136.9 (C), 144.8 (C), 150.8 (C), 151.3 (C), 162.4 (CO), 163.6 (CO); MS m/z = 363 [M + 1].
Synthesis of pyrazolo[4,3-d]pyridazine-4,7-diones 5 a,d A mixture of 4a, d (12 × 10−3 mol) and hydrazine hydrate (3 g, 6 × 10−2 mol) was refluxed for 8 hours in ethanol, after cooling the reaction mixture was poured onto ice. The solid obtained was filtered, dried and crystallized from methanol.
3-Amino-2-(4-chlorophenyl)-5,6-dihydro-2H-pyrazolo
[4,3-d]pyridazine-4,7-dione (5a). Yield: 94%, mp: 167˚C - 169˚C; IR (KBr, cm−1): 3380, 3345 (NH, NH2), 1665 (CO), 1670 (CO); 1H NMR (DMSO-d6): δ 6.46 (s, 2H, NH2), 7.54 (d, 2H, J = 8.1 Hz), 7.62 (d, 2H, J = 8.1 Hz); 10.05 (s, 1H, NH), 12.11 (s, 1H, NH); 13C NMR (DMSO-d6): δ 93.2 (C), 126.3 (2CH), 129.9 (2CH), 132.7 (C), 136.7 (C), 147.5 (C), 150.8 (C), 159.4 (C), 163.7 (C); MS m/z = 278 [M + 1], 280 [M + 3].
3-Amino-2-(2-bromo-4-fluorophenyl)-5,6-dihydro- 2H-pyrazolo[4,3-d]pyridazine-4,7-dione (5d). Yield:
86%, mp:178˚C - 180˚C; IR (KBr, cm−1): 3385, 3360 (NH, NH2), 1652 (CO), 1670 (CO); 1H NMR (DMSO-d6): δ 6.82 (s, 2H, NH2), 7.46 (dd, 1H, J = 8.4 and 2.7 Hz), 7.69 (d, 1H, J = 8.4 Hz), 7.86 (d, 1H, J = 2.7 Hz), 10.39 (s, 1H, NH), 12.59 (s, 1H, NH); 13C NMR (DMSO-d6): δ 94.3 (C), 116.7 (CH), 121.4 (CH), 123.8 (C), 132.8 (CH), 141.1 (C), 153.0 (C), 154.3 (C), 159.4 (C), 160.3 (C), 162.5 (C), 164.6 (C); MS m/z = 341 [M + 1], 343 [M + 3].
5. Cytotoxic Activity
The cytotoxic activity was studied against P815 (murine mastocytoma cell line) using colorimetric MTT assay as described and modified by Tim Mossman [21]. Cells were washed by centrifugation in PBS (Phosphate Buffered Saline), and incubated in 96-well microtiter plates (Bioster Italy) at a density of 1.5 × 105 cells/ml in 100 µl per well of culture medium (D-MEM) supplemented with 5% of foetal calf serum, and 100 UI/ml of penicillin and 100 µg/ml streptomycin, 0.2% sodium bicarbonate).
Then 100 µl of fresh culture medium containing appropriate serial concentrations of the tested compounds were added in each well. After incubation for 48 h at 37˚C and 5% CO2, 100 µl of medium were carefully aspirated from each well and replaced by 20 µl of MTT solution (5 mg/ml PBS). After incubation during 4 h in the same conditions, the plates were treated with a mixture of HCl/Isopropanol (24:1) to dissolve the blue intracellular formazan product. One hour later, the optical density in the wells was read on a MicroElisa reader using dualwavelength mode (540-630 nm). The cytotoxicty (%) = 100 × (1-ODt/ODo), where ODo and ODt are respectively the optical density of control and treated wells, respectively. Three independent sets of experiments performed in duplicate were evaluated.
6. Acknowledgements
The synthetic work was supported by a grant of the University of Sultan Moulay Slimane, Béni-Mellal and the National Centre for Scientific and Technical Research (CNRST), Rabat, Morocco. The authors thank Prof. A. Zyad (Laboratory of Biological Engineering, Faculty of Sciences and Technology, Beni-Mellal, Morocco) for antitumor tests.
REFERENCES
- B. K. Srivastava, A. Joharapurkar, S. Raval, J. Z. Patel, R. Soni, P. Raval, A. Gite, A. Goswami, N. Sadhwani, N. Gandhi, H. Patel, B. Mishra, M. Solanki, B. Pandey, M. R. Jain and P. R. Patel. “Diaryl Dihydropyrazole-3-carboxamides with Significant in Vivo Antiobesity Activity Related to CB1 Receptor Antagonism: Synthesis, Biological Evaluation, and Molecular Modeling in the Homology Model,” Journal of Medicinal Chemistry, Vol. 50, No. 24, 2007, pp. 5951-5966. doi:10.1021/jm061490u
- S. Yamamoto, N. Tomita, Y. Suzuki, T. Suzaki, T. Kaku, T. Hara, M. Yamaoka, N. Kanzaki, A. Hasuoka, A. Baba and M. Ito, “Design, Synthesis, and Biological Evaluation of 4-Arylmethyl-1-phenylpyrazole and 4-Aryloxy-1- phenylpyrazole Derivatives as Novel Androgen Receptor Antagonists,” Bioorganic & Medicinal Chemistry, Vol. 20, No. 7, 2012, pp. 2338-2352. doi:10.1016/j.bmc.2012.02.005
- C. E. Mowbray, C. Burt, R. Corbau, M. Perros, I. Tran, P. A. Stupple, R. Webster and A. Wooda, “Pyrazole NNRTIs 1: Design and Initial Optimisation of a Novel Template,” Bioorganic & Medicinal Chemistry Letters, Vol. 19, No. 19, 2009, pp. 5599-5602. doi:10.1016/j.bmcl.2009.08.039
- G. R. Bebernitz, G. Argentieri, B. Battle, C. Brennan, B. Balkan, B. F. Burkey, M. Eckhardt, J. Gao, P. Kapa, R. J. Strohschein, H. F. Schuster, M. Wilson and D. D. Xu, “The Effect of 1,3-Diaryl-[1H]-pyrazole-4-acetamides on Glucose Utilization in ob/ob Mice,” Journal of Medicinal Chemistry, Vol. 44, No. 16, 2001, pp. 2601-2611. doi:10.1021/jm010032c
- A. A. Bekhit and T. Abdel-Aziem, “Design, Synthesis and Biological Evaluation of Some Pyrazole Derivatives as Anti-Inflammatory-Antimicrobial Agents,” Bioorganic & Medicinal Chemistry, Vol. 12, No. 8, 2004, 1935-1945. doi:10.1016/j.bmc.2004.01.037
- D. V. Dekhane, S. S. Pawar, S. Gupta, M. S. Shingare, C. R. Patil and S. N. Thore, “Synthesis and Anti-Inflammatory Activity of Some New 4,5-Dihydro-1,5-diaryl-1Hpyrazole-3-substituted-heteroazole Derivatives,” Bioorganic & Medicinal Chemistry Letters, Vol. 19, No. 21, 2011, pp. 6527-6532. doi:10.1016/j.bmcl.2011.08.061
- F. R. Souza, V. T. Souza, V. Ratzlaff, L. P. Borges, M. R. Oliveira, H. G. Bonacorso, N. Zanatta, M. A. P. Martins and C. F. Mello, “Hypothermic and Antipyretic Effects of 3-Methyland 3-Phenyl-5-hydroxy-5-trichloromethyl-4,5- dihydro-1H-pyrazole-1-carboxyamides in Mice,” European Journal of Pharmacology, Vol. 451, No. 2, 2002, pp. 141-147. doi:10.1016/S0014-2999(02)02225-2
- M. Iovu, C. Zalaru, F. Dumitrascu, C. Draghici, M. Moraru and E. Criste, “New Substituted 2-(Pyrazol-1-yl)- dialkylacetanilides with Potential Local Anesthetic and Antiarrhythmic Action,” Il Farmaco, Vol. 58, 2003, pp. 301-307.
- X.-F. Huang, X. Lu, Y. Zhang, G.-Q. Song, Q.-L. He, Q.-S. Li, X.-H. Yang, Y. Wei and H.-L. Zhu, “Synthesis, Biological Evaluation, and Molecular Docking Studies of N-((1,3-Diphenyl-1H-pyrazol-4-yl)methyl)aniline Derivatives as Novel Anticancer Agents,” Bioorganic & Medicinal Chemistry, Vol. 20, 2012, pp. 4895-4900.
- X. Li, X. Lu, M. Xing, X.-H. Yang, T.-T. Zhao, H.-B. Gong and H.-L. Zhu, “Synthesis, Biological Evaluation, and Molecular Docking Studies of N,1,3-Triphenyl-1Hpyrazole-4-carboxamide Derivatives as Anticancer Agents,” Bioorganic & Medicinal Chemistry Letters, Vol. 22, 2012, pp. 3589-3593.
- F. Chimenti, R. Fioravanti, A. Bolasco, F. Manna, P. Chimenti, D. Secci, O. Befani, P. Turini, F. Ortuso and S. Alcaro, “Monoamine Oxidase Isoform-Dependent Tautomeric Influence in the Recognition of 3,5-Diaryl Pyrazole Inhibitors,” Journal of Medicinal Chemistry, Vol. 50, No. 3, 2007, pp. 425-428. doi:10.1021/jm060868l
- L.-L. Xu, C.-J. Zheng, L.-P. Sun, J. Miao and H.-R. Piao, “Synthesis of Novel 1,3-Diaryl Pyrazole Derivatives Bearing Rhodanine-3-fatty Acid Moieties as Potential Antibacterial Agents,” European Journal of Medicinal Chemistry, Vol. 48, 2012, pp. 174-178. doi:10.1016/j.ejmech.2011.12.011
- J. Wen, Y. Fu, R.-Y. Zhang, J. Zhang, S.-Y. Chen and X.-Q. Yu, “A Simple and Efficient Synthesis of Pyrazoles in Water,” Tetrahedron, Vol. 67, No. 49, 2011, pp. 9618- 9621. doi:10.1016/j.tet.2011.09.074
- M. V. Patel, R. Bell, S. Majest, R. Henry and T. Kolasa, “Synthesis of 4,5-Diaryl-1H-pyrazole-3-ol Derivatives as Potential COX-2 Inhibitors,” Journal of Organic Chemistry, Vol. 69, No. 21, 2004, pp. 7058-7065. doi:10.1021/jo049264k
- S. T. Heller and S. R. Natarajan, “1,3-Diketones from Acid Chlorides and Ketones: A Rapid and General One-Pot Synthesis of Pyrazoles,” Organic Letters, Vol. 8, 2006, pp. 2675-2678.
- P. Conti, A. Pinto, L. Tamborini, V. Rizzo and C. D. Micheli, “A Regioselective Route to 5-Substituted Pyrazoleand Pyrazoline-3-phosphonic Acids and Esters,” Tetrahedron, Vol. 63, No. 25, 2007, pp. 5554-5560. doi:10.1016/j.tet.2007.04.027
- V. K. Aggarwal, J. D. Vicente and R. V. Bonnert, “A Novel One-Pot Method for the Preparation of Pyrazoles by 1,3-Dipolar Cycloadditions of Diazo Compounds Generated in Situ,” Journal of Organic Chemistry, Vol. 68, No. 13, 2003, pp. 5381-5383. doi:10.1021/jo0268409
- D. B. Sharp and C. S. Hamilton, “Derivatives of 1,2,4- Triazole and of Pyrazole,” Journal of the American Chemical Society, Vol. 68, No. 4, 1946, pp. 588-591. doi:10.1021/ja01208a018
- S. Abouricha, E. M. Rakib, N. Benchat, M. Alaoui, H. Allouchi and B. El Bali, “Facile Synthesis of New Spirothiadiazolopyridazines by 1,3-Dipolar Cycloaddition,” Synthetic Communication, Vol. 35, No. 16, 2005, pp. 2213-2221; doi:10.1080/00397910500182697
- H. Sekkak, S. Mojahidi, E. M. Rakib, S. Abouricha, A. Kerbal, C. Aiello and M. Viale, “Synthesis and Antiproliferative Evaluation Of Spirothiadiazolopyridazine Derivatives,” Letters in Drug Design & Discovery, Vol. 7, No. 10, 2010, pp. 743-746. doi:10.2174/1570180811007010743
- T. Mossman, “Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays,” Journal of Immunological Methods Vol. 65, No. 1-2, 1983, pp. 55-63. doi:10.1016/0022-1759(83)90303-4
NOTES
*Corresponding author.

