Journal of Crystallization Process and Technology
Vol.4 No.1(2014), Article ID:41733,7 pages DOI:10.4236/jcpt.2014.41003
Plasma-Assisted Chemical Vapor Deposition of Titanium Oxide Layer at Room-Temperature
Department of Biomolecular Functional Engineering, Ibaraki University, Hitachi, Japan.
Email: *ysatoshi@mx.ibaraki.ac.jp
Copyright © 2014 Satoshi Yamauchi et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In accordance of the Creative Commons Attribution License all Copyrights © 2014 are reserved for SCIRP and the owner of the intellectual property Satoshi Yamauchi et al. All Copyright © 2014 are guarded by law and by SCIRP as a guardian.
Received November 9th, 2013; revised December 9th, 2013; accepted December 16th, 2013
Keywords:PCVD; Amorphous Titanium-Oxide; FTIR; Hydroxyl; Hydrophilicity
ABSTRACT
Plasma-assisted chemical vapor deposition (PCVD) at pressure as low as 3 mtorr using titanium-tetra-isopropoxide (TTIP) and oxygen mixed gas plasma generated by 13.56 MHz radio frequency power (RF-power) below 70 W were applied to deposit titanium-oxide layer at temperature under 40˚C. Plasma optical emission spectroscopy and FTIR indicated that density of OH group in the amorphous layer was related to the density of OH or H2O in the plasma and the species was formed on electrode to induce the RF-power. Hydrophilicity on the layer was dependent on the density of chemisorbed OH, but was degraded by the excess OH. The PCVD-TiOx coating was demonstrated on polyethylene terephthalate and showed good hydrophilic property with the contact angle of water about 5˚.
1. Introduction
Titanium dioxide (TiO2) has been extensively investigated in view of photo-induced applications using the photo-catalytic reactions and the hydrophilicity [1,2], in addition to electronic and optoelectronic applications [3,4]. Especially, anatase-TiO2 is preferred for the photo-induced applications because of higher surface reaction during UV-irradiation than another crystal structures (brookite, rutile) [5]. It has been well recognized that the photocatalytic reaction inducing UV-light irradiation on the TiO2 surface is related to the Ti3+-sites which are reduced from the surface Ti4+ by photoexcited electrons, accompanying oxygen vacancies generated by the photo-excited holes [6]. The Ti3+-sites at the photo-activated TiO2 surface, which adsorb OH by the redox reaction for H2O molecules and generate bridging -OH, resulted in the super-hydrophilicity. In the sequence, it is basically important to control the crystallinity of TiO2 including impurity concentration and surface morphology. For the synthesis, a low wet- [7,8] or dry-process [9-11] has been applied to enhance the photo-induced property. Plasma-assisted chemical vapor deposition (PCVD) that is expected to have some advantages for the low temperature deposition, control of the grain structure, step coverage and so on has also been a candidate for suitable synthesis process of the TiO2 films. In the process, titanium tetra-iso-propoxide (TTIP) seems to be more sufficient as preliminary precursor than titanium tetrachloride (TiCl4) in view of contamination in the layers [12]. Previously, we demonstrated the PCVD of anatase-TiO2 for highly hydrophilic performance at 380˚C using TTIP and O2 [13], in which the deposition was achieved in low pressure of 3 mtorr by the plasma-cracked precursors and the thermal dissociation of TTIP on the surface. However, the hydrophilic property was degraded with decreasing deposition temperature and not responsible to UV-irradiation on the amorphous layer deposited at low temperatures under 280˚C. On the other, Nakamura et al. reported an interesting result that amorphous TiOx layers deposited by PCVD at low temperatures around 150˚C show good hydrophilicity after UV-irradiation [14]. They suggested that the hydroxyls including in the layer play an important role for the hydrophilicity. The result expects that amorphous TiOx layer with hydrophilic conversion by UV-irradiation can be deposited at roomtemperature, which is required to perform hydrophilic coatings on organic materials such as PET, resin etc., but the deposition condition has to be precisely optimized with investigation of the decomposition sequence because the adsorptions such as water, hydroxyl and/or hydrocarbons frequently occur with high sticking-coefficient.
In this paper, amorphous TiOx layer at near roomtemperature under 50˚C using TTIP and oxygen gas was demonstrated by PCVD in the low pressure of 3 mtorr investigating the reactive species to enhance the decomposition of TTIP optimizing the deposition condition for the hydrophilic performance. FTIR measurements were also carried out to evaluate residual impurities such as hydroxyls and hydrocarbons.
2. Experimental
2.1. Deposition of TiOx Layer
A conventional bell-jar type reactor with the base pres-sure under 1 × 10−5 torr was used for plasmaassisted chemical vapor deposition (PCVD) of titaniumoxide. Schematic details of the apparatus was shown elsewhere [13]. DC-coil was settled around the bell-jar to induce DC-magnetic field during the deposition. Titanium tetra-iso-propoxide (TTIP, Ti(O-i-C3H7)4: 99.7%- purity) used as preliminary precursor was charged into a quartz cell and vaporized at 70˚C to introduce into the reactor through a stainless tube without any carrier gas, where the liquid-phase TTIP was preliminary purified at 50˚C in vacuum for 3 hrs before the deposition. Pure oxygen gas (99.9999%-purity) was also introduced for the deposition. The supply ratio of O2/TTIP was controlled by monitoring the reactor pressure using Shultz gage when the TTIP and the O2 was individually introduced into the reactor. Radio-frequency power (RFpower) at 13.56 MHz was applied through an inductively coupled rf-electrode to discharge the gases in the reactor, where the mixed gas plasma was generated in the pressure as low as 3 mtorr and stabilized by DC-magnetic field of 3000 Gauss. Si-wafers with mirror-surface used as substrates were rinsed in deionized water and dried after removal of contamination on the surface by methanol, and then mounted on a holder with a water cooling sys-tem. Prior to the deposition, the reactor was cleaned by oxygen gas plasma excited by 100 W rf-power with closing shutter settled above substrate to remove residual gases such as adsorbed water on the inner wall, then the substrates were exposed in oxygen-gas plasma excited by 50 W RF-power for 5 min at roomtemperature. TiOx layer with the thickness about 400 nm was deposited at the temperature under 40˚C which was monitored by a thermo-couple.
2.2. Evaluation
Thickness of the layer was checked by a surface profiler (DEKTAK150). Hydrophilicity on the layer was evaluated by the contact angle of water when 10 μL deionized water was drop on the layer in air with 50%-humidity at 2˚C. A black-light with the peak wavelength at 365 nm and the light power density of 1 mW/cm2 was used for UV-irradiation in air on the TiOx surface. Residual impurities such as hydroxyls and hydrocarbons in the layers were evaluated by a FTIR system (JASCO FT/IR-420). Optical emission spectroscopy of plasma in the reactor was also carried out by UV-Vis spectrometer (OCEAN OPTICS: USB-2000) though a quartz-window on the cell.
3. Results and Discussions
3.1. Dependence on O2/TTIP Supply Ratio
Figure 1 shows deposition rate of TiOx layer by induced RF-power of 30 W as a function of TTIP pressure (PTTIP) in the bottom axis and O2 pressure (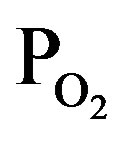 ) in the top axis during the deposition. In the process, the layers were deposited in 3 mtorr with a constant exhaust rate, that is, the deposition in the PTTIP of 3 mtorr was performed without O2 supply. The deposition rate was uniquely increased with the PTTIP, which indicates the deposition was limited by TTIP supply rate, but saturated above 2 mtorr. Plasma optical emission spectra was quite different by O2 supply as shown in Figure 2, in which the spectrum of O2 plasma is shown in 1/5-scale and the
) in the top axis during the deposition. In the process, the layers were deposited in 3 mtorr with a constant exhaust rate, that is, the deposition in the PTTIP of 3 mtorr was performed without O2 supply. The deposition rate was uniquely increased with the PTTIP, which indicates the deposition was limited by TTIP supply rate, but saturated above 2 mtorr. Plasma optical emission spectra was quite different by O2 supply as shown in Figure 2, in which the spectrum of O2 plasma is shown in 1/5-scale and the
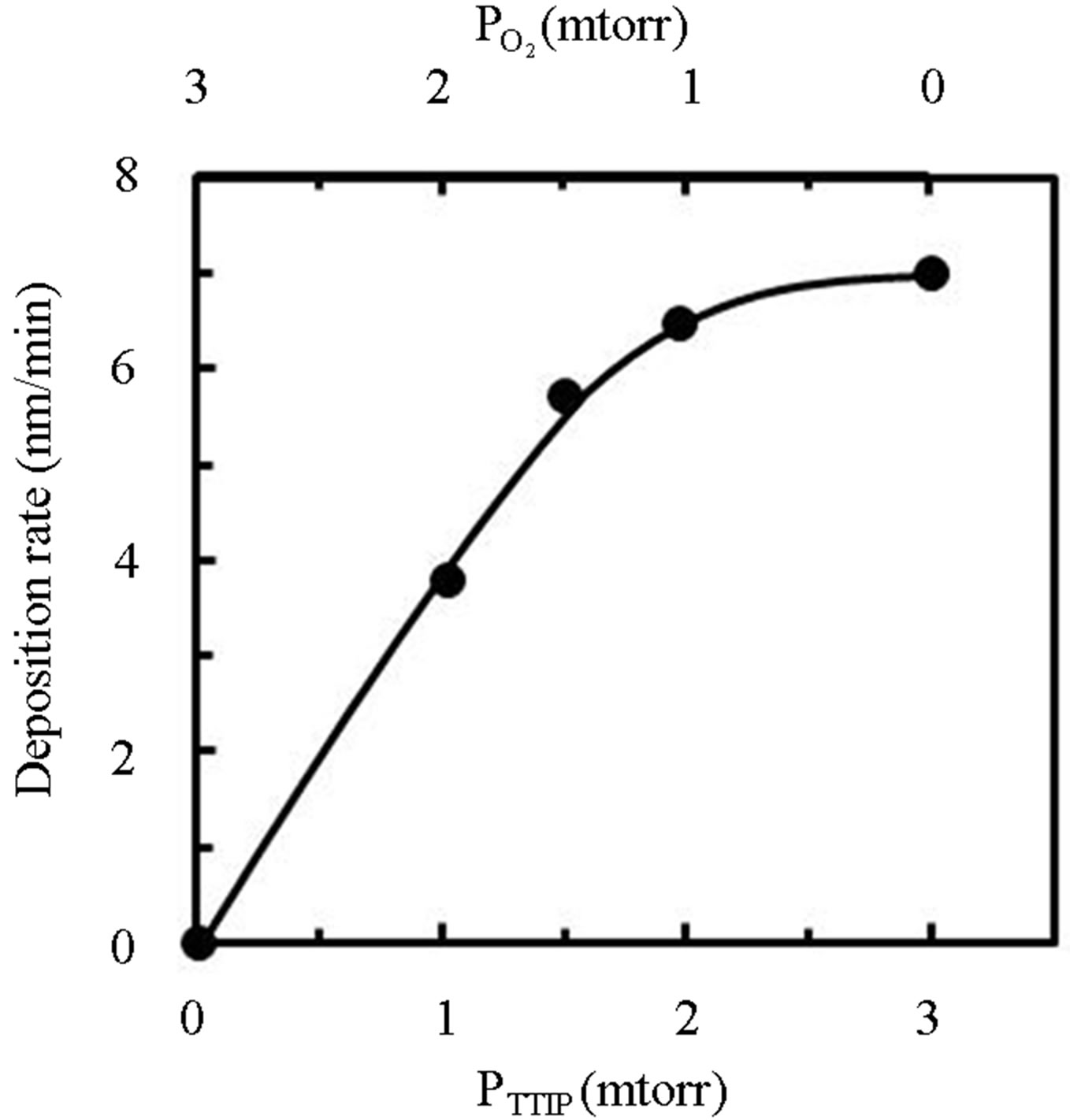
Figure 1. Dependence of TiOx deposition rate on TTIPpressure (PTTIP: bottom axis) and O2-pressure (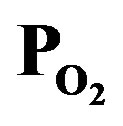 : top axis).
: top axis).
TTIP + O2 plasma was generated by the PTTIP of 1.5 mtorr (PTTIP/ = 1). Typical emissions due to excited oxygen, hydrogen, carbon-oxides and hydroxyl were observed in the TTIP + O2 plasma, however, any emission due to excited oxygen could not be detected in the TTIP plasma. The emission spectra suggested TTIP was not only dissociated in TiO-R but also in Ti-OR because the CO2 and OH emissions could be observed in the TTIP plasma without the emissions due to O and O2. It is considered that the decomposition sequence was similar to the previous report for PCVD performed in 350 mtorr, in which it was suggested that TTIP can be decomposed by electron impact to Ti and TiOx in vapor-phase of TTIP + O2 plasma and then oxidized by O-radical in the vapor phase or on the deposition surface [15]. However, TTIP was scarcely dissociated in the vapor phase at low pressure of 3 mtorr as discussed by the dependence on RF-power in Section 3.2. Figure 3 shows dependence of the emission intensity due to the excited species on the
= 1). Typical emissions due to excited oxygen, hydrogen, carbon-oxides and hydroxyl were observed in the TTIP + O2 plasma, however, any emission due to excited oxygen could not be detected in the TTIP plasma. The emission spectra suggested TTIP was not only dissociated in TiO-R but also in Ti-OR because the CO2 and OH emissions could be observed in the TTIP plasma without the emissions due to O and O2. It is considered that the decomposition sequence was similar to the previous report for PCVD performed in 350 mtorr, in which it was suggested that TTIP can be decomposed by electron impact to Ti and TiOx in vapor-phase of TTIP + O2 plasma and then oxidized by O-radical in the vapor phase or on the deposition surface [15]. However, TTIP was scarcely dissociated in the vapor phase at low pressure of 3 mtorr as discussed by the dependence on RF-power in Section 3.2. Figure 3 shows dependence of the emission intensity due to the excited species on the
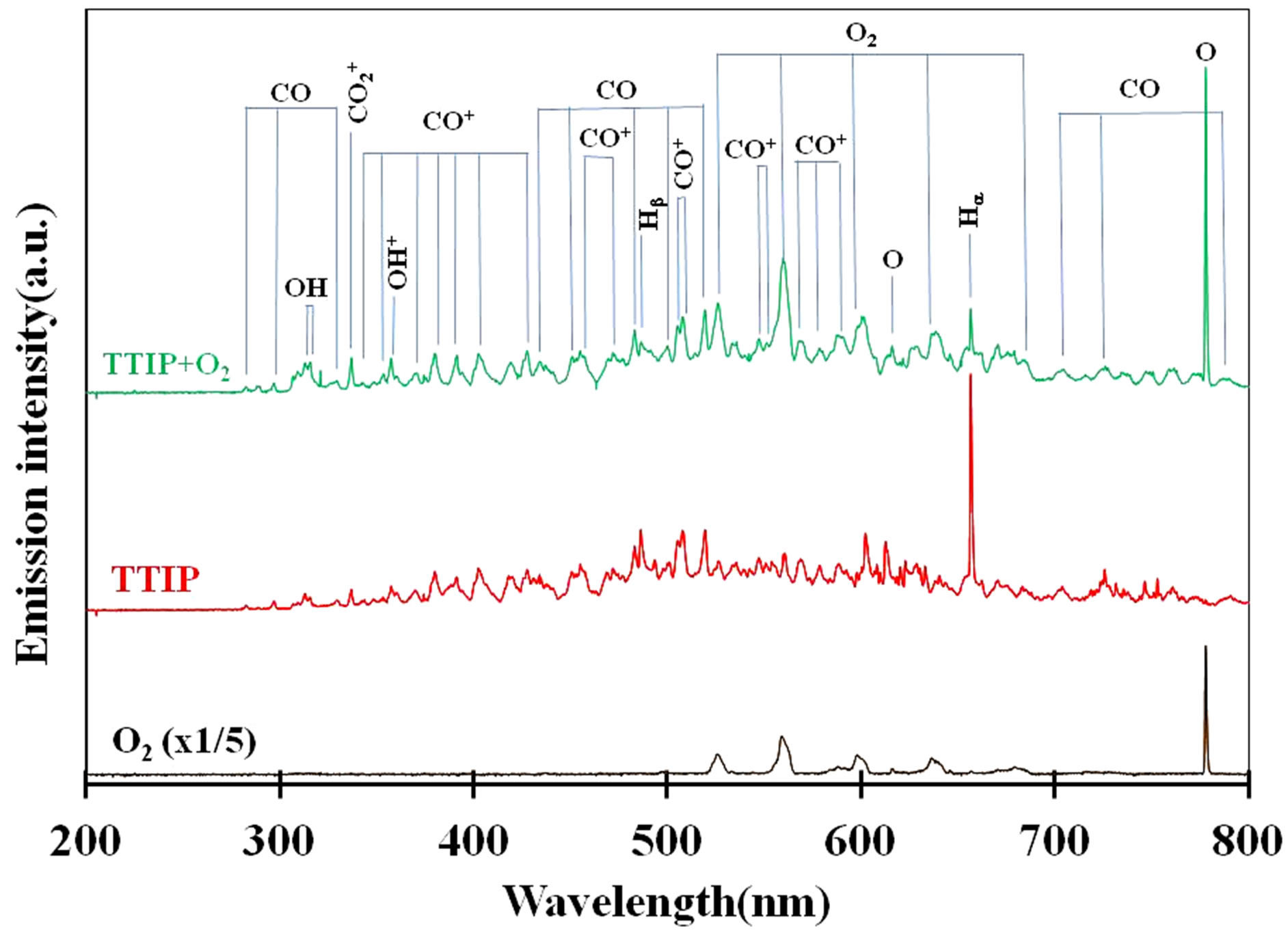
Figure 2. Optical emission spectra of O2 plasma (black-line), TTIP plasma (red-line) and O2 + TTIP plasma (green-line).
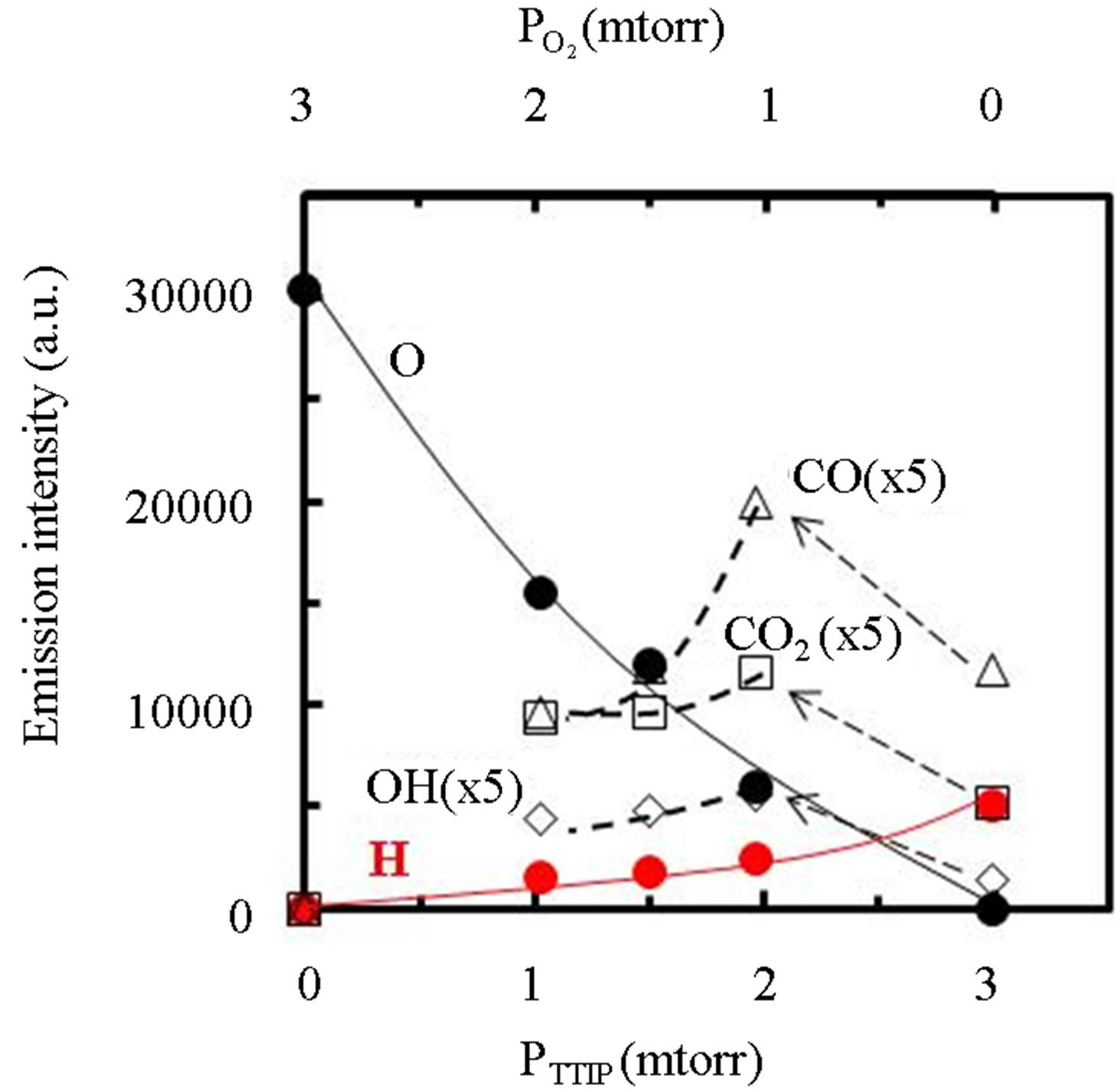
Figure 3. Dependence of optical emission spectra due to excited species in the plasma on the PTTIP and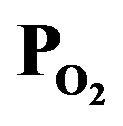 .
.
gas supply rate shown by PTTIP and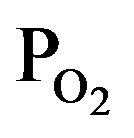 , where the intensities of CO, CO2 and OH were extended by 5-times. The intensity of O and H were gradually increased and decreased with
, where the intensities of CO, CO2 and OH were extended by 5-times. The intensity of O and H were gradually increased and decreased with 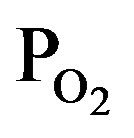 (decreasing PTTIP), respectively. In contrast, the emissions due to CO, CO2 and OH were significantly increased by O2 supply comparing to the TTIP plasma as shown by bow-lines and then gradually decreased with decreasing TTIP supply rate. The result could be recognized that CO and H were oxidized by excited oxygen. Commonly, it is expected that OH or H2O can play a role to enhance TTIP dissociation as demonstrated in Sol-Gel process, which is resulted in the formation of Ti-OH and the oxo-bridging of Ti-O-Ti [16]. It is however seemed in this work that not only OH but also the other species influenced to the dissociation because the deposition rate by using TTIP plasma was comparable in the plasma to that by using TTIP + O2 plasma with the PTTIP = 2.0 mtorr whereas the emission due to OH was significantly increased in the TTIP + O2 plasma comparing to that in the TTIP plasma. Previously, it was reported by FTIR studies for TTIP dissociation that atomic oxygen radicals supplied from oxygen remote-plasma enhance the dissociation of TTIP and the dissociation energy is decreased to 27.3 kJ/mol [17]. In contrast, the energy for the dissociation in the PCVD was 4.5 kJ/mol as shown elsewhere [13]. The significantly low dissociation energy in the PCVD comparing to the remote-plasma assisted deposition suggested that charged particles such as electrons and ions were also contributed to the dissociation.
(decreasing PTTIP), respectively. In contrast, the emissions due to CO, CO2 and OH were significantly increased by O2 supply comparing to the TTIP plasma as shown by bow-lines and then gradually decreased with decreasing TTIP supply rate. The result could be recognized that CO and H were oxidized by excited oxygen. Commonly, it is expected that OH or H2O can play a role to enhance TTIP dissociation as demonstrated in Sol-Gel process, which is resulted in the formation of Ti-OH and the oxo-bridging of Ti-O-Ti [16]. It is however seemed in this work that not only OH but also the other species influenced to the dissociation because the deposition rate by using TTIP plasma was comparable in the plasma to that by using TTIP + O2 plasma with the PTTIP = 2.0 mtorr whereas the emission due to OH was significantly increased in the TTIP + O2 plasma comparing to that in the TTIP plasma. Previously, it was reported by FTIR studies for TTIP dissociation that atomic oxygen radicals supplied from oxygen remote-plasma enhance the dissociation of TTIP and the dissociation energy is decreased to 27.3 kJ/mol [17]. In contrast, the energy for the dissociation in the PCVD was 4.5 kJ/mol as shown elsewhere [13]. The significantly low dissociation energy in the PCVD comparing to the remote-plasma assisted deposition suggested that charged particles such as electrons and ions were also contributed to the dissociation.
Figure 4 shows FTIR absorption spectra of the TiOx layers deposited by PTTIP of (a) 2.0, (b) 1.5, and (c) 1.0 mtorr, where the 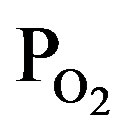 /PTTIP was 0.5, 1.0 and 2.0 respectively. Broad-absorptions around 500 cm−1, which are recognized due to short-range O-Ti-O network in amorphous TiOx [15], were observed in the all samples and the intensity was scarcely dependent on the
/PTTIP was 0.5, 1.0 and 2.0 respectively. Broad-absorptions around 500 cm−1, which are recognized due to short-range O-Ti-O network in amorphous TiOx [15], were observed in the all samples and the intensity was scarcely dependent on the 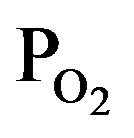
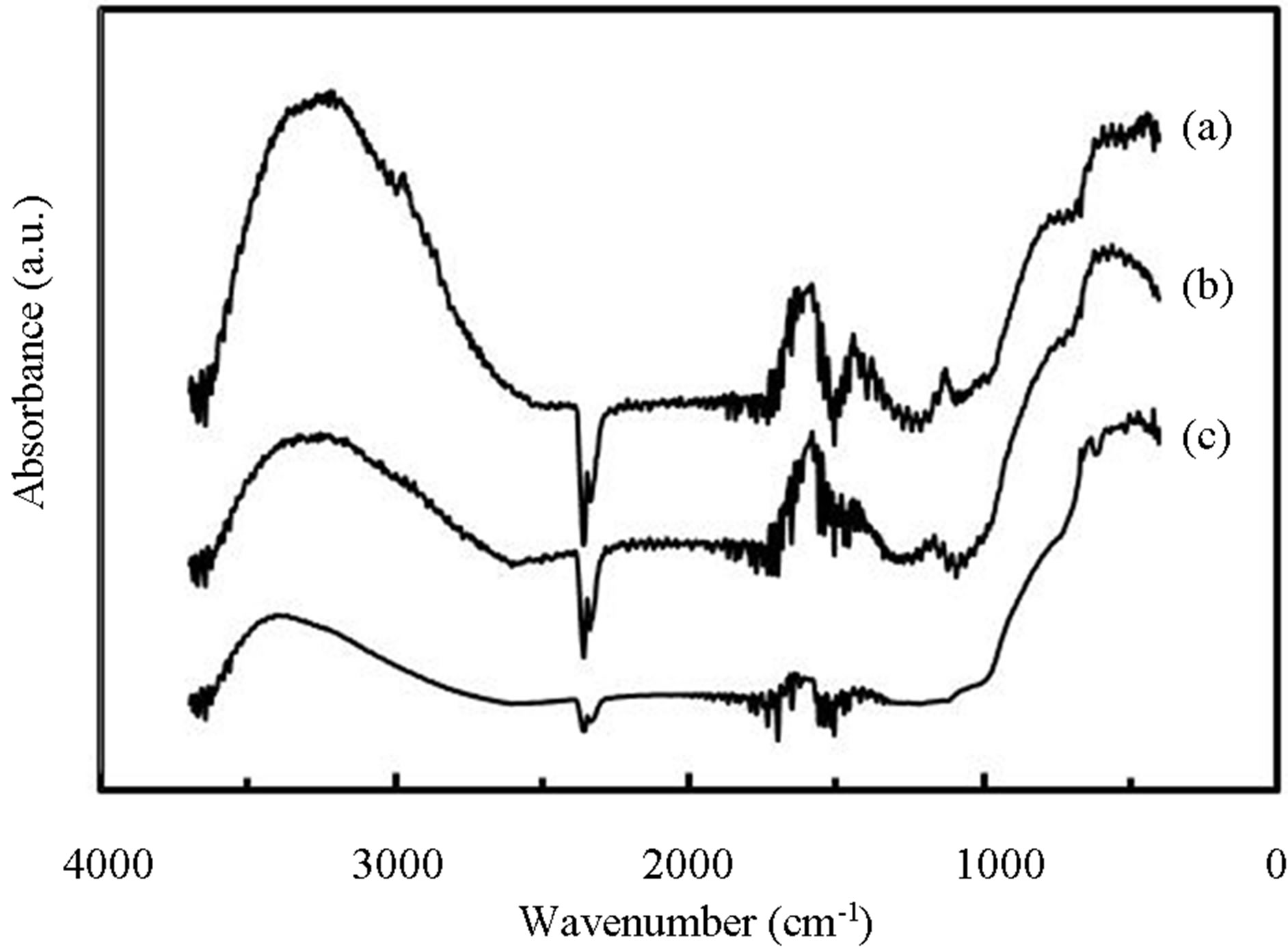
Figure 4. FTIR spectra of TiOx layers deposited by PTTIP of (a) 2.0, (b) 1.5 and (c) 1.0 mtorr.
/PTTIP ratio. In addition, absorptions at 1410, 2850 and 2950 cm−1, which is attributed to -CH2 (bending), -CH3 (stretching) and -CH2 (stretching) respectively [18], were observed in the spectrum (a), but the intensity were notably decreased with increasing the 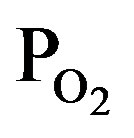 /PTTIP ratio and could not be observed in the spectrum (b) and (c). The broad-spectrum below 2600 cm−1 can be assigned to absorptions due to H2O or OH [18]. In addition, broad-spectrum below 2600 cm−1 was clearly observed and the feature was significantly decreased with increasing
/PTTIP ratio and could not be observed in the spectrum (b) and (c). The broad-spectrum below 2600 cm−1 can be assigned to absorptions due to H2O or OH [18]. In addition, broad-spectrum below 2600 cm−1 was clearly observed and the feature was significantly decreased with increasing 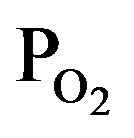 /PTTIP ratio. It is noted that the broad-spectrum was shifted toward higher wavenumber with increasing the ratio, which indicated the spectrum consists of some spectra. Figure 5 shows the absorption spectra above 2600 cm−1 in the layers deposited by PTTIP of (a) 2.0, (b) 1.5 and (c) 1.0 mtorr with the deconvoluted spectra (blue-line) and the fitted curve (red-line), where Gaussian function was used for the deconvolution. The spectrum could be well reconstructed by four spectra peak at 3015, 3251, 3418 and 3530 cm−1 which could be attributed to the absorptions due to physisorbed H2O (around 3008 cm−1) [19], chemisorbed OH to Ti (around 3200 cm−1, 3400 cm−1) [20] and H2O2 (around 3537 cm−1) [21]. Figure 6(a) shows dependence of the integrated intensity related to the hydroxyls on PTTIP. The absorptions were significantly increased with TTIP supply rate (decreasing O2/TTIP ratio). The feature could be simply described by OH emission intensity in the plasma as shown in Figure 6(b), where the absorption intensity (ABS) was divided by the deposition period (Depo. period). The result linearly dependent on the OH emission intensity clearly indicated the absorption species due to hydroxyls were formed by OH or H2O in the plasma. In addition, the (ABS)/(Depo. period) for the species was asymptotic to
/PTTIP ratio. It is noted that the broad-spectrum was shifted toward higher wavenumber with increasing the ratio, which indicated the spectrum consists of some spectra. Figure 5 shows the absorption spectra above 2600 cm−1 in the layers deposited by PTTIP of (a) 2.0, (b) 1.5 and (c) 1.0 mtorr with the deconvoluted spectra (blue-line) and the fitted curve (red-line), where Gaussian function was used for the deconvolution. The spectrum could be well reconstructed by four spectra peak at 3015, 3251, 3418 and 3530 cm−1 which could be attributed to the absorptions due to physisorbed H2O (around 3008 cm−1) [19], chemisorbed OH to Ti (around 3200 cm−1, 3400 cm−1) [20] and H2O2 (around 3537 cm−1) [21]. Figure 6(a) shows dependence of the integrated intensity related to the hydroxyls on PTTIP. The absorptions were significantly increased with TTIP supply rate (decreasing O2/TTIP ratio). The feature could be simply described by OH emission intensity in the plasma as shown in Figure 6(b), where the absorption intensity (ABS) was divided by the deposition period (Depo. period). The result linearly dependent on the OH emission intensity clearly indicated the absorption species due to hydroxyls were formed by OH or H2O in the plasma. In addition, the (ABS)/(Depo. period) for the species was asymptotic to
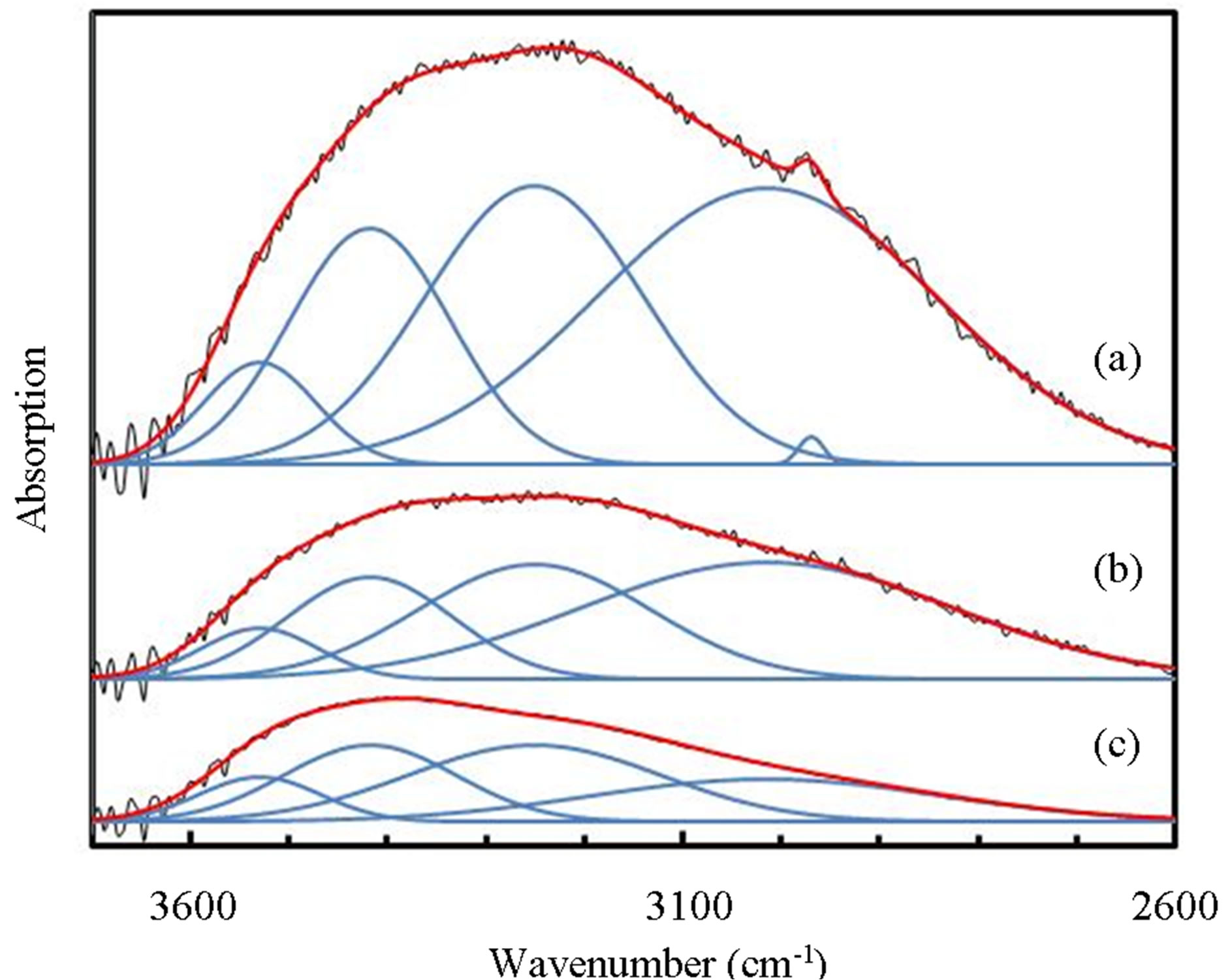
Figure 5. Broad absorption spectra above 2600 cm−1 in TiOx layer deposited by PTTIP of (a) 2.0, (b) 1.5 and (c) 1.0 mtorr (black-line) with the deconvoluted spectra (blue-line) and the fitted curve (red-line).
the OH emission intensity about 850, which indicated that OH or H2O was also contributed to the deposition without the absorption species.
3.2. Dependence on Induced RF-Power
Figure 7 shows dependence of (a) deposition rate and (b) optical emission intensity on the induced RF-power, where the PTTIP/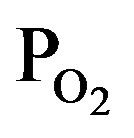 ratio was kept to 1.0 (PTTIP =
ratio was kept to 1.0 (PTTIP = 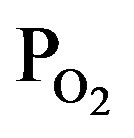 = 1.5 mtorr). Although any deposit could not be observed when the RF-power was not induced, the deposition rate was increased with the RF-power and then decreased by the power above 70 W. In general, TTIP can be dissociated to the metal-oxides in the gas-phase and on the deposition surface. It was previously reported for amorphous TiOx deposition by PCVD using TTIP and O2 that the deposition rate is dependent on the density of atomic oxygen radical and decreased with increasing the induced RF-power from 5 W to 20 W in 350 mtorr because of increasing the gas-phase dissociation in the plasma [22]. In contrast, the deposition rate was increased with the RF-power below 50 W in this work, which is considered to be derived from the increased mean-free path in the low pressure of 3 mtorr. On the other, intensity of optical emission from the plasma was linearly increased with the RF-power. If TTIP was dissociated in the gas phase of the plasma, density of hydrogen and hydroxyls should be increased with the RF-
= 1.5 mtorr). Although any deposit could not be observed when the RF-power was not induced, the deposition rate was increased with the RF-power and then decreased by the power above 70 W. In general, TTIP can be dissociated to the metal-oxides in the gas-phase and on the deposition surface. It was previously reported for amorphous TiOx deposition by PCVD using TTIP and O2 that the deposition rate is dependent on the density of atomic oxygen radical and decreased with increasing the induced RF-power from 5 W to 20 W in 350 mtorr because of increasing the gas-phase dissociation in the plasma [22]. In contrast, the deposition rate was increased with the RF-power below 50 W in this work, which is considered to be derived from the increased mean-free path in the low pressure of 3 mtorr. On the other, intensity of optical emission from the plasma was linearly increased with the RF-power. If TTIP was dissociated in the gas phase of the plasma, density of hydrogen and hydroxyls should be increased with the RF-
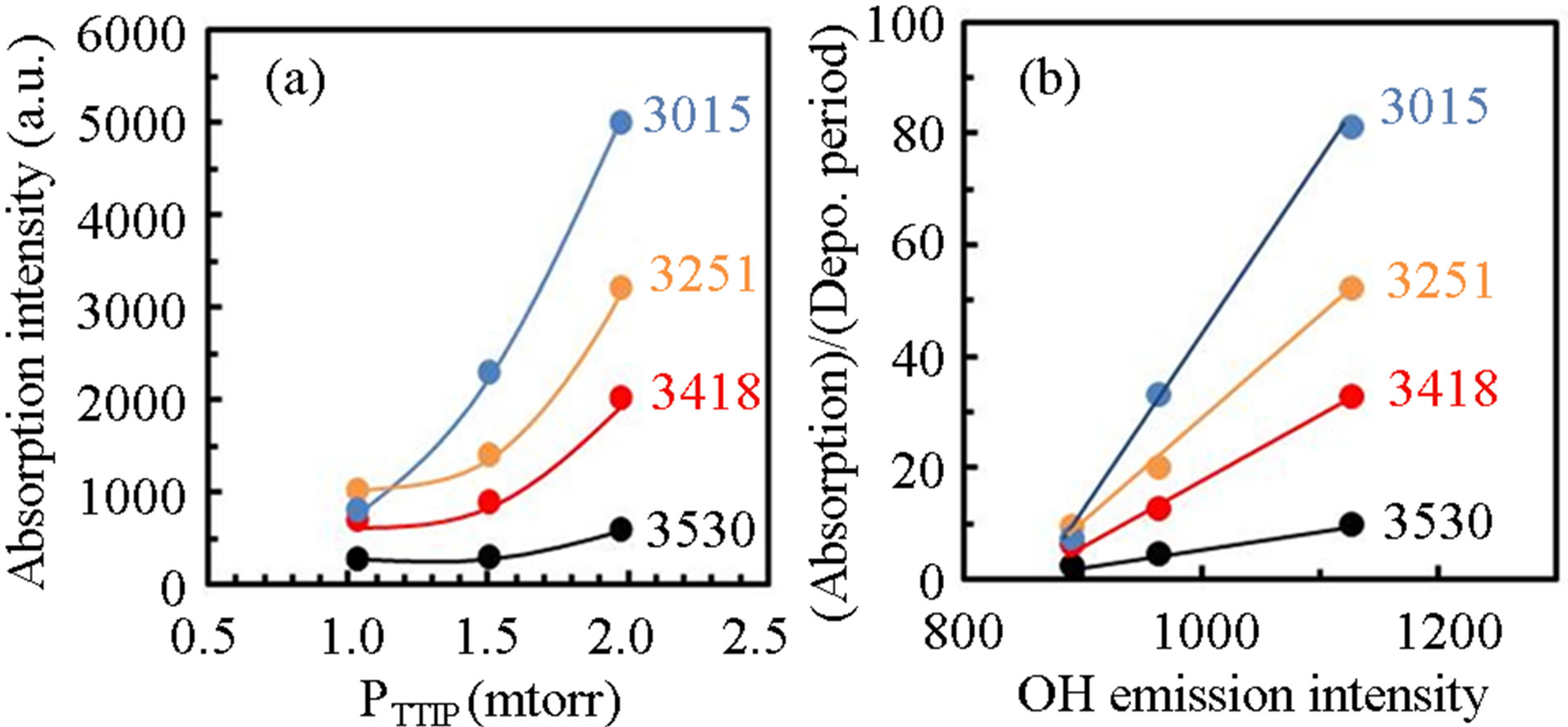
Figure 6. (a) Integrated intensity of absorptions concerned to hydroxyls in TiOx layers for PTTIP and (b) the absorption intensity divided by the deposition period for OH emission intensity in the plasma.
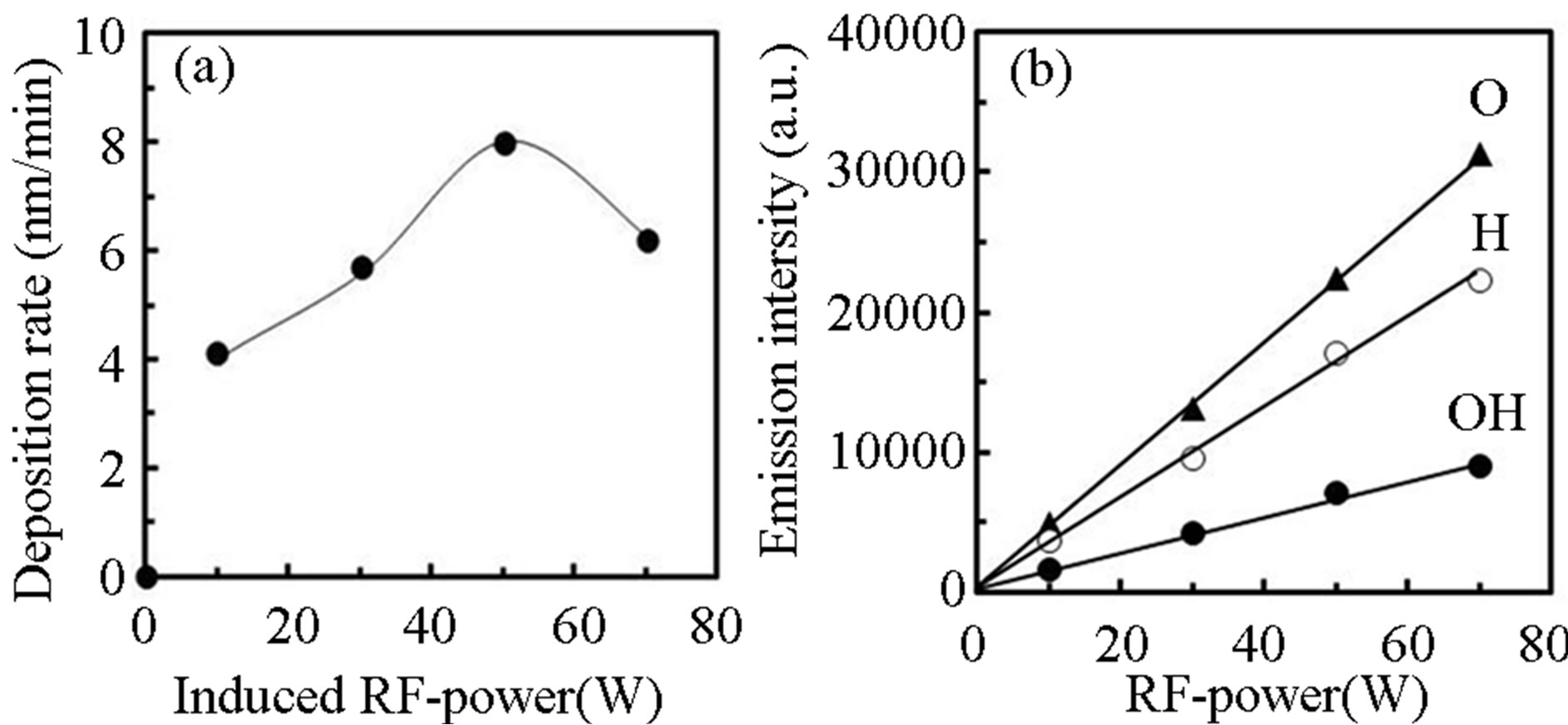
Figure 7. Dependence of (a) deposition rate and (b) optical emission intensity on the induced RF power, where the supply rate of PTTIP/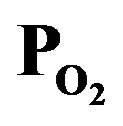 was 1.0 (PTTIP =
was 1.0 (PTTIP = 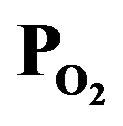 = 1.5 mtorr).
= 1.5 mtorr).
power, and the emission intensity should be super-linearly increased with the RF-power because the emission intensity was expected to be in proportion to the density of particle and the electron density in the gas phase. Therefore, it is concluded from the result that not only oxygen density but also hydrogen and hydroxyls density were not dependent on the RF-power. In the process introducing RF-power through the electrode settled in reactor, the gases can be efficiently dissociated on the electrode, in which the density of the dissociated species are fundamentally dependent on the density of precursor in the reactor.
Figure 8 show FTIR spectra of PCVD-TiOx layers deposited by RF-power of (a) 10, (b) 30, (c) 50 and (d) 70 W, where the PTTIP was 0.15 mtorr. The feature of the broad-absorption below 2600 cm−1 was dependent on the RF-power. Figure 9 show variations of (a) integrated intensity of deconvoluted FTIR spectra for the OH-related absorptions and (b) (ABS)/(Depo. Period) for the RF-power, where the thickness of layers were about 400 nm in the samples. Absorptions due to CHx were not observed in the samples. The absorption due to physisorbed H2O around 3530 cm−1 was gradually decreased
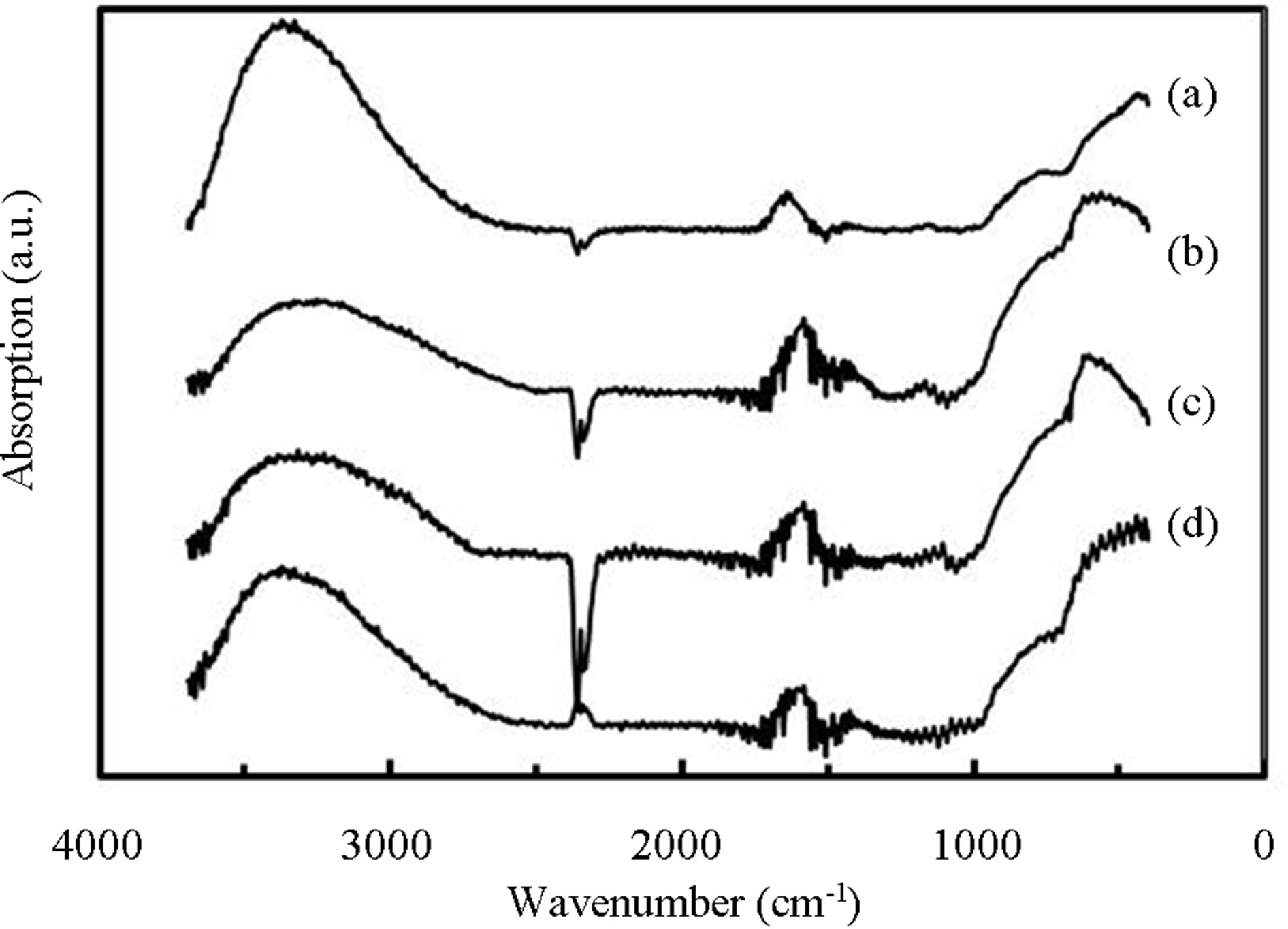
Figure 8. FTIR spectra of TiOx layers deposited by RFpower of (a) 10, (b) 30, (c) 50 and (c) 70 W, where PTTIP was 1.5 mtorr.
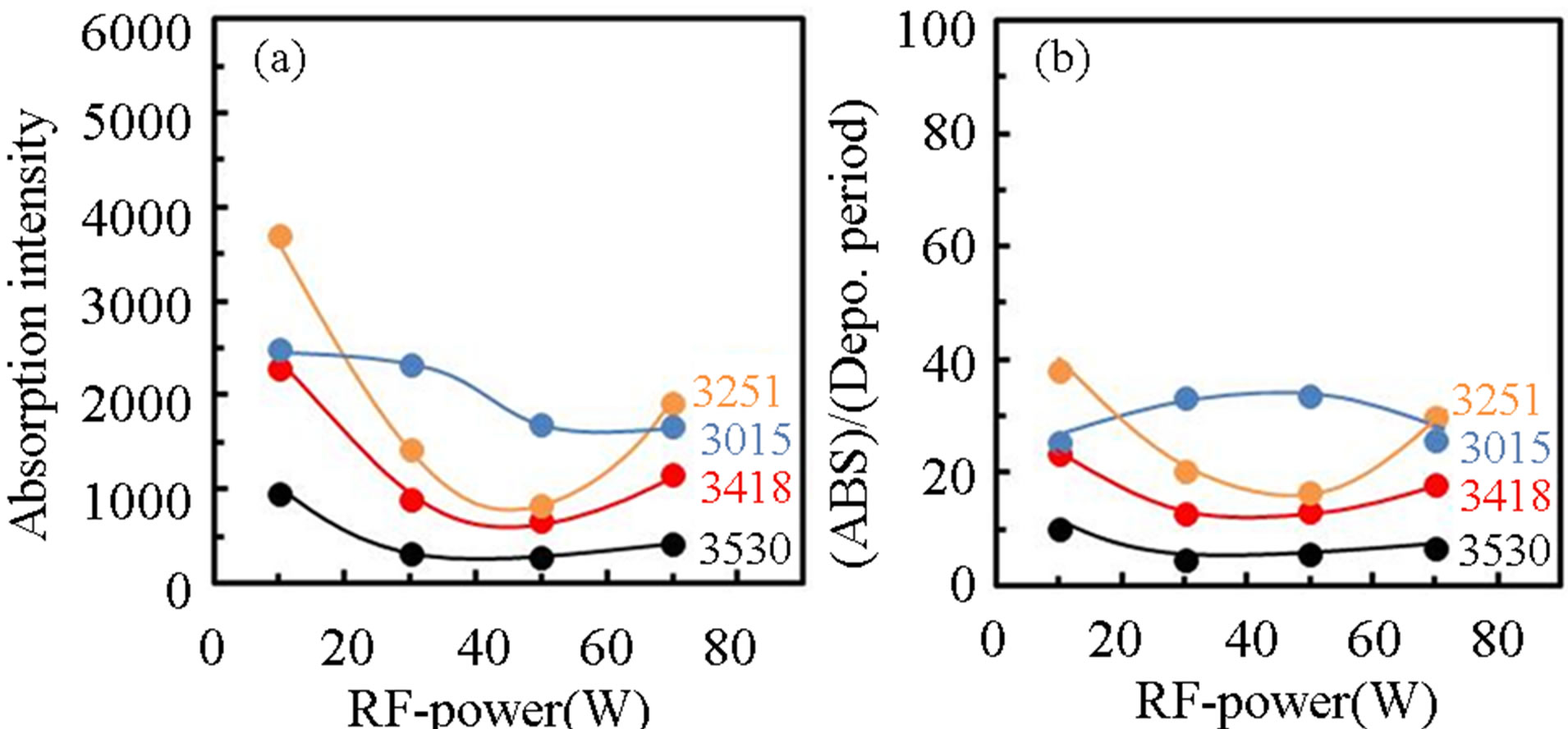
Figure 9. Variation of (a) integrated intensity of deconvoluted OH-related absorptions in TiOx layers and the intensity divided by the deposition rate for induced RF-power.
with increasing RF-power whereas density of OH or H2O was not dependent on the RF-power. In contrast, the other absorptions were decreased with increasing RF-power but increased by the RF-power of 70 W. For the (ABS)/ (Depo. Period), the physisorbed H2O was slightly increased with the RF-power but decreased by the RFpower of 70 W. On the other, the (ABS)/(Depo. Period) due to chemisorbed OH were minimized by the RFpower of 30 - 50 W. It has been well recognized that TTIP is easily dissociated to Ti-(OH)x at room-temperature by water contribution [16]. The hydrolysis was also included in the PCVD process, especially, in the case of low RF-power such as 10 W, in which the supplied H2O was partially consumed to dissociate TTIP. On the other, it is considered that the contribution of electron and/or excited species such as radicals and ions should be taken into account to the deposition sequence, for example, in Ti-O-Ti network formation from Ti-OH and in dissociation of supplied H2O on the deposition surface. Therefore, the density of Ti-OH was decreased with the RF-power and that of H2O was decreased by the RF-power of 70 W. It is noted here that when electrons are supplied to the deposition surface, however, Ti-O-Ti is dissociated to Ti-O, Ti be-cause the Ti4+ is reduced to Ti3+, and then Ti-OH is formed by supplied H and OH. As a result, density of Ti-OH was increased with the RF-power above 50 W.
3.3. Hydrophilicity
Figures 10 show contact angle of water (θwater) on TiOx layer deposited by (a) various TTIP supply rate (PTTIP) and (b) various RF-power, where the θwater was evaluated after UV-irradiation for 1 hour (black-circles) and then after exposure in air for 2-weeks (red-circles). θwater was decreased with decreasing PTTIP (increasing 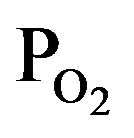 /PTTIP). In contrast, θwater was decreased with the RF-power but increased on the layers deposited by the RF-power above 50 W. Although amorphous TiOx deposited at relatively high temperature above 250˚C is not in response to UV-irradiation [16] for the hydrophilicity,
/PTTIP). In contrast, θwater was decreased with the RF-power but increased on the layers deposited by the RF-power above 50 W. Although amorphous TiOx deposited at relatively high temperature above 250˚C is not in response to UV-irradiation [16] for the hydrophilicity,
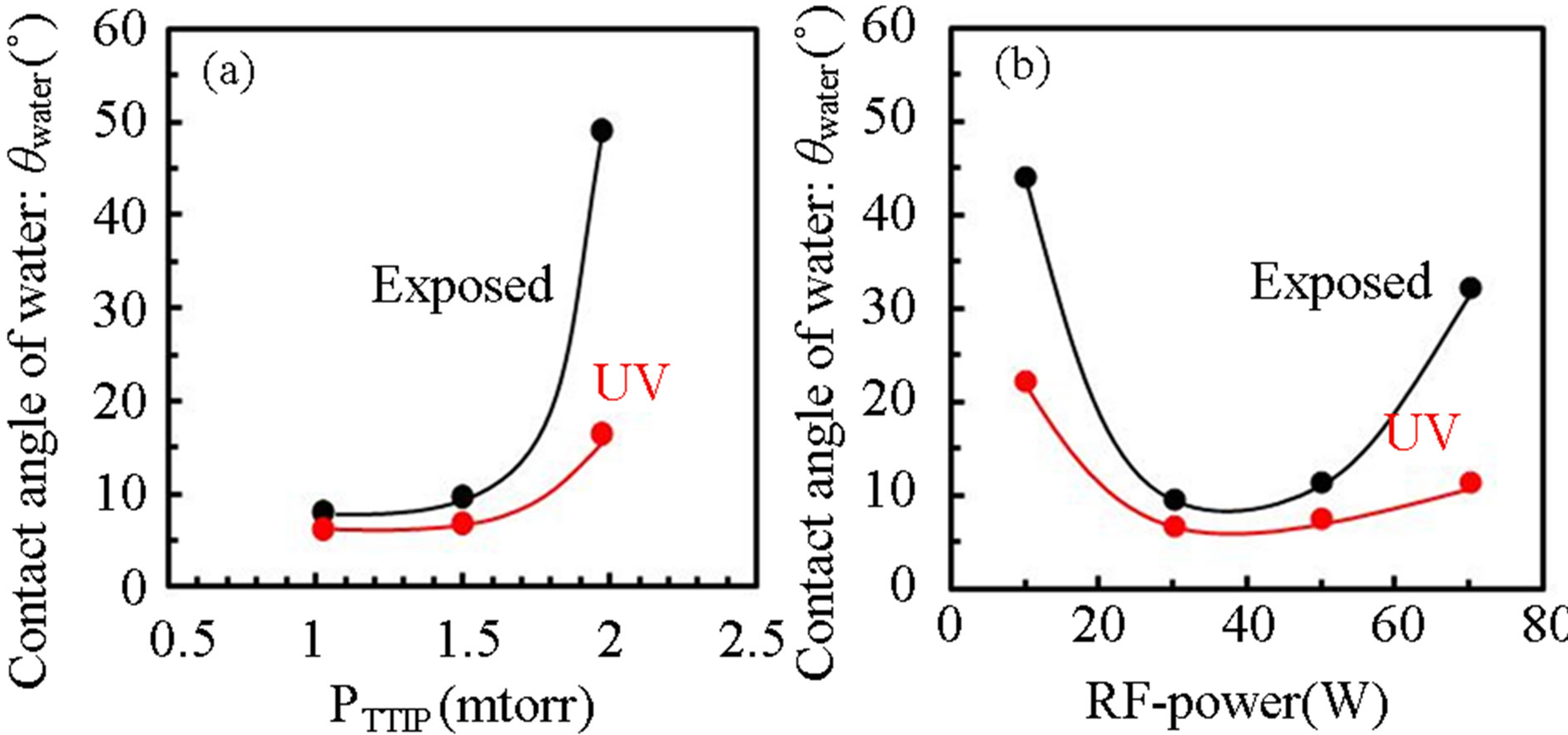
Figure 10. Contact angle of water on TiOx layer deposited by various (a) PTTIP and (b) RF power after UV-irradiation (red-circles) and after exposure for 2-weeks in air (blackcircles).
the contact angle of water OH-included amorphous TiOx layer is significantly decreased by UV-irradiation [17]. Therefore, it is considered that OH species play a role for hydrophilicity on the layers deposited in this work. Figure 11 shows dependence of Cos(θwater) on the integrated absorption intensity in TiOx layers grown by various PTTIP and RF-power, in which the absorption intensity is divided by the layer thickness to estimate the density of the species on the surface. In this work, the Cos(θwater) was linearly increased with decreasing chemisorbed OH density but seemed to be not dependent on the physisorbed H2O. In addition, the intensity due to H2O2 (3530) was asymptotic to zero at the Cos(θwater) = 1.0 but the intensity due to chemisorbed OH (3418, 3251) was not asymptotic to zero at the Cos(θwater) = 1.0. The result indicated that chemisorbed OH is necessary for the super-hydrophilicity but the excess species are not suitable for the purpose because of decreasing the Ti-O-Ti network. Especially, H2O2 which is expected to be included as Ti-OH-HO-Ti should be removed from the layers.
The PCVD-TiOx coating at room-temperature was demonstrated on polyethylene terephthalate (PET). Figure 12 shows features of water droplets on (a) PET and (b) PCVD-TiOx layer deposited on PET by optimized condition (PTTIP = 1.5 mtorr, RF power = 30 W), where the water was dropped on the TiOx layer after UV-irradiation for 1 hour. It is noted that any deformation of the
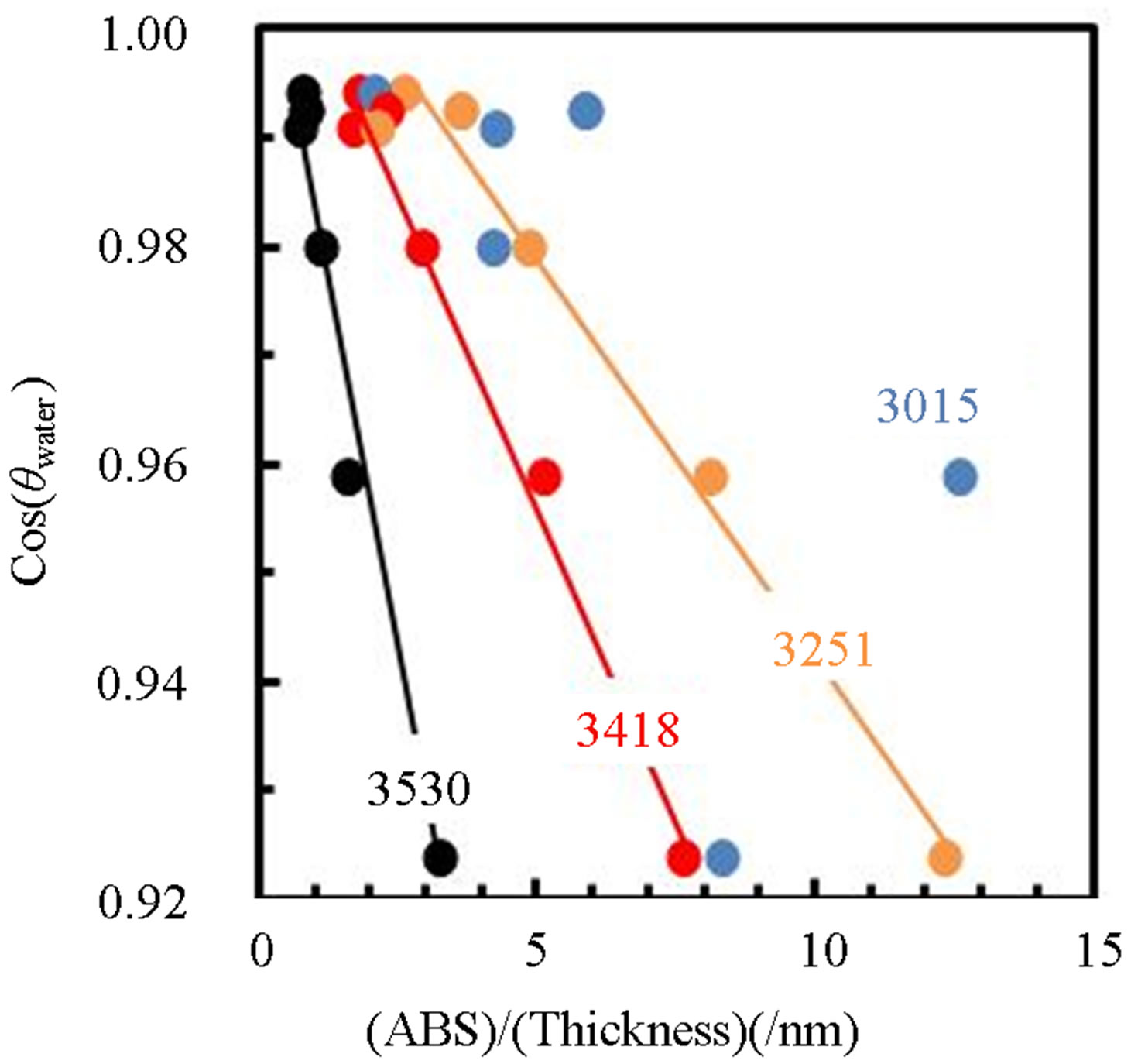
Figure 11. Dependence of Cos(qwater) on OH-related absorption intensity, where the absorption intensity in the layer (ABS) is divided by the layer thickness (thickness).

Figure 12. Feature of water droplets on (a) PET and (b) PCVD-TiOx/PET, in which water of 10 μL was dropped on the PCVD-TiOx/PET after UV-irradiation.
PET was not observed after the TiOx deposition. Whereas the θwater was 75˚ on the PET, the angle was decreased to 5˚ on the TiOx layer. The θwater on the TiOx layer is slightly small comparing to that on TiOx layer deposited on Si substrate, which was probably derived from the difference of surface roughness. As demonstrated here, the PCVD process at room-temperature can be concluded to be useful for hydrophilic coating on the materials with poor thermal resistance.
4. Conclusion
Plasma-assisted deposition of TiOx layer at room temperature using TTIP and O2 gas plasma was studied by FTIR and plasma optical emission spectroscopy. OH content in the layer was significantly related to OH density in the plasma. Optical emission spectroscopy indicated that OH density in the plasma was increased by O2 supply but not dependent on the induced RF-power, which suggested that TTIP was scarcely dissociated in the plasma at pressure as low as 3 mtorr but efficiently dissociated on RF-electrode. Chemisorbed OH, especially H2O2 in the layer was significantly influenced on the hydrophilicity. The PCVD-TiOx coating was demonstrated on PET and the contact angle of water was decreased to 5˚ compared with 75˚ on PET.
REFERENCES
- R. Wang, K. Hashimoto and A. Fujishima, “Light-Induced Amphiphilic Surfaces,” Nature, Vol. 388, No. 6641, 1997, pp. 431-432. http://dx.doi.org/10.1038/41233
- A. Mills, A. Lepre, N. Elliott, A. Bhopal, I. P. Parkin and S. A. Neill, “Characterisation of the Photocatalyst Pilkington ActivTM: A Reference Film Photocatalyst?” Journal of Photochemistry and Photobiology A: Chemistry, Vol. 160, No. 3, 2003, pp. 213-224. http://dx.doi.org/10.1016/S1010-6030(03)00205-3
- S. A. Campbell, H. S. Kim, D. C. Gilmer, B. He, T. Ma and W. L. Gladfelter, “Titanium Dioxide (TiO2)-Based Gate Insulators,” IBM Journal of Research and Development, Vol. 43, No. 3, 1999, pp. 383-392. http://dx.doi.org/10.1147/rd.433.0383
- C. Martinet, V. Paillard, A. Gagnaire and J. Joseph, “Deposition of SiO2 and TiO2 Thin Films by Plasma Enhanced Chemical Vapor Deposition for Antireflection Coating,” Journal of Non-Crystalline Solids, Vol. 216, 1997, pp. 77-82. http://dx.doi.org/10.1016/S0022-3093(97)00175-0
- H. Gerischer and H. Heller, “The Role of Oxygen in Photooxidation of Organic Molecules on Semiconductor Particles,” The Journal of Physical Chemistry, Vol. 95, No. 13, 1991, pp. 5261-5267. http://dx.doi.org/10.1021/j100166a063
- R. Wang, K. Hashimoto, A. Fujishima, M. Chikuni, E. Kojima, A. Kitamura, M. Shimohigoshi and T. Watanabe, “Photogeneration of Highly Amphiphilic TiO2 Surfaces,” Advanced Materials, Vol. 10, No. 2, 1998, pp. 135-138. http://dx.doi.org/10.1002/(SICI)1521-4095(199801)10:2<135::AID-ADMA135>3.0.CO;2-M
- N. Kotov, F. Meldrum and J. H. Fendler, “Monoparticulate Layers of Titanium Dioxide Nanocrystallites with Controllable Interparticle Distances,” The Journal of Physical Chemistry, Vol. 98, No. 36, 1994, pp. 8827-8830. http://dx.doi.org/10.1021/j100087a002
- M. Okuya, N. A. Prokudina, K. Mushika and S. Kaneko, “TiO2 Thin Films Synthesized by the Spray Pyrolysis Deposition (SPD) Technique,” Journal of the European Ceramic Society, Vol. 19, No. 6, 1999, pp. 903-906. http://dx.doi.org/10.1016/S0955-2219(98)00341-0
- M. H. Suhail, G. Mohan Rao and S. Mohan, “dc Reactive Magnetron Sputtering of Titanium-Structural and Optical Characterization of TiO2 Films,” Journal of Applied Physics, Vol. 71, No. 3, 1992, pp. 1421-1427. http://dx.doi.org/10.1063/1.351264
- P. Lobl, M. Huppertz and D. Mergel, “Nucleation and Growth in TiO2 Films Prepared by Sputtering and Evaporation,” Thin Solid Films, Vol. 251, No. 1, 1994, pp. 72-79. http://dx.doi.org/10.1016/0040-6090(94)90843-5
- V. Gauthier, S. Bourgeois, P. Sibillot, M. Maglione and M. Sacilotti, “Growth and Characterization of AP-MOCVD Iron Doped Titanium Dioxide Thin Films,” Thin Solid Films, Vol. 340, No. 1, 1999, pp. 175-182. http://dx.doi.org/10.1016/S0040-6090(98)01469-2
- S. Mathur and P. Kuhn, “CVD of Titanium Oxide Coatings: Comparative Evaluation of Thermal and Plasma Assisted Processes,” Surface and Coatings Technology, Vol. 201, No. 3-4, 2006, pp. 807-814. http://dx.doi.org/10.1016/j.surfcoat.2005.12.039
- S. Yamauchi and Y. Imai, “Plasma-Assisted Chemical Vapor Deposition of TiO2 Thin Films for Highly Hydrophilic Performance,” Crystal Structure Theory and Applications, Vol. 2, No. 1, 2013, pp. 1-7. http://dx.doi.org/10.4236/csta.2013.21001
- M. Nakamura, S. Kato, T. Aoki, L. Sirghi and Y. Hatanaka, “Role of Terminal OH Groups on the Electrical and Hydrophilic Properties of Hydro-Oxygenated Amorphous TiOx:OH Thin Films,” Journal of Applied Physics, Vol. 90, No. 7, 2001, pp. 3391-3395. http://dx.doi.org/10.1063/1.1398599
- L. Sirghi, T. Aoki and Y. Hatanaka, “Hydrophilicity of TiO2 Thin Films Obtained by Radio Frequency Magnetron Sputtering Deposition,” Thin Solid Films, Vol. 422, No. 1, 2002, pp. 55-61. http://dx.doi.org/10.1016/S0040-6090(02)00949-5
- M. Gotić, M. Ivanda, A. Sekulić, S. Musić, S. Popovi, A. Turković and K. Furić, “Microstructure of Nanosized TiO, Obtained by Sol-Gel Synthesis,” Materials Letters, Vol. 28, No. 1-3, 1996, pp. 225-229. http://dx.doi.org/10.1016/0167-577X(96)00061-4
- K. H. Ahn, Y. B. Park and D. W. Park, “Kinetic and Mechanistic Study on the Chemical Vapor Deposition of Titanium Dioxide Thin Films by in Situ FT-IR Using TTIP,” Surface and Coatings Technology, Vol. 171, No. 1, 2003, pp. 198-204.
- T. Tsuchiya, A. Watanabe, Y. Imai, H. Niino, I. Yamaguchi, T. Manabe, T. Kumagai and S. Mizuta, “Direct Conversion of Titanium Alkoxide into Crystallized TiO2 (Rutile) Using Coating Photolysis Process with ArF Excimer Laser,” Japanese Journal of Applied Physics, Vol. 38, No. 7B, 1999, pp. L823-L825. http://dx.doi.org/10.1143/JJAP.38.L823
- J. Araña, C. Garriga i Cabo, J. M. Doña-Rodríguez, O. González-Díaz, J. A. Herrera-Melián and J. Pérez-Peña, “FTIR Study of Formic Acid Interaction with TiO2 and TiO2 Doped with Pd and Cu in Photocatalytic Processes,” Applied Surface Science, Vol. 239, No. 1, 2004, pp. 60- 71.
- D. A. Panayotov and J. T. Yates Jr., “Depletion of Conduction Band Electrons in TiO2 by Water ChemisorptionIR Spectroscopic Studies of the Independence of Ti-OH Frequencies on Electron Concentration,” Chemical Physics Letters, Vol. 410, No. 1, 2005, pp. 11-17. http://dx.doi.org/10.1016/j.cplett.2005.03.146
- M. Pettersson, S. Tuominen and M. Räsänen, “Matrix Isolation and Quantum Chemical Studies on the H2O2- SO2 Complex,” Physical Chemistry Chemical Physics, Vol. 6, No. 19, 2004, pp. 4607-4613.
- W. Yang and C. A. Wolden, “Plasma-Enhanced Chemical Vapor Deposition of TiO2 Thin Films for Dielectric Applications,” Thin Solid Films, Vol. 515, No. 4, 2006, pp. 1708-1713. http://dx.doi.org/10.1016/j.tsf.2006.06.010
NOTES
*Corresponding author.

