Paper Menu >>
Journal Menu >>
 Materials Sciences and Applications, 2010, 1, 247-252 doi:10.4236/msa.2010.14036 Published Online October 2010 (http://www.SciRP.org/journal/msa) Copyright © 2010 SciRes. MSA Effect of Solution Concentration on the Electrospray/Electrospinning Transition and on the Crystalline Phase of PVDF Lígia Maria Manzine Costa, Rosário Elida Suman Bretas, Rinaldo Gregorio, Jr. Department of Materials Engineering, Federal University of São Carlos, Rod. Washington Luís, São Carlos-SP, Brazil. Email: gregorio@ufscar.br Received March 19th, 2010; revised June 17th, 2010; accepted June 21st, 2010. ABSTRACT A study was conducted regarding the effect of concentration of poly (vinylidene fluoride) (PVDF)/N,N-dimethylformamide (DMF) and PVDF/DMF/acetone solutions on the transition between electrospray and electrospinning and on the for- mation of the and crystalline phases of PVDF. The crystalline phases present in the samples, crystallinity and morphology were determined by Fourier transform infrared spectroscopy (FTIR), differential scanning calorimetry (DSC) and scanning electron microscopy (SEM), respectively. Low concentration solutions resulted in films consisting of small droplets (electrospray) containing predominantly the phase. High concentration solutions resulted in a non-woven mesh of nano-to-micron diameter fibers (electrospinning) containing exclusively the phase. These results showed that, the formation of this phase in the electrospinning is related mainly to the solvent evaporation rate, and not to drawing experienced by the polymer during the process. Solvent type affected the amount of crystalline phase present, the boundary concentration between the two processes and the average diameter of fibers. Meshes processed by elec- trospinning display a degree of crystallinity higher than the films obtained by electrospray. Keywords: Poly(Vinylidene Fluoride) (PVDF), Electrospinning, Electrospray, Crystalline Phase, Fibers, Morphology 1. Introduction PVDF, poly(vinylidene fluoride), is a widely investigated polymer due to its excellent mechanical properties, chemical stability and ferroelectricity. This polymer has a simple chemical formula, -CH2-CF2-, and may crystal- lize in at least four crystalline phases known as α, β, γ and δ. The structure and property of these phases are well documented in the literature [1]. Each crystalline phase confers characteristic properties to the polymer and therefore distinct applications. The β phase is that which displays the strongest pyro and piezoelectric activities and is therefore technologically more interesting. Many techniques have been used to obtain this phase, being the most common uni or biaxial drawing of originally α phase films [2-6]. Crystallization of PVDF from solution (casting) may also result in the phase, depending on the evaporation rate of the solvent [7-9]. Low rates result predominantly in the phase, whereas high rates favor the phase. Electrospinning [10,11] also produces pre- dominantly the phase [12-18]. In this technique the polymer solution is added to a capillary (which can be a syringe with needle). The solution forms a droplet at the tip of the needle due to surface tension. If a sufficiently high electric voltage (5-30 kV) is applied to the solution electric charges accumulate in the droplet. When the electrostatic repulsion between the charges overcomes the surface tension and viscoelasticity of the droplet this assumes the shape of a cone. When the force exerted by the electric field, formed between the droplet and a grounded collector, overcomes the surface tension, a thin jet is formed. The jet of the charged solution is acceler- ated towards the collector under the action of the electric field. If the concentration of the solution is sufficiently high (viscous) to stabilize the jet, the polymer solution is severely stretched and the solvent evaporates to form ultrathin fibers which solidify and are deposited on the collector forming a non-woven mesh of fibers. Some authors [13-17] attribute the formation of β phase in electrospun PVDF meshes to this severe drawing ex-  Effect of Solution Concentration on the Electrospray/Electrospinning Transition and on the Crystalline Phase of PVDF Copyright © 2010 SciRes. MSA 248 perienced by the polymer jet solution. This elongation process would be similar to the well known transi- tion caused by mechanical drawing at T 90℃ [2-6]. A variant of electrospinning is electrospray [19]. The basic difference between these two processes lies in the concentration of the solution. In electrospray the concen- tration is sufficiently low to destabilize the charged jet which then breaks down into small spherical droplets that solidify during the course and are deposited on the col- lector. In this case the polymer solution does not experi- ence severe drawing and the formed film consists of small droplets instead of fibers. The objectives of the current investigation were: 1) determine the solution concentration at which the transi- tion between electrospray and electrospinning of PVDF occurs and 2) verify through the electrospray technique, where no stretching occurs, that the formation of the phase depends fundamentally on the solvent evaporation rate. The effect of the type of solvent in the process was also verified. 2. Experimental Methods 2.1. Materials The PVDF used was Foraflon® 4000HD, from Elf Ato- chem. The solvents used were N,N-dimethylformamide (DMF, Merck 99.5%) and acetone (Merck, 99.7%). So- lutions were prepared at concentrations of 5, 7, 10 and 15 wt% PVDF using as solvent pure DMF and a 3:1 v/v mixture of DMF with acetone. The ratio of mixture DMF and acetone was selected because they produce thinner and more homogeneous nanofibers in the electrospinning process [13]. Solubilization was carried out at 70℃ un- der stirring for one hour. Table 1 shows some properties of the solvents used. 2.2. Electrospray/Electrospinning A scheme of the system used in the electrospray/electro- spinning process is shown in Figure 1. A 20-mL glass syringe was used with a steel needle with internal di- ameter of 0.7 mm. The distance between needle and col- lector was 3 cm and the electric voltage applied was 10 kV, using a Bertan 210 30R (0-30 kV) high voltage source. The collector used was an aluminum disk with diameter of 15 cm and width of 5 cm, at an angular velocity of 60 rpm. The process was carried out at 25℃ and RH 55%. 2.3. Characterizations The crystalline phases present in the samples were char- acterized by transmission infrared spectroscopy (FTIR, Perkin Elmer Spectrum 1000) in the range between 400 and 1000 cm-1 and with resolution of 2 cm-1. Calorimetric analyses were conducted in a Perkin Elmer DSC-7, cali- Table 1. Properties of the solvents used (20℃). Solvent Formula Density (g/mL) Boiling point (℃) Viscosity (mPa.s) Acetone C3H6O 0.786 56.5 0.326 DMF C3H7NO 0.944 153 0.820 Figure 1. Scheme of the setup used in the Electrospinning/ Electrospray process. brated with In, using samples of 8 mg and heating rate of 10℃/min. Degree of crystallinity was determined by comparing the enthalpy of fusion of the sample obtained by DSC with that of 100% crystalline PVDF- (104.5 J/g) [20]. Sample surface morphology was observed by using a scanning electron microscope (Philips 30 FEG Model XL, operating at 15 kV) after gold coating. Size distribu- tion of the fibers diameters was determined by the soft- ware “image J” developed by the National Institute of Health. 3. Results and Discussions The micrographs in Figures 2(a) to (d) and 3(a) to (d) present the morphology of the samples processed with PVDF/DMF and PVDF/DMF/acetone solutions at dif- ferent concentrations, respectively. One can observe in Figures 2(a)-(b) (5 and 7 wt% in DMF) and 3(a) (5 wt% in DMF/acetone) the formation of a film consisting of small droplets, characteristic of the electrospray process. The morphology of this film depends among other things on droplet volume at impact and on evaporation rate of the solvent [21]. In Figures 2(c)-(d) (10, 15 wt% in DMF) and 3(b)-(d) (7, 10 and 15 wt% in DMF/acetone) fibers predominate, characteristic of electrospinning. In micrographs 2(c) (10 wt% in DMF) and 3( b) (7 wt% in DMF/acetone) the electrospun sheets present some small droplets, demonstrating that concen- tration was not yet ideal for the formation of homogene- ous fibers. These results show that the boundary concen- tration between electrospray and electrospinning ranges from 7 to10 wt% PVDF in DMF and from 5 to 7 wt% in 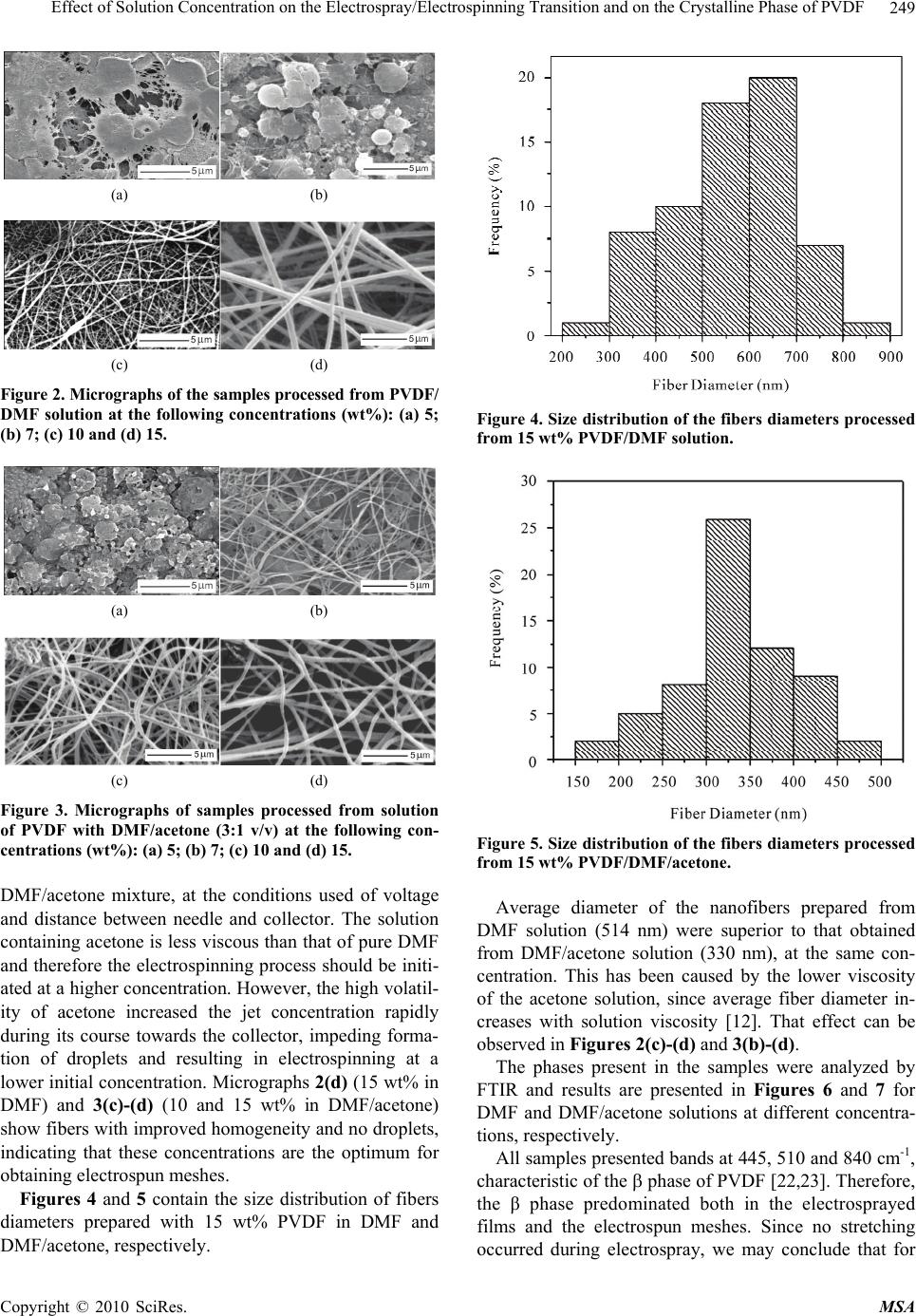 Effect of Solution Concentration on the Electrospray/Electrospinning Transition and on the Crystalline Phase of PVDF Copyright © 2010 SciRes. MSA 249 (a) (b) (c) (d) Figure 2. Micrographs of the samples processed from PVDF/ DMF solution at the following concentrations (wt%): (a) 5; (b) 7; (c) 10 and (d) 15. (a) (b) (c) (d) Figure 3. Micrographs of samples processed from solution of PVDF with DMF/acetone (3:1 v/v) at the following con- centrations (wt%): (a) 5; (b) 7; (c) 10 and (d) 15. DMF/acetone mixture, at the conditions used of voltage and distance between needle and collector. The solution containing acetone is less viscous than that of pure DMF and therefore the electrospinning process should be initi- ated at a higher concentration. However, the high volatil- ity of acetone increased the jet concentration rapidly during its course towards the collector, impeding forma- tion of droplets and resulting in electrospinning at a lower initial concentration. Micrographs 2(d) (15 wt% in DMF) and 3(c)-(d) (10 and 15 wt% in DMF/acetone) show fibers with improved homogeneity and no droplets, indicating that these concentrations are the optimum for obtaining electrospun meshes. Figures 4 and 5 contain the size distribution of fibers diameters prepared with 15 wt% PVDF in DMF and DMF/acetone, respectively. Figure 4. Size distribution of the fibers diameters processed from 15 wt% PVDF/DMF solution. Figure 5. Size distribution of the fibers diameters processed from 15 wt% PVDF/DMF/acetone. Average diameter of the nanofibers prepared from DMF solution (514 nm) were superior to that obtained from DMF/acetone solution (330 nm), at the same con- centration. This has been caused by the lower viscosity of the acetone solution, since average fiber diameter in- creases with solution viscosity [12]. That effect can be observed in Figures 2(c)-(d) and 3(b)-(d) . The phases present in the samples were analyzed by FTIR and results are presented in Figures 6 and 7 for DMF and DMF/acetone solutions at different concentra- tions, respectively. All samples presented bands at 445, 510 and 840 cm-1, characteristic of the phase of PVDF [22,23]. Therefore, the β phase predominated both in the electrosprayed films and the electrospun meshes. Since no stretching occurred during electrospray, we may conclude that for 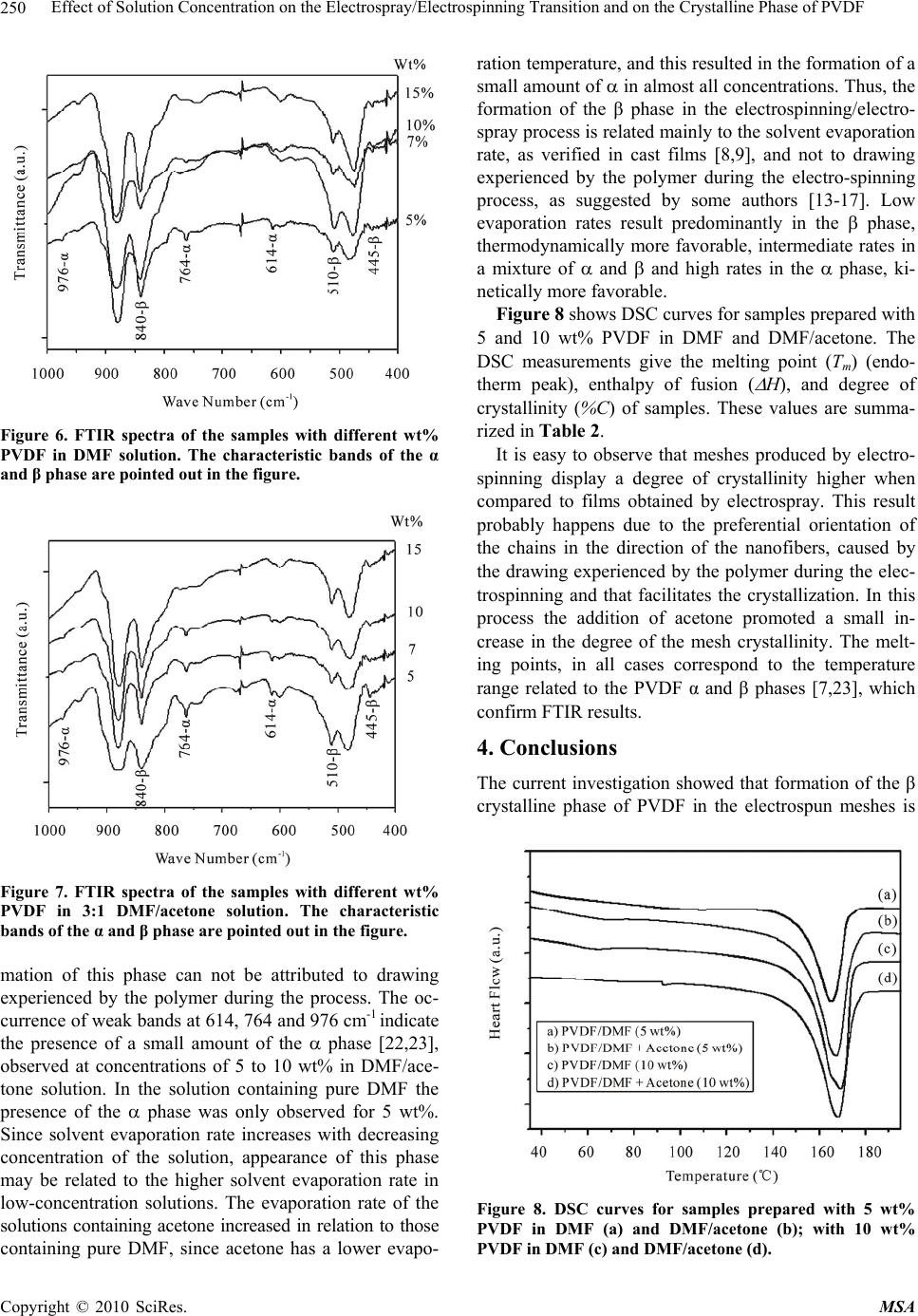 Effect of Solution Concentration on the Electrospray/Electrospinning Transition and on the Crystalline Phase of PVDF Copyright © 2010 SciRes. MSA 250 Figure 6. FTIR spectra of the samples with different wt% PVDF in DMF solution. The characteristic bands of the α and β phase are pointed out in the figure. Figure 7. FTIR spectra of the samples with different wt% PVDF in 3:1 DMF/acetone solution. The characteristic bands of the α and β phase are pointed out in the figure. mation of this phase can not be attributed to drawing experienced by the polymer during the process. The oc- currence of weak bands at 614, 764 and 976 cm-1 indicate the presence of a small amount of the phase [22,23], observed at concentrations of 5 to 10 wt% in DMF/ace- tone solution. In the solution containing pure DMF the presence of the phase was only observed for 5 wt%. Since solvent evaporation rate increases with decreasing concentration of the solution, appearance of this phase may be related to the higher solvent evaporation rate in low-concentration solutions. The evaporation rate of the solutions containing acetone increased in relation to those containing pure DMF, since acetone has a lower evapo- ration temperature, and this resulted in the formation of a small amount of in almost all concentrations. Thus, the formation of the β phase in the electrospinning/electro- spray process is related mainly to the solvent evaporation rate, as verified in cast films [8,9], and not to drawing experienced by the polymer during the electro-spinning process, as suggested by some authors [13-17]. Low evaporation rates result predominantly in the phase, thermodynamically more favorable, intermediate rates in a mixture of and and high rates in the phase, ki- netically more favorable. Figure 8 shows DSC curves for samples prepared with 5 and 10 wt% PVDF in DMF and DMF/acetone. The DSC measurements give the melting point (Tm) (endo- therm peak), enthalpy of fusion ( H), and degree of crystallinity (%C) of samples. These values are summa- rized in Table 2. It is easy to observe that meshes produced by electro- spinning display a degree of crystallinity higher when compared to films obtained by electrospray. This result probably happens due to the preferential orientation of the chains in the direction of the nanofibers, caused by the drawing experienced by the polymer during the elec- trospinning and that facilitates the crystallization. In this process the addition of acetone promoted a small in- crease in the degree of the mesh crystallinity. The melt- ing points, in all cases correspond to the temperature range related to the PVDF α and β phases [7,23], which confirm FTIR results. 4. Conclusions The current investigation showed that formation of the β crystalline phase of PVDF in the electrospun meshes is Figure 8. DSC curves for samples prepared with 5 wt% PVDF in DMF (a) and DMF/acetone (b); with 10 wt% PVDF in DMF (c) and DMF/acetone (d).  Effect of Solution Concentration on the Electrospray/Electrospinning Transition and on the Crystalline Phase of PVDF Copyright © 2010 SciRes. MSA 251 Table 2. Melting point (Tm), enthalpy of fusion ( H) and degree of cristallinity (%C) of the samples processed with PVDF/DMF and PVDF/DMF/acetone solutions at different concentrations. Sample Process Tm (℃) H (J/g) %C 5 wt% PVDF/DMF 166 46.2 44.6 5 wt% PVDF/DMF/acetone Electrospray 167 46.8 45.2 10 wt% PVDF/DMF 168 53.4 51.1 10 wt% PVDF/DMF/acetone Electrospinning 167 55.0 53.1 not related to drawing experienced by the polymer during the process, as suggested by some authors. Formation of the and phase is related primarily to the evaporation rate of the solvent. High rates favor formation of the phase, whereas low rates favor the phase. Under the same conditions of voltage and needle-collector distance, the boundary concentration between electrospray and electrospinning depends on the solvent used. For PVDF this concentration lies between 7 and 10 wt% with DMF as solvent and between 5 and 7 wt% for the 3:1 DMF/ acetone mixture. Moreover, addition of acetone to the solution reduced average fiber diameter. Meshes pro- duced by electrospinning present degree of crystallinity higher than the films obtained by electrospray, for both used solvents. 5. Acknowledgements The following agencies are acknowledged for financial support: CAPES, CNPq and FAPESP. REFERENCES [1] K. Tashiro, “Crystal Structure and Phase Transition of PVDF and Related Copolymers,” In: H. S. Nalwa, Ed., Ferroelectric Polymers: Chemistry, Physics and Applica- ons, Marcel Dekker, New York, 1995, pp. 63-180. [2] R. Gregorio, Jr. and E. M. Ueno, “Effect of Crystalline Phase, Orientation and Temperature on the Dielectric Properties of Poly(Vinylidene Fluoride) (PVDF),” Jour- nal of Materials Science, Vol. 34, 1999, pp. 4489-4500. [3] V. Sencadas, V. M. Moreira, S. Lanceros-Mendez, A. S. Pouzada and R. Gregorio, Jr., “Alpha-to-Beta Transfor- mation on PVDF Films Obtained by Uniaxial Stretch,” Advanced Materials Forum III, Vol. 514-516, 2006, 872- 876. [4] P. Sajkiewicz, A. Wasiak and Z. Goclowski, “Phase Tran- sitions during Stretching of Poly(Vinylidene Fluoride),” European Polymer Journal, Vol. 35, No. 3, pp. 423-429. [5] D. T. Grubb and F. R. Kearney, “Phase Transitions dur- ing Stretching of Poly(Vinylidene Fluoride),” Journal of Polymer Science Part B: Polymer Physics, Vol. 28, No. 11, 1990, pp. 2071-2078. [6] M. C. Branciforti, V. Sencadas, S. Lanceros-Mendez and R. Gregorio, Jr., “New Technique of Processing Highly Oriented Poly(Vinylidene Fluoride) Films Exclusively in the Phase,” Journal of Polymer Science Part B: Poly- mer Physics, Vol. 45, 2007, pp. 2793-2801. [7] R. Gregorio, Jr., and M. Cestari, “Effect of Crystallization Temperature on the Crystalline Phase Content and Mor- phology of Poly(Vinylidene Fluoride),” Journal of Poly- mer Science Part B: Polymer Physics, Vol. 32, No. 5, pp. 859-870. [8] R. Gregorio, Jr. and D. S. Borges, “Effect of Crystalliza- tion Rate on the Formation of the Polymorphs of Solution Cast Poly(Vinylidene Fluoride),” Polymer, Vol. 49, No. 18, 2008, pp. 4009-4016. [9] D. L. Chinaglia, R. Gregorio, Jr., J. C. Stefanello, R. A. P. Altafin, W. Wirges, F. Wang and R. Gerhard, “Influence of the Solvent Evaporation Rate on the Crystalline Phases of Solution-Cast Poly(Vinylidene Fluoride) Films,” Jour- nal of Applied Polymer Science, Vol. 116, No. 2, 2010, pp. 785-791. [10] A. Formhals, “Process and Apparatus for Preparing Artificial Threads,” U.S. Patent 1,975,504, 1934. [11] J. Doshi and D. H. Reneker, “Electrospinning Process and Application of Electrospun Fibers,” Journal of Electro- statics, Vol. 35, No. 2-3, 1995, pp. 151-160. [12] M. Nasir, H. Matsumoto, T. Danno, M. Minagawa, T. Irisawa, M. Shioya and A. Tanioka, “Control of Diameter, Morphology, and Structure of PVDF Nanofiber Fabri- cated by Electrospray Deposition,” Journal of Polymer Science Part B: Polymer Physics, Vol. 44, No. 5, pp. 779- 786. [13] W. A. Yee, M. Kotaki, Y. Liu and X. Lu, “Morphology, Polymorphism Behavior and Molecular Orientation of Electrospun Poly(Vinylidene Fluoride) Fibers,” Polymer, Vol. 48, No. 2, 2007, pp. 512-521. [14] E. Ogut, O. S. Yordem, Y. Z. Menceloglu and M. Papila, “Poly(Vinylidene Fluoride)/Zinc Oxid Smart Composite Material,” Proceeding of SPIE, Vol. 6526, 2007, p. 65260Q-1-10 [15] J. Zheng, A. He, J. Li and C. C. Han, “Polymorphism Control of Poly(Vinylidene Fluoride) through Electro- spinning,” Macromolecular Rapid Communications, Vol. 28, No. 22, 2007, pp. 2159-2162. [16] W. A. Yee, A. C. Nguyen, P. S. Lee, M. Kotaki, Y. Liu, B. T. Tan, S. Mhaisalkar and X. Lu, “Stress-Induced Struc- tural Changes in Electrospun Polyvinylidene Difluoride Nanofibers Colleted Using a Modified Rotating Disk,” Polymer, Vol. 49, No. 19, 2008, pp. 4196-4203.  Effect of Solution Concentration on the Electrospray/Electrospinning Transition and on the Crystalline Phase of PVDF Copyright © 2010 SciRes. MSA 252 [17] J. S. Andrew and D. R. Clarke, “Effect of Electrospin- ning on the Ferroelectric Phase Content of Polyvinylidene Difluoride Fibers,” Langmuir, Vol. 24, No. 3, 2008, pp. 670-672. [18] H. Na, Y. Zhao, C. Zhao, C. Zhao and X. Yuan, (2008) Effect of Hot-Press on Electrospun Poly(Vinylidene Fluo- ride) Membranes,” Polymer Engineering and Science, Vol. 48, No. 5, pp. 934-940. [19] G. Taylor, “Electrospray,” Proceedings of Royal Society of London, London, Vol. A280, 1964, pp. 383-387. [20] K. Nakagawa and Y. Ishida, “Dielectric Relaxations and Molecular Motions in Poly(Vinylidene Fluoride) with Crystal Form II,” Journal of Polymer Science Part B: Polymer Physics, Vol. 11, No. 8, pp. 1503-1533. [21] I. B. Rietveld, K. Kobayashi, H. Yamada and K. Matsu- shige, “Model Supported Morphology Control of Elec- trospray Deposited Poly(Vinylidene Fluoride) Film,” Macromolecular Symposium, 2007, pp. 249-250, 322- 329. [22] M. Kobayashi, K. Tashiro and H. Tadokoro, “Molecular Vibrations of Three Crystal Forms of Poly(Vilidene Fluo- ride),” Macromolecules, Vol. 8, No. 2, 1975, pp. 158-170. [23] R. Gregorio, Jr., “Determination of the , and Crys- talline Phases of Poly(Vinylidene Fluoride) Films Pre- pared at Different Conditions,” Journal of Applied Poly- mer Science, Vol. 100, No. 4, 2006, pp. 3272-3279. |

