Flash microbiocide: A Rapid and Economic Method for Determination of MBC and MFC
Copyright © 2013 SciRes. AJPS
852
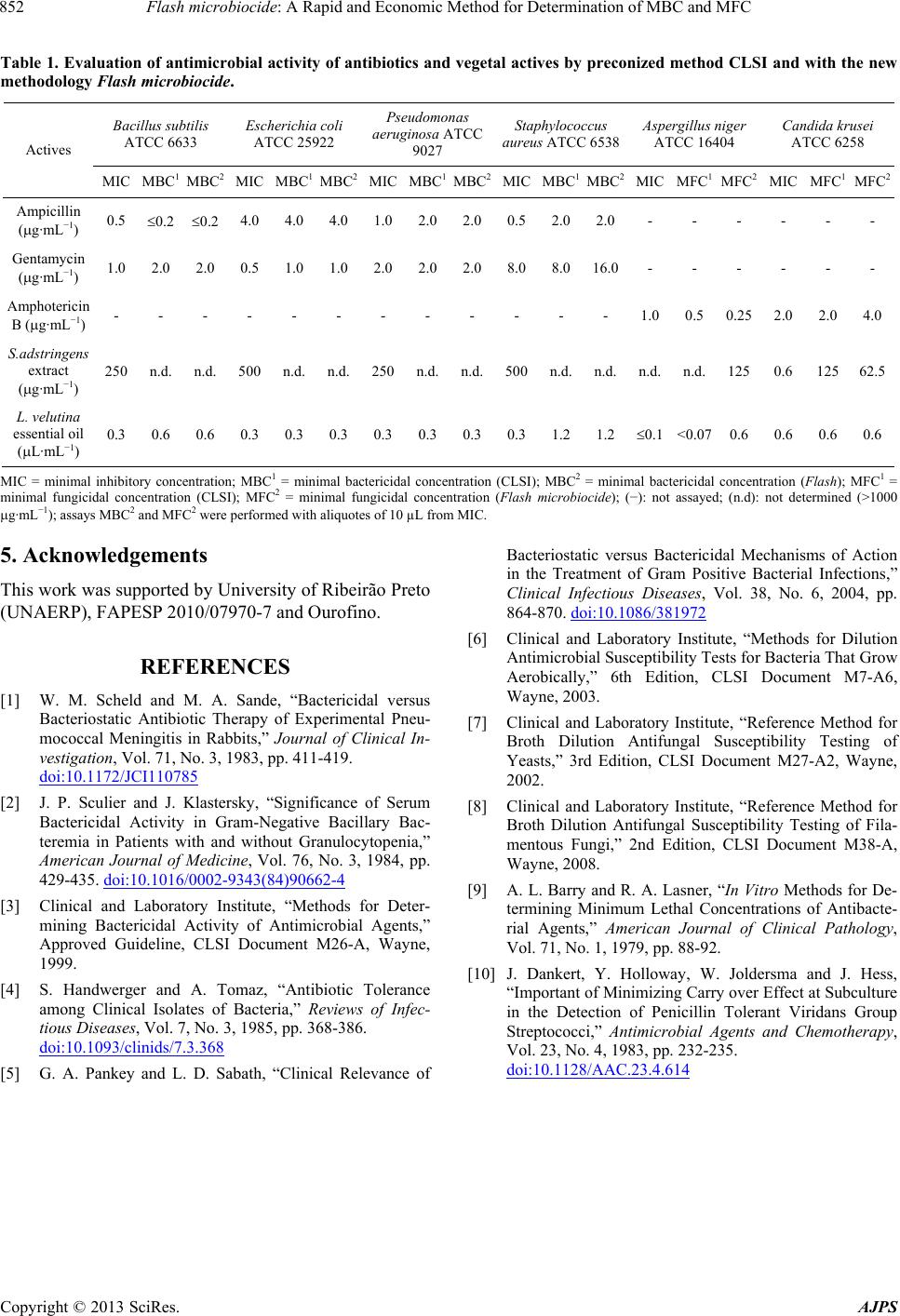
Table 1. Evaluation of antimicrobial activity of antibiotics and vegetal actives by preconized method CLSI and with the new
methodology Flash microbiocide.
Bacillus subtilis
ATCC 6633
Escherichia coli
ATCC 25922
Pseudomonas
aeruginosa ATCC
9027
Staphylococcus
aureus ATCC 6538
Aspergillus niger
ATCC 16404
Candida krusei
ATCC 6258
Actives
MIC MBC1 MBC2MIC MBC1 MBC2MIC MBC1MBC2MIC MBC1MBC2MIC MFC1 MFC2 MIC MFC1MFC2
Ampicillin
(g·mL−1) 0.5 0.2 0.2 4.0 4.0 4.0 1.02.02.00.52.02.0- - - - - -
Gentamycin
(g·mL−1) 1.0 2.0 2.0 0.5 1.0 1.0 2.0 2.0 2.0 8.0 8.016.0- - - - - -
Amphotericin
B (g·mL−1) - - - - - - - - - - - - 1.00.5 0.25 2.0 2.04.0
S.adstringens
extract
(g·mL−1)
250 n.d. n.d. 500 n.d. n.d. 250 n.d.n.d.500 n.d. n.d. n.d. n.d. 125 0.6 12562.5
L. velutina
essential oil
(L·mL−1)
0.3 0.6 0.6 0.3 0.3 0.3 0.3 0.3 0.30.3 1.2 1.20.1 <0.07 0.6 0.6 0.60.6
MIC = minimal inhibitory concentration; MBC1 = minimal bactericidal concentration (CLSI); MBC2 = minimal bactericidal concentration (Flash ); MFC1 =
minimal fungicidal concentration (CLSI); MFC2 = minimal fungicidal concentration (Flash microbiocide); (−): not assayed; (n.d): not determined (>1000
g·mL−1); assays MBC2 and MFC2 were performed with aliquotes of 10 µL from MIC.
5. Acknowledgements
This work was supported by University of Ribeirão Preto
(UNAERP), FAPESP 2010/07970-7 and Ourofino.
REFERENCES
[1] W. M. Scheld and M. A. Sande, “Bactericidal versus
Bacteriostatic Antibiotic Therapy of Experimental Pneu-
mococcal Meningitis in Rabbits,” Journal of Clinical In-
vestigation, Vol. 71, No. 3, 1983, pp. 411-419.
doi:10.1172/JCI110785
[2] J. P. Sculier and J. Klastersky, “Significance of Serum
Bactericidal Activity in Gram-Negative Bacillary Bac-
teremia in Patients with and without Granulocytopenia,”
American Journal of Medicine, Vol. 76, No. 3, 1984, pp.
429-435. doi:10.1016/0002-9343(84)90662-4
[3] Clinical and Laboratory Institute, “Methods for Deter-
mining Bactericidal Activity of Antimicrobial Agents,”
Approved Guideline, CLSI Document M26-A, Wayne,
1999.
[4] S. Handwerger and A. Tomaz, “Antibiotic Tolerance
among Clinical Isolates of Bacteria,” Reviews of Infec-
tious Diseases, Vol. 7, No. 3, 1985, pp. 368-386.
doi:10.1093/clinids/7.3.368
[5] G. A. Pankey and L. D. Sabath, “Clinical Relevance of
Bacteriostatic versus Bactericidal Mechanisms of Action
in the Treatment of Gram Positive Bacterial Infections,”
Clinical Infectious Diseases, Vol. 38, No. 6, 2004, pp.
864-870. doi:10.1086/381972
[6] Clinical and Laboratory Institute, “Methods for Dilution
Antimicrobial Susceptibility Tests for Bacteria That Grow
Aerobically,” 6th Edition, CLSI Document M7-A6,
Wayne, 2003.
[7] Clinical and Laboratory Institute, “Reference Method for
Broth Dilution Antifungal Susceptibility Testing of
Yeasts,” 3rd Edition, CLSI Document M27-A2, Wayne,
2002.
[8] Clinical and Laboratory Institute, “Reference Method for
Broth Dilution Antifungal Susceptibility Testing of Fila-
mentous Fungi,” 2nd Edition, CLSI Document M38-A,
Wayne, 2008.
[9] A. L. Barry and R. A. Lasner, “In Vitro Methods for De-
termining Minimum Lethal Concentrations of Antibacte-
rial Agents,” American Journal of Clinical Pathology,
Vol. 71, No. 1, 1979, pp. 88-92.
[10] J. Dankert, Y. Holloway, W. Joldersma and J. Hess,
“Important of Minimizing Carry over Effect at Subculture
in the Detection of Penicillin Tolerant Viridans Group
Streptococci,” Antimicrobial Agents and Chemotherapy,
Vol. 23, No. 4, 1983, pp. 232-235.
doi:10.1128/AAC.23.4.614