 Vol.2, No.10, 1164-1170 (2010) Natural Science http://dx.doi.org/10.4236/ns.2010.210144 Copyright © 2010 SciRes. OPEN ACCESS The factorial structure of self-reported androgen-promoted physiological traits Lee Ellis1, Shyamal Das2 1University Malaya, Department of Anthropology, Kuala Lumpur, Malaysia; lee.ellis@hotmail.com; 2Elizabeth City State University, Elizabeth City, USA; sdas@mail.ecsu.edu. Received 27 June 2010; revised 29 July 2010; accepted 4 August 2010. ABSTRACT Androgens make major contributions to aver- age sex differences in anatomy, physiology, and behavior. Despite having established their cru- cial role in sexual differentiation, much remains to be learned about how androgens coordinate their influences. The present study was under- taken to shed light on androgenic effects on the body using self-reported survey data. We ana- lyzed the ratings provided by over 11,000 col- lege students on the magnitude of eleven traits that previous research has shown to be influ- enced by testosterone or other androgens. Pre- dictably, the average values for all eleven traits were significantly greater in males than in fe- males. Nevertheless, when data were analyzed separately according to sex of the respondents, some of the traits failed to positively correlate with one another, suggesting that not all an- drogen-influenced traits differentiate in a simple fashion. Factor analysis of these eleven traits by sex reinforced this view by identifying four fac- tors. In men, the primary factor loaded most heavily on: masculine body build, masculine mannerisms, overall physical strength, upper body strength, and lower body strength. The primary factor for women was limited to: upper body strength, lower body strength, and overall physical strength. In both sexes, the primary factor was interpreted as reflecting the influ- ence of perinatal and postpubertal testosterone exposure. The other three factors may reflect the effects of other androgens (e.g., andros- tenediol), or the influence of female hormones such as estradiol. Findings were discussed in terms of future use of self-reported physiologi- cal measures for assessing androgenic effects on the human body. Keywords: Androgen-promoted physical traits; Testosterone; Masculinization; Physical strength; Factorial structure; Sex differences 1. INTRODUCTION A recent literature review provided evidence that the sexes differ in a mired of ways, ranging from easy-to- measure traits (e.g., birth weights and adult body size) to many complex characteristics (e.g., susceptibility to nu- merous diseases, detailed biochemistry, neurology, per- ceptual sensitivities, motor coordination, and even many cognitive and behavioral patterns) [1]. This evidence raises questions about how sex differences are produced. Although the details are still far from fully understood, numerous studies have implicated bodily exposure to androgens as primarily responsible for sex differences in traits [2-5]. In broad terms, the sexual differentiation of animals occurs as follows: The default sex at least for mammals, is female, meaning that males are a genetic variant on the female sex [6-9]. Early in the gestation process of nearly all males, the would-be ovaries are made to begin differentiating into testes instead by genes located on the Y-chromosome [10]. As this occurs in humans during the first five months of gestation, the genitals of males gradually take on a masculine rather than a feminine appearance [11-12]. The gestational aspects of sexual differentiation are referred to as its organizational stage, a stage in humans extending from the first month of gestation into about the fourth month following birth [13]. The second phase of sexual differentiation is known as the activational (or postpubertal) stage. It is marked by the appearance of so-called secondary sex characteristics, but also includes enlargement of the penis and testes in males [14-17]. The traits that are masculinized by bodily exposure to androgens are numerous. They include the following: Growth of body hair [18-20] Darkening of the iris of the eye [21,22] Facial acne [23-25]  L. Ellis et al. / Natural Science 2 (2010) 1164-1170 Copyright © 2010 SciRes. OPEN ACCESS 116 1165 Darkening of hair color [26,27] Increase in height [28-32] Lowering of the voice [33-37] Increase in upper body strength [15,38,39] Increase in lower body strength [40,42] Increase in masculine body appearance [43-44] Increase in masculine mannerisms [45,46] Despite the abundant evidence that androgens mascu- linize many aspects of human development, the details are still only vaguely understood. The purpose of the present study was to examine the eleven androgen-in- fluenced traits listed above using self-ratings, with the following three questions in mind. First, are all of these traits in fact sexually dimorphic? Second, within each sex, how well do the eleven traits correlate with one an- other? Third, do the within-sex expressions of these traits cluster together, thereby suggesting that they may be resulting from a limited number of similar androgenic regimens? 2. METHODS As part of a broad-ranging investigation, a standar- dized questionnaire was completed by a large sample of college students at twenty United States and two Cana- dian universities between 1988 and 1998 involving 3,786 males and 7,697 females [47]. Subjects ranged in age from 18 to 56, with a mean of 22 for both sexes. In terms of race and ethnicity, the subjects were 85% white, 4% black, 2% Native American, 2% Asian / Pacific Islander, 1% Hispanic, and 6% providing no answer. Eight of the androgen-promoted physiological traits were measured by asking subjects to rate themselves regarding each trait using a 1 to 100 scale, with 100 rep- resenting maximum expression of each trait. These eight traits were: masculine mannerisms, masculine body ap- pearance, physical strength, low deep voice, upper body strength, lower body strength, body-hair development, and facial acne. Height was measured simply in terms of feet and inches (converted to inches). Eye color and hair color were measured, first, by asking subjects to give a one- or two-word description of their eye color and natural hair color. These descriptions were then inter- preted and transcribed into four categories. From highest to lowest values, eye color was coded as being Black, Brown, Hazel / Green, and Blue. For hair color, the four categories were Black, Dark Brown, Light Brown, and Blond. (A copy of the questionnaire is available upon request) Analysis was carried out in three stages. First, the sexes were compared regarding their average scores on all eleven traits using a t-test. Second, to determine how well the eleven traits correlated with one another, a cor- relation matrix was created for the sexes separately. Third, factorial analysis was performed on the eleven traits to assess whether or not some of the traits would form into clusters. 3. RESULTS Table 1 shows that all eleven androgen-promoted traits are significantly more pronounced in men than in wo- men, with p = 0.000 in all cases except for eye color (which attained significance only at the 0.05 level). This is entirely predictable, given that the levels of testost- erone (and other androgens) are higher for males than for females throughout both the organizational and acti- vational stages of sexual differentiation ([1], pp. 89-93). Table 2, however, reveals that within each sex, some of the androgen-promoted traits are not positively corre- lated with one another. Such a rather surprising finding can be interpreted as suggesting that the enhancement of androgen-promoted traits does not occur through a uni- tary process. Most notably, the variables of eye color and especially adolescent facial acne correlate negatively with many of the other androgen-influenced traits among both sexes. The results for factor analyzing responses regarding the eleven androgen-promoted traits are presented in Table 3 for each sex separately. Regarding males (Table 3(a)), the first factor to emerge was named masculinity / strength since it was comprised of masculine manner- isms, masculine body build, and all three of the physical strength measures. We named the second factor pigment because it only loaded strongly on hair color and eye color. Body hair development and adolescent facial acne loaded most heavily on the third factor (with some sec- ondarily strong loadings on upper and lower body strength), which was named dark pigmentation. Finally, height and low-deep voice comprised a fourth factor, which we named physical prowess since both height and low-deep voice are likely to have evolved primarily to intimidate rivals (and possibly impress prospective mates). Turning to females (Table 3(b)), four factors also emerged. The first factor had to do with stren g th. Mascu- line mannerisms and body build loaded along with low-deep voice onto a second factor; therefore, we called it female masculinity. The remaining two female factors were identical to those in males, a dark pigmentation fac- tor and a skin-hair factor. It is interesting to note that height loaded heavily on the physical prowess factor in males but failed to load on any factor among females. 4. CONCLUSIONS While there is no doubt that androgens play a pivotal role in differentiating males from females, much remains 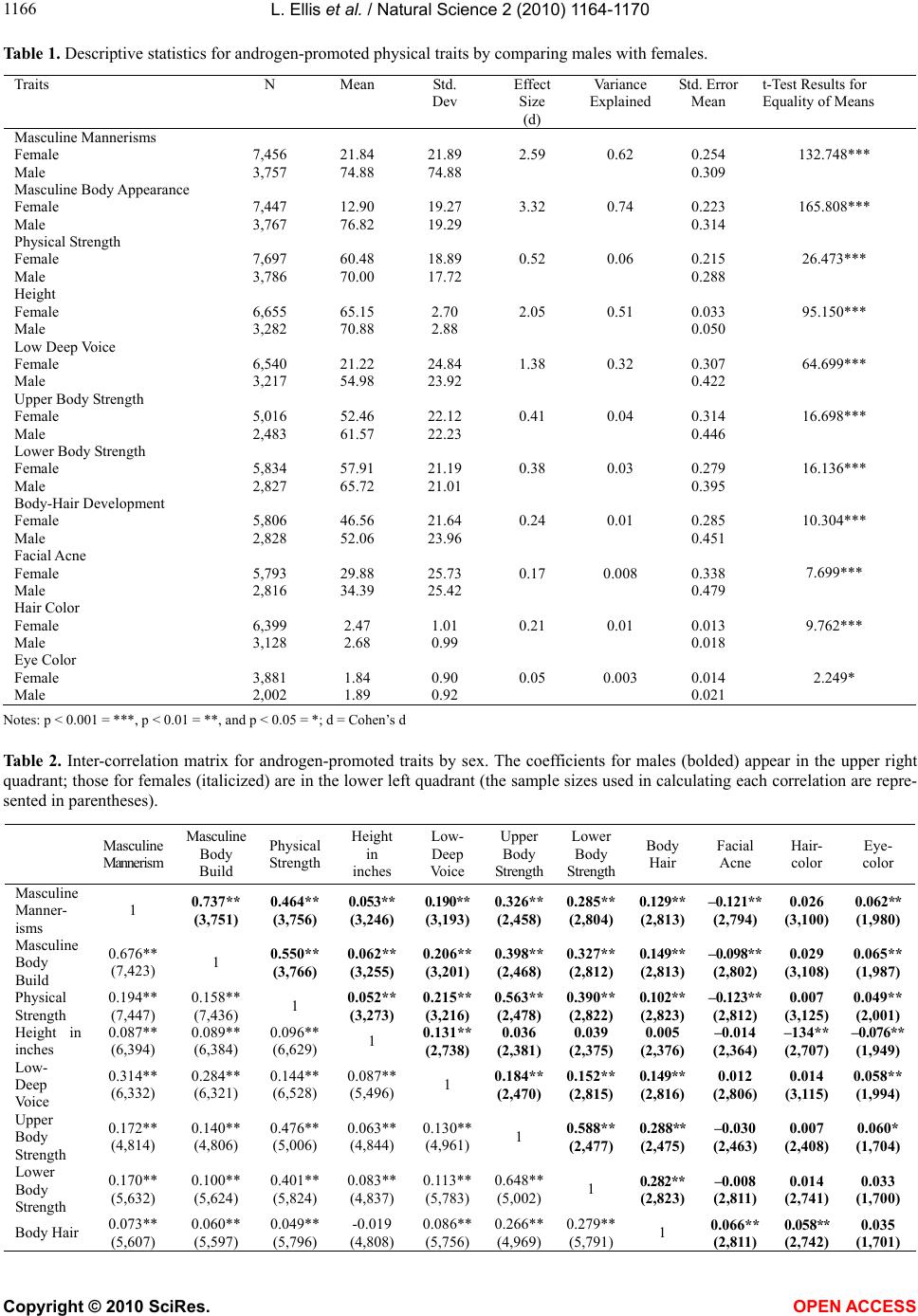 L. Ellis et al. / Natural Science 2 (2010) 1164-1170 Copyright © 2010 SciRes. OPEN ACCESS 1166 Table 1. Descriptive statistics for androgen-promoted physical traits by comparing males with females. Traits N Mean Std. Dev Effect Size (d) Variance Explained Std. Error Mean t-Test Results for Equality of Means Masculine Mannerisms Female Male 7,456 3,757 21.84 74.88 21.89 74.88 2.59 0.62 0.254 0.309 132.748*** Masculine Body Appearance Female Male 7,447 3,767 12.90 76.82 19.27 19.29 3.32 0.74 0.223 0.314 165.808*** Physical Strength Female Male 7,697 3,786 60.48 70.00 18.89 17.72 0.52 0.06 0.215 0.288 26.473*** Height Female Male 6,655 3,282 65.15 70.88 2.70 2.88 2.05 0.51 0.033 0.050 95.150*** Low Deep Voice Female Male 6,540 3,217 21.22 54.98 24.84 23.92 1.38 0.32 0.307 0.422 64.699*** Upper Body Strength Female Male 5,016 2,483 52.46 61.57 22.12 22.23 0.41 0.04 0.314 0.446 16.698*** Lower Body Strength Female Male 5,834 2,827 57.91 65.72 21.19 21.01 0.38 0.03 0.279 0.395 16.136*** Body-Hair Development Female Male 5,806 2,828 46.56 52.06 21.64 23.96 0.24 0.01 0.285 0.451 10.304*** Facial Acne Female Male 5,793 2,816 29.88 34.39 25.73 25.42 0.17 0.008 0.338 0.479 7.699*** Hair Color Female Male 6,399 3,128 2.47 2.68 1.01 0.99 0.21 0.01 0.013 0.018 9.762*** Eye Color Female Male 3,881 2,002 1.84 1.89 0.90 0.92 0.05 0.003 0.014 0.021 2.249* Notes: p < 0.001 = ***, p < 0.01 = **, and p < 0.05 = *; d = Cohen’s d Table 2. Inter-correlation matrix for androgen-promoted traits by sex. The coefficients for males (bolded) appear in the upper right quadrant; those for females (italicized) are in the lower left quadrant (the sample sizes used in calculating each correlation are repre- sented in parentheses). Masculine Mannerism Masculine Body Build Physical Strength Height in inches Low- Deep Voice Upper Body Stre ng th Lower Body Stre ng th Body Hair Facial Acne Hair- color Eye- color Masculine Manner- isms 1 0.737** (3,751) 0.464** (3,756) 0.053** (3,246) 0.190** (3,193) 0.326** (2,458) 0.285** (2,804) 0.129** (2,813) –0.121** (2,794) 0.026 (3,100) 0.062** (1,980) Masculine Body Build 0.676** (7,423) 1 0.550** (3,766) 0.062** (3,255) 0.206** (3,201) 0.398** (2,468) 0.327** (2,812) 0.149** (2,813) –0.098** (2,802) 0.029 (3,108) 0.065** (1,987) Physical Strength 0.194** (7,447) 0.158** (7,436) 1 0.052** (3,273) 0.215** (3,216) 0.563** (2,478) 0.390** (2,822) 0.102** (2,823) –0.123** (2,812) 0.007 (3,125) 0.049** (2,001) Height in inches 0.087** (6,394) 0.089** (6,384) 0.096** (6,629) 1 0.131** (2,738) 0.036 (2,381) 0.039 (2,375) 0.005 (2,376) –0.014 (2,364) –134** (2,707) –0.076 ** (1,949) Low- Deep Voice 0.314** (6,332) 0.284** (6,321) 0.144** (6,528) 0.087** (5,496) 1 0.184** (2,470) 0.152** (2,815) 0.149** (2,816) 0.012 (2,806) 0.014 (3,115) 0.058** (1,994) Upper Body Strength 0.172** (4,814) 0.140** (4,806) 0.476** (5,006) 0.063** (4,844) 0.130** (4,961) 1 0.588** (2,477) 0.288** (2,475) –0.030 (2,463) 0.007 (2,408) 0.060* (1,704) Lower Body Strength 0.170** (5,632) 0.100** (5,624) 0.401** (5,824) 0.083** (4,837) 0.113** (5,783) 0.648** (5,002) 1 0.282** (2,823) –0.008 (2,811) 0.014 (2,741) 0.033 (1,700) Body Hair 0.073** (5,607) 0.060** (5,597) 0.049** (5,796) -0.019 (4,808) 0.086** (5,756) 0.266** (4,969) 0.279** (5,791)1 0.066** (2,811) 0.058** (2,742) 0.035 (1,701) 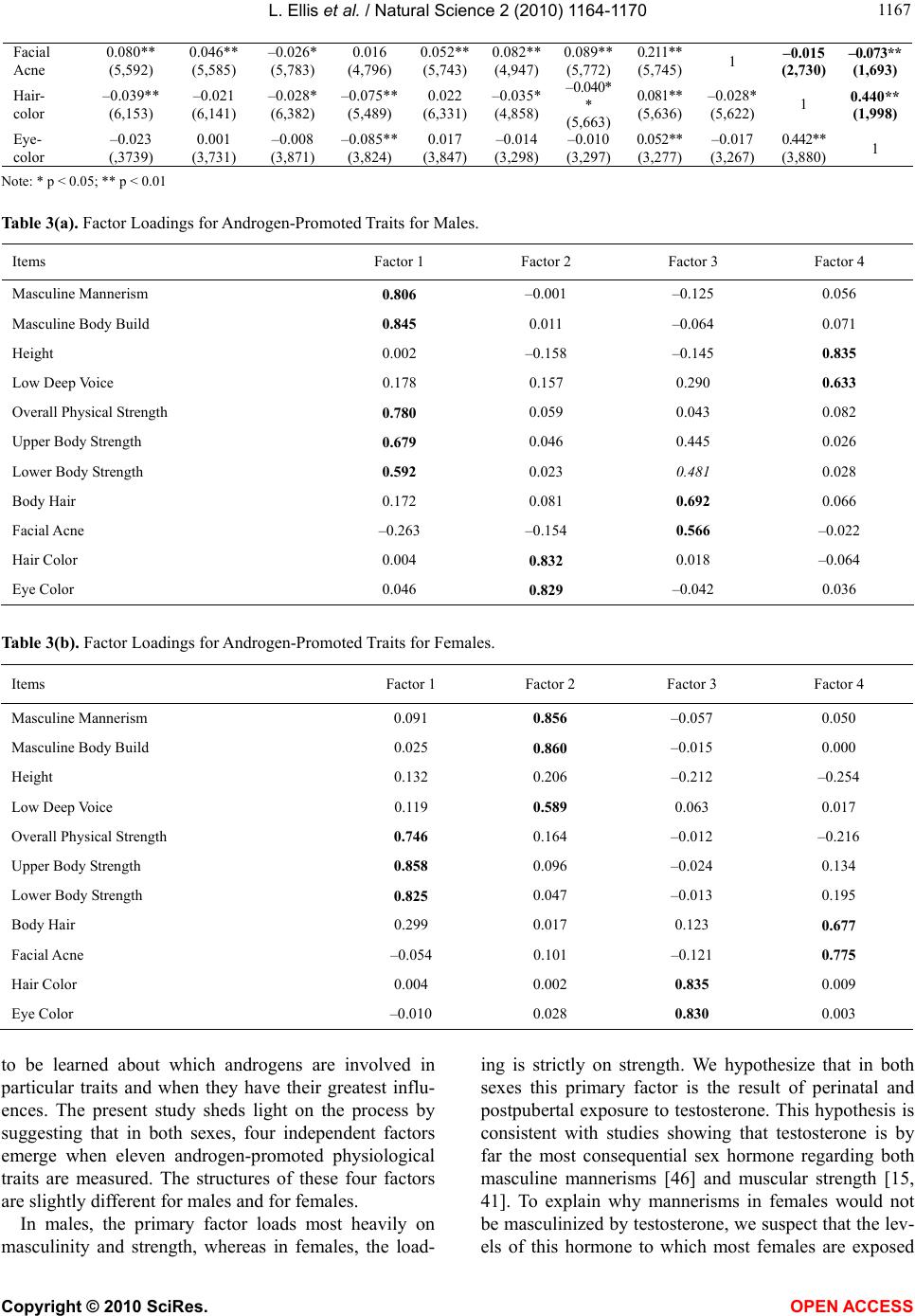 L. Ellis et al. / Natural Science 2 (2010) 1164-1170 Copyright © 2010 SciRes. OPEN ACCESS 116 1167 Facial Acne 0.080** (5,592) 0.046** (5,585) –0.026* (5,783) 0.016 (4,796) 0.052** (5,743) 0.082** (4,947) 0.089** (5,772) 0.211** (5,745) 1 –0.015 (2,730) –0.073* * (1,693) Hair- color –0.039** (6,153) –0.021 (6,141) –0.028* (6,382) –0.075** (5,489) 0.022 (6,331) –0.035* (4,858) – 0.040* * (5,663) 0.081** (5,636) –0.028* (5,622) 1 0.440** (1,998) Eye- color –0.023 (,3739) 0.001 (3,731) –0.008 (3,871) –0.085** (3,824) 0.017 (3,847) –0.014 (3,298) –0.010 (3,297) 0.052** (3,277) –0.017 (3,267) 0.442** (3,880)1 Note: * p < 0.05; ** p < 0.01 Table 3(a). Factor Loadings for Androgen-Promoted Traits for Males. Items Factor 1 Factor 2 Factor 3 Factor 4 Masculine Mannerism 0.806 –0.001 –0.125 0.056 Masculine Body Build 0.845 0.011 –0.064 0.071 Height 0.002 –0.158 –0.145 0.835 Low Deep Voice 0.178 0.157 0.290 0.633 Overall Physical Strength 0.780 0.059 0.043 0.082 Upper Body Strength 0.679 0.046 0.445 0.026 Lower Body Strength 0.592 0.023 0.481 0.028 Body Hair 0.172 0.081 0.692 0.066 Facial Acne –0.263 –0.154 0.566 –0.022 Hair Color 0.004 0.832 0.018 –0.064 Eye Color 0.046 0.829 –0.042 0.036 Table 3(b). Factor Loadings for Androgen-Promoted Traits for Females. Items Factor 1 Factor 2 Factor 3 Factor 4 Masculine Mannerism 0.091 0.856 –0.057 0.050 Masculine Body Build 0.025 0.860 –0.015 0.000 Height 0.132 0.206 –0.212 –0.254 Low Deep Voice 0.119 0.589 0.063 0.017 Overall Physical Strength 0.746 0.164 –0.012 –0.216 Upper Body Strength 0.858 0.096 –0.024 0.134 Lower Body Strength 0.825 0.047 –0.013 0.195 Body Hair 0.299 0.017 0.123 0.677 Facial Acne –0.054 0.101 –0.121 0.775 Hair Color 0.004 0.002 0.835 0.009 Eye Color –0.010 0.028 0.830 0.003 to be learned about which androgens are involved in particular traits and when they have their greatest influ- ences. The present study sheds light on the process by suggesting that in both sexes, four independent factors emerge when eleven androgen-promoted physiological traits are measured. The structures of these four factors are slightly different for males and for females. In males, the primary factor loads most heavily on masculinity and strength, whereas in females, the load- ing is strictly on strength. We hypothesize that in both sexes this primary factor is the result of perinatal and postpubertal exposure to testosterone. This hypothesis is consistent with studies showing that testosterone is by far the most consequential sex hormone regarding both masculine mannerisms [46] and muscular strength [15, 41]. To explain why mannerisms in females would not be masculinized by testosterone, we suspect that the lev- els of this hormone to which most females are exposed  L. Ellis et al. / Natural Science 2 (2010) 1164-1170 Copyright © 2010 SciRes. OPEN ACCESS 1168 are insufficient to significantly affect this trait while muscular strength responds to even low amounts of tes- tosterone. Another possibility is that high (female-typical) exposure to estradiol or other female hormones may counteract the effects of testosterone on masculine man- nerisms. The fact that the same factors for the dark pigmenta- tion factor and the skin-hair factor emerged in both sexes suggests that these traits are a) the result of androgens other than testosterone, and b) that the androgen(s) pri- marily responsible for hair growth and facial acne is different from those influencing hair and eye color. Regarding the three questions posed in the introduc- tion, one can conclude the following: First, all eleven traits that other studies have shown to be androgen- promoted are, as expected, more pronounced in males than in females. Second, within each sex, most of the eleven traits are positively correlated. The fact that there are exceptions leads one to expect that different andro- gens are operating in somewhat different ways within each sex. Third, factor analysis supports this expectation by demonstrating that there are four clusters of andro- gen-promoted traits amongst the eleven traits examined in the present study. We named these four factors and hypothesized that testosterone is responsible for the first (and most prominent) factor for both sexes. In males, this primary factor involved both strength and masculine mannerisms, while in females it only involved strength. Research is needed to verify these four factors and to look for other androgen-promoted traits within each sex. In future studies, direct measurement would almost cer- tainly provide more reliable data than self-reports. How- ever, the time required for obtaining direct measures with a sufficiently large sample of subjects needed for factor analysis could be prohibitive. It can also be said that the extent to which people can provide accurate in- formation about themselves may surpass expectations. In this regard, we compared the average heights of our subjects to estimates recently given by the Center for Disease Control based on direct measurements [48]. The results were very similar: 70.88 inches or 5’9.1” tall in our male sample compared to 5’9.2” for the national sample, and 65.15 inches or 5’4.3” tall in our female sample compared to 5’3.8” for the national sample. If the four factor structure of androgen-promoted traits revealed in the present study can be replicated, the next phase in this line of research would be to identify each of their specific causes. In other words, what are the ac- tual androgens involved in producing each factor and what is the developmental timing involved? REFERENCES [1] Ellis, L., Hershberger, S., Field, E., Wersinger, S., Pellis, S., Geary, D., Palmer, C., Hoyenga, K., Hetsroni, A. and Karadi, K. (2008) Sex differences: Summarizing more than a century of scientific research. Psychology Press, New York. [2] Alexander, G.M., Welcox, T. and Farmer, M.E. (2009) Hormone-behavior associations in early infancy. Hor- mones and Behavior, 56, 498-502. [3] Cooke, B., Hegstrom, C.D., Villeneuve, L.S. and Breed- love, S.M. (1998) Sexual differentiation of the vertebrate brain: Principles and mechanisms. Frontiers in Neuro- endocrinology, 19, 323-362. [4] Lutchmaya, S., Baron-Cohen, S., Raggatt, P., Knickmeyer, R. and Manning, J. (2004) 2nd to 4th digit ratios, fetal testosterone and estradiol. Early Human Development, 77, 23-28. [5] Zucker, K.J., Beaulieu, N., Bradley, S.J., Grimshaw, G.M. and Wilcox, A. (2001) Handedness in boys with gender identity disorder. Journal of Child Psychology and Psy- chiatry, 42, 767-776. [6] Dennis, C. (2004) Brain development: The most impor- tant sexual organ. Nature, 427, 390-392. [7] Goodfellow, P.N. and Lovell-Badge, R. (1993) SRY and sex determination in mammals. Annual Review of Genet- ics, 27, 71-92. [8] Jost, A., Price, D. and Edwards, R.G. (1970) Hormonal factors in the sexual differentiation of the mammalian foetus. Transactions of the Royal Society of London, 259, 119-130. [9] Woodson, J.C. and Gorski, R.A. (2000) Structural sex differences in the mammalian brain: Reconsidering the male / female dichotomy. In: Malsumoto, A. Ed., Sexual Differentiation of the Brain, CRC Press, Boca Raton, 229- 239. [10] Vergnaud, G., Page, D.C., Simmler, M.C., Brown, L., Rouyer, F., Noel, B., et al. (1986) A deletion map of the human Y chromosome based on DNA hybridization. American Journal of Human Genetics, 38, 109-124. [11] Jost, A. (1983) Genetic and hormonal factors in sex diffe- rentiation of the brain. Psychoneuroendocrinology, 8, 183- 193. [12] Carruth, L.L., Reisert, I. and Arnold, A.P. (2002) Sex chromosome genes directly affect brain sexual differen- tiation. Nature Neuroscience, 5, 933-934. [13] Ellis, L. (1996) The role of perinatal factors in determin- ing sexual orientation. In: Savin-Williams, R.C. and Co- hen, K.M. Eds., The Lives of Lesbians, Gays, and Bi- sexuals: Children to Adults, Harcourt Brace, New York, 35-70. [14] Cohen-Bendahan, C.C., van de Beek, C. and Berenbaum, S.A. (2005) Prenatal sex hormone effects on child and adult sex-typed behavior: Methods and findings. Neuro- science and Biobehavior Review, 29, 353-384. [15] Fink, B., Thanzami, V., Seydel, H. and Manning, J.T. (2006) Digit ratio and hand-grip strength in German and Mizos men: Cross-cultural evidence for an organizing effect of prenatal testosterone on strength. American Jour- nal of Human Biology, 18, 776-782. [16] Seale, J.V., Wood, S.A., Atkinson, H.C., Lightman, S.L. and Harbuz, M.S. (2001) Organizational role for testos- terone and estrogen on adult hypothalamic-pituitary- ad- renal axis activity in the male rat. Endocrinology, 146, 1973-1982.  L. Ellis et al. / Natural Science 2 (2010) 1164-1170 Copyright © 2010 SciRes. OPEN ACCESS 116 1169 [17] Sisk, C.L. and Zehr, J.L. (2005) Pubertal hormones or- ganize the adolescent brain and behavior. Frontiers in Neuroendocrinology, 26, 163-174. [18] Marshall, W.A. and Tanner, J.M. (1970) Variations in the pattern of pubertal changes in boys. Archives of Disease in Childhood, 45, 13-23. [19] Lookingbill, D.P., Demers, L.M., Wang, C., Leung, A., Rittmaster, R.S. and Santen, R.J. (1991) Clinical and biochemical parameters of androgen action in normal healthy caucasian versus chinese subjects. Journal of Cli- nical Endocrinology and Metabolism, 72, 1242-1248. [20] Giltay, E.J. and Gooren, L.J. (2000) Effects of sex steroid deprivation / administration on hair growth and skin se- bum production in transsexual males and females. Jour- nal of Clinical Endocrinology & Metabolism, 85, 2913- 2921. [21] Morejohn, G.V. and Genelly, R.E. (1961) Plumage dif- ferentiation of normal and sex-anomalous ring-necked pheasants in response to synthetic hormone implants. The Condor, 63, 101-110. [22] Coplan, R.J., Coleman, B. and Rubin, K.H. (1998) Shy- ness and little boy blue: Iris pigmentation, gender, and social wariness in preschoolers. Developmental Psycho- biology, 32, 37-44. [23] Farthing, M., Mattei, A.M., Edwards, C.R.W. and Daw- son, A.M. (1982) Relationship between plasma testos- terone and dihydrotestosterone concentrations and male facial hair growth. British Journal of Dermatology, 107, 559-564. [24] de Waal, W.J., Torn, M., de Muinck Keizer-Schrama, S.M.P.F., Aarsen, R.S.R. and Drop, S.L.S. (1995) Long term sequelae of sex steroid treatment in the management of constitutionally tall stature. Archives of Disease in Chi- ldhood, 73, 311-315. [25] Thiboutot, D. (2003) Acne: Hormonal concepts and the- rapy. Clinics in Dermatology, 22, 419-428. [26] Bubenik, G.A. and Bubenik, A.B. (1985) Seasonal varia- tions in hair pigmentation of white-tailed deer and their relationship to sexual activity and plasma testosterone. Journal of Experimental Zoology, 235, 387-395. [27] Toro, J., Turner, M. and Gahl, W.A. (1999) Dermatologic manifestations of hermansky-pudlak syndrome in pa- tients with and without a 16-base pair duplication in the hps1 gene. Archives of Dermatology, 135, 774-780. [28] Cassorla, F.G., Skerda, M.C., Valk, I.M., Hung, W., Cut- ler, G.B. and Loriaux, D.L. (1984) The effects of sex ste- riods on ulnar growth during adolescence. Journal of Clinical Endocrinology and Metabolism, 58, 717-720. [29] Bourguignon, J.P., Vandeweghe, M., Vanderschueren- Lodeweyckx, M., Malvaux, P., Wolter, R., Du Caju, M., et al. (1986) Pubertal growth and final height in hypo- pituitary boys: A minor role of bone age at onset of pu- berty. Journal of Clinical Endocrinology and Metabolism, 63, 376-382. [30] Zemel, B.S. and Katz, S.H. (1986) The contribution of adrenal and gonadal androgens to the growth in height of adolescent males. American Journal of Physical Anthro- pology, 71, 459-466. [31] Martin, M.M., Martin, A.L. and Mossman, K.L. (1986) Testosterone treatment of constitutional delay in growth and development: Effect of dose on predicted versus de- finitive height. Acta Endocrinology Supplement, 279, 147-152. [32] Jassal, S.K., Barrett-Connor, E. and Edelstein, S.L. (1995) Low bioavailable testosterone levels predict future height loss in postmenopausal women. Journal of Bone and Mineral Research, 10, 650-654. [33] Butler, G., Walker, R.F., Walker, R.V., Teague, P., Riad, F. and Ratcliffe, S. (1989) Salivary testosterone levels and the progress of puberty in the normal boy. Clinical En- docrinology, 30, 487-596. [34] Harries, M., Hawkins, S., Hacking, J. and Hughes, I.A. (1998) Changes in the male voice at puberty: Vocal fold length and its relationship to the fundamental frequency of the voice. Journal of Laryngology and Otology, 112, 451-454. [35] Dabbs, J.M. and Mallinger, A. (1999) High testosterone levels predict low voice pitch among men. Personality and Individual Differences, 27, 801-804. [36] Nieschlag, E. and Zitzmann, M. (2001) Testosterone levels in healthy men and the relation to behavioural and physical characteristics: Facts and constructs. European Journal of Endocrinology, 144, 183-197. [37] Bruckert, L., Lienard, J.-S., Lacroix, A., Kreutzer, M. and Leboucher, G. (2006) Women use voice parameters to assess men’s characteristics. Proceedings of the Royal Society B, 273, 83-89. [38] Hamilton, J.B. (1948) The role of testicular secretions as indicated by the effects of castration in man and by stud- ies of pathological conditions and the short lifespan as- sociated with maleness. Recent Progress of Hormonal Research, 3, 257-322. [39] Wang, C., Eyre, D.R., Clark, R., Kleinberg, D., Newman, C., Iranmanesh, A., et al. (1996) Sublingual testosterone replacement improves muscle mass and strength, de- creases bone resorption, and increases bone formation markers in hypogonadal men: A clinical research center study. Journal of Clinical Endocrinology and Metabolism, 81, 3654-3662. [40] Joubert, Y., Tobin, C. and Lebart, C. (1994) Testoster- one-induced masculinization of the rat levator ani muscle during puberty. Developmental Biology, 162, 104-110. [41] Perry, H.M., Miller, D.K., Patrick, P. and Morley, J.E. (2000) Testosterone and leptin in older African-American men: Relationship to age, strength, function, and season. Metabolism, 49, 1085-1091. [42] Iannuzzi-Sucich, M., Prestwood, K.M. and Kenny, A.M. (2002) Prevalence of sarcopenia and predictors of skele- tal muscle mass in healthy, older men and women. Jour- nals of Gerontology Series A: Biological Sciences and Medical Sciences, 57, M772-M777. [43] Bosinski, H.A.G., Schroder, I., Peter, M., Arndt, R., Wille, R. and Sippell, W.G. (1997) Anthropometrical measure- ments and androgen levels in males, females, and hor- monally untreated female-to-male transsexuals. Archives of Sexual Behavior, 26, 143-157. [44] Wang, C., Swerdloff, R.S. and Iranmanesh, A. (2000) Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parame- ters in hypogonadal men. Journal of Clinical Endocri- nology and Metabolism, 85, 2839-2853. [45] Whalen, R.E. and Edwards, D.A. (1967) Hormonal de- terminants of the development of masculine and feminine behavior in male and female rats. Anatomical Record,  L. Ellis et al. / Natural Science 2 (2010) 1164-1170 Copyright © 2010 SciRes. OPEN ACCESS 1170 157, 173-180. [46] Goy, R.W., Bercovitch, F.B. and McBrair, M.C. (1988) Behavioral masculinization is independent of genital masculinization in prenatally androgenized female rhesus macaques. Hormones and Behavior, 22, 552-571. [47] Ellis, L. and Cole-Harding, S. (2001) The effects of pre- natal stress, and of prenatal alcohol and nicotine expo- sure, on human sexual orientation. Physiology and Be- havior, 74, 213-226. [48] Ogden, C.L., Fryar, C.D., Carroll, M.D. and Flegal, K.M. (2004) Mean body weight, Height, and body mass index, United States 1960-2002. Advance Data from Vital and Health Statistics, 347, 1-18.
|