 Vol.2, No.10, 1155-1163 (2010) Natural Science http://dx.doi.org/10.4236/ns.2010.210143 Copyright © 2010 SciRes. OPEN ACCESS Distribution of polychaetes in the shallow, sublittoral zone of Admiralty Bay, King George Island, Antarctica in the early and late austral summer Letícia de Souza Barbosa1, Abílio Soares-Gomes1, Paulo Cesar Paiva2* 1Department of Marine Biology, Fluminense Federal University, Niterói, Brazil; leticiadsb@gmail.com; abiliosg@vm.uff.br; 2Department of Zoology, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil; *Corresponding Author: paulo.paiva@gmail.com. Received 20 July 2010; revised 25 August 2010; accepted 28 August 2010. ABSTRACT This study assessed the spatial distribution pa- ttern of soft-sediment polychaetes on the near- shore of Admiralty Bay, King George Island, Antarctica. In the early and late summer of 2003 /04, seven sites at three different depths (20, 30 and 60 meters) were sampled using a van Veen grab. 8,668 individuals all told, belonging to 67 species and 23 families, were identified. The families Terebellidae, Syllidae and Maldanidae were the most speciose. Mean densities ranged from 45.2 to 388.1 ind. 0.1 m-2 in the early sum- mer, and from 29 to 183 ind.0.1m-2 in the late. The species Aphelochaeta cincinnata, Levin- senia gracilis and Rhodine antarctica were the most frequent and abundant. Initially, mean biomass ranged from 0.11 to 5.27 g.0.1 m-2, in the early season and from 0.35 to 5.86 g.0.1 m-2 towards the end. Aglaophamus trissophyllus, Eupolymnia sp. and Barrukia cristata were the species with the highest biomass. Polychaete taxocoenosis structure remained similar in both periods. In the early summer, mean densities, biomass and number of species were lower at 30 meters and higher at 60, whereas in the late, these differences were higher among transects. Ice impacts, mainly anchor-ice, in the early sum- mer, as well as icebergs later on, most likely caused the differences encountered. Keywords: Polychaeta; Soft-Sediment; Benthic Structure; South Shetland Islands; Antarctic Peninsula 1. INTRODUCTION The Antarctic benthos is characterized by pronounced endemism and a marked dependence on physical condi- tions, such as sediment patterns, waves and ice effects [1]. Distribution of the benthic community in shallow waters (up to 100 m) could be influenced by depth [2]. According to Sahade et al. [3], benthic density is the highest at 25 meters. From here down to 50 meter depth, there is a decrease [4]. Below this, the community is free from the impacts of icebergs and storms, thereby reach- ing an advanced stage in development. Besides depth, distribution is also influenced by habitat heterogeneity, bottom topography and hydrodynamics, among other factors [2]. Low and stable water temperatures, low fluctuations in salinity during the summer, reduced terri- genous sediment input and the seasonality of food re- sources, could also exert an influence on both the struc- ture and distribution of the Antarctic fauna [1]. Never- theless, according to Barnes & Conlan [5], ice remains as one of the foremost agents of disturbance in shallow water benthos. The benthic fauna of the Southern Ocean is well known, the polychaetes being one of the most represen- tative groups in soft-sediment habitats [6-8]. The group can account for over 50% of the macrofauna in several Antarctic areas, such as Chile Bay, Greenwich Island [9], Port Foster, Deception Island [10], Arthur Harbour, An- vers Island [11], McMurdo Sound [12] and Admiralty Bay, King George Island [6]. Polychaete composition and distribution in Admiralty Bay was already studied by several authors [6,13-19] and can be summarized in the following zonation patterns: the dominance of Leito- scoloplos kerguelensis, Ophryotrocha notialis and Mi- crospio cf. moorei on shallow bottoms (down to 12 m) and higher densities of Aphelochaeta cincinnata, Apis- tobranchus glacierae, Rhodine antarctica and Levin- senia gracilis further down. According to Conlan et al. [20], certain polychaetes, such as Ophryotrocha notialis, Capitella perarmata, Aphelochaeta sp. and Leitoscolop- los kerguelensis, are dominant in areas under the impact of sea-waste disposal, besides being capable of coloniz-  L. de S. Barbosa et al. / Natural Science 2 (2010) 1155-1163 Copyright © 2010 SciRes. OPEN ACCESS 1156 ing ice-disturbed areas [21,22]. The aim of this survey was to investigate polychaete spatial distribution in the nearshore soft-sediments at three depths in Admiralty Bay, during the early and late austral summer. 2. STUDY AREA Admiralty Bay, the largest bay in King George Island, is approximately 122 km², with depths exceeding 500 meters [23]. The fjord-like shaped bay has three inlets, Mackellar and Martel located in the northern portion, and Ezcurra located in the western [17]. The bay re- ceives water from the Bransfield Strait through a 500- meters-deep channel. Coarse sediments mixed with fine mud occur down to a depth of 50 meters, the rest con- sisting mainly of fine mud [24]. The sediment in front of the Brazilian Antarctic station contained high concentra- tions of trace metals (B, Mo, Pb, V, Zn, Ni, Cu, Mg and Mn), organic matter and oil contaminants. However, despite the evidence of contamination, the low bioavail- ability of these pollutants is an indication of low envi- ronmental risk [25]. Variation in temperature and salinity is slight, ranging from −0.4°C to 0.9°C and 33.8 to 33.4, respectively, at the bottom [24]. The phytoplankton from Admiralty Bay is dominated by diatoms, under the in- fluence of benthic species from sediment resuspension or ice defrosting [26]. 3. MATERIAL AND METHODS Seven transects located in the Mackellar and Martel inlets were sampled (Figure 1): Research Station “Co- mandante Ferraz” (CFA, CFB and CFC), Botany Point (BP), Hennequin Point (HE), Machu Picchu (MP) and Thomas Point (AR), during the austral summer of 2003- 2004. At each site, samples were collected at three dep- ths (20, 30 and 60 meters) with a van Veen grab (0.056 Figure 1. Sampling sites at Admiralty Bay. m²). In the early summer (November and December, 2003) three replicates were collected, whereas four were in the late season (February and March, 2004). Samples were sieved through a 0.5 mm mesh. Specimens were fixed in 4% formaldehyde and preserved in 70% alcohol. The polychaetes were identified at the species level. Unidentifiable individuals were included in the analysis as morphotypes. Biomass was estimated by the meas- urement of wet-weight (± 0.01 mg). Dry-sieve and pi- pette methodologies were used for grain-size analysis, as described by Suguio [27]. Calcium carbonate content was determined by dry-weight difference after HCl 10% attack, and that of total carbon and nitrogen by using an Elemental Analyser CHNS/O Perkin Elmer (2400 Series II), with a detection limit of 0.02% for C and 0.03% for N [28]. Species densities (ind. 0.1 m² ± standard-error) were used to calculate species dominance, according to the formula: Do = (Na/N) * 100 where Do = dominance of species A, Na = density of species A and N = sum of all species densities. Species occurrence frequencies were calculated by using the following formula: Fa = (Pa/P) * 100 where Fa = frequency of species A, Pa = number of samples in which species A occurred, and P = total of samples. Species with higher than 50% occurrence were con- sidered constant, those between 50% and 10%, common, and those with less than 10%, rare. Density data were transformed (square root), and Two-way ANOVA em- ployed to check differences between early and late sum- mer surveys. Cluster analysis was with the UPGMA al- gorithm using a Bray Curtis similarity index calculated by densities. Diagrams of ordination were produced through non-metric Multi-Dimensional Scaling (nMDS) analysis. The significance of differences among depths during early and late summer was tested by One-Way Analysis of Variance by Similarities-ANOSIM [29]. Canonical Correspondence Analysis (CCA) was ap- plied with a matrix of 10 abiotic variables (gravel, coarse sand, medium sand, fine sand, silt, clay, carbonate, total carbon, organic carbon and total nitrogen), together with the most frequent species in each of the summer periods. The transect AR 60 m was not considered, due to the lack of grain-size data. Statistical analysis was under- taken with the Statistica 6.0 program, multivariate ana- lysis with the Primer 6, program, and CCA by using the Biplot 1.1 add-in routine for Excel [30]. 4. RESULTS 4.1. Abiotic Variables The sediment consisted mainly of silt and clay (more  L. de S. Barbosa et al. / Natural Science 2 (2010) 1155-1163 Copyright © 2010 SciRes. OPEN ACCESS 1157 than 60%) at most transects and depths, although the percentages of fine sand were higher (ca. 30%) in some stations like HE and AR. At 60 meters, slightly lower percentages of both gravel and coarse sand were ob- served, when compared to shallower transects. Calcium carbonate content was slightly higher in the late summer (more than 12%), whereas in the early season, this was lower at 20 and 30 meters, with the lowest (6.5%) at MP. Carbon and nitrogen content were very low (< 1%). CFA, CFB and CFC presented the highest percentages of car- bon content (0.54% to 0.75%), and MP the lowest (0.28% to 0.46%). There was no apparent variation of either variable with the increase in depth. 4.2. Polychaete Composition A total of 8,668 individuals were collected throughout the period. The set of samples yielded 67 species and four morphotypes (Cirratulidae gen. sp.1, Cirratulidae gen. sp.2, Maldanidae gen. sp. and Terebellidae gen. sp.), belonging to 23 families (Table 1). 21 species were col- lected in each sampling survey. The most speciose fami- lies were Terebellidae and Syllidae with seven species each, and Maldanidae with six. The families Glyceridae and Sabellidae were exclusive to the early summer, whereas Nereididae and Serpulidae were to the late sum- mer. The families Nereididae, Serpulidae and Glyceridae were each represented by only one species, viz., Nicon ehlersi, Helicosiphon biscoensis and Glycera capitata, respectively. The sabellids were represented by three species: Euchone pallida, Perkinsiana litorallis and Perkinsiana milae, all somewhat scarce during the sum- mer. 4.3. Dominance and Frequency In terms of density, the species Aphelochaeta cincin- nata, Levinsenia gracilis, Cirratulidae gen. sp. 1, Apis- tobranchus glacierae and Rhodine antarctica dominated, throughout the whole period studied. The exceptions were Cirratulidae gen. sp. 1 and A. glacierae, dominant only at the beginning. Throughout, Aphelochaeta cin- cinnata was the most frequent species, with 93.55% in the early summer and 85.71% in the late. The species Levinsenia gracilis and Rhodine antarctica were also constant during the whole study period, with 69.35% and 56.45%, respectively, in the first part, and both 65.48% towards the end. These three species were responsible for 33.7% of total polychaetes in the early summer and 34.7% in the late. Aricidea (Acmira) strelzovi, Leito- scloplos geminus, Brada villosa, Apistobranchus glaci- erae, Barrukia cristata and Cirrophorus brevicirratus were considered common throughout. Scalibregma in- flatum, besides being a low-frequency species during the whole period (8.06% in the early part and 5.95% in the late), occurred only at 60 meters (Table 1). 4.4. Density and Biomass Polychaete density ranged from 45.24 to 388.10 ind.0.1 m-2 in the early summer. Apistobranchus glaci- erae, with the highest score all told, was also responsible for the high result observed at CFC (20 m) (173.21 ± 76.79 ind. 0.1 m-2). The species Aphelochaeta cincinnata at CFB (60 m), Rhodine antarctica at CFC (20 m) and Levinsenia gracilis at CFC (60 m) also presented high values (Figure 2). In the late summer, polychaete den- sity varied from 29.02 to 183.93 ind.0.1 m-2. The highest densities, attributed to R. antarctica, were observed at the MP transect at 20 and 30 meters (Figure 3). During sampling, a significant variation in density among depths was observed (ANOVA, p < 0.002) (Ta- ble 2), with lower densities at 30 meters when compared to both 20 and 60 (Tukey test, p < 0.005), although no differences among transects were detected (p = 0.599). Nevertheless, this pattern seems to be rather complex, since the interaction between transect and depth was significant (p < 0.02). This interaction occurred at tran- sects CFA and CFC, with no clear bathymetric pattern. On the contrary, in late summer, significant differences were found only among transects (p < 0.002), but not depths (Table 2), with MP and AR presenting higher densities than CFA and CFC (Tukey test, p < 0.05). These differences occurred due to the high densities of cirratulids (Aphelochaeta cincinnata and Cirratulidae gen. sp.1), paraonids (Levinsenia gracilis, Aricidea (Ac- mira) strelzovi and Cirrophorus brevicirratus) and the maldanid Rhodine antarctica, at MP and AR. On the other hand, biomass encountered at both CFA and CFC was low. Surprisingly, polychaete density at CFB was similar to that observed at MP and AR. In the early summer, biomass means (± standard-error) ranged from 5.27 ± 4.19 g.0.1 m-2 at CFC-20 m, to 0.11 ± 0.06 g.0.1 m-2 at BP-30 m (Figure 4). In the late sea- son, the highest biomass mean was observed at CFB-30 m (5.86 ± 4.72 g.0.1 m-2) and the lowest at BP-30 m (0.35 ± 0.27 g.0.1 m-2) (Figure 5). The species Aglao- phamus ornatus, Eupolymnia sp. and Barrukia cristata presented the highest values. Although Rhodine antarc- tica biomass was not high, it remained constant at MP, all through the later part of summer, this constancy probably contributing to the proximity of values found at all depths. 4.5. Multivariate Analysis In the early summer, the samples were grouped through cluster analysis, according to depth. The results indicated that in the first group, composed of transects MP, BP, AR at 20 meters, and MP and AR at 30 meters,  L. de S. Barbosa et al. / Natural Science 2 (2010) 1155-1163 Copyright © 2010 SciRes. OPEN ACCESS 1158 Table 1. Frequency (Fo) and dominance (Do) of polychaete species in early and late summer, and species codes used in canonical correspondence analysis. Species Code Family Early summer Late summer Fo (%) Do Fo (%) Do Levinsenia gracilis Lev 69.35 17.51 65.48 28.64 Aricidea (Acmira) strelzovi Ari 35.48 4.45 33.33 4.45 Cirrophorus brevicirratus Aph Paraonidae 12.90 0.85 23.81 3.94 Leitoscoloplos kerguelensis Lek 22.58 1.02 16.67 0.95 Leitoscoloplos geminus Leg 29.03 1.76 21.43 1.13 Scoloplos (Leodamas) marginatus 3.23 0.04 2.38 0.08 Orbinia minima Orbiniidae - - 2.38 0.08 Scalibregma inflatum Scalibregmatidae 8.06 0.38 5.95 0.43 Ophelina syringopyge 4.84 0.22 4.76 0.33 Ophelina breviata 3.23 0.07 1.19 0.03 Ophelina sp. Ophellidae 1.61 0.09 2.38 0.08 Capitella sp.1 1.61 0.02 4.76 0.13 Capitella sp. 2 Capitellidae - - 1.19 0.08 Asychis ampliglypta Asy 11.29 0.44 21.43 1.21 Maldane sarsi antarctica Mal 11.29 0.58 11.90 0.60 Lumbriclymenella robusta Lur 1.61 0.02 10.71 0.38 Rhodine antarctica Rho 56.45 9.50 65.48 20.30 Praxillella sp. 1.61 0.02 - - Maldanidae gen. sp. Mas Maldanidae 24.19 0.76 19.05 0.98 Austrolaenilla antarctica 4.84 0.07 5.95 0.13 Barrukia cristata Bar 22.58 0.47 21.43 0.73 Harmothoe sp. Polynoidae - - 1.19 0.03 Glycera capitata Glyceridae 1.61 0.02 - - Eulalia varia - - 2.38 0.05 Eulalia sp. - - 4.76 0.13 Eteone sculpta 1.61 0.02 2.38 0.05 Genetyllis polyphylla 3.23 0.07 3.57 0.15 Anaitides sp. Phyllodocidae 1.61 0.04 1.19 0.03 Sphaerodoropsis arctowskyensis 6.45 0.20 2.38 0.05 Sphaerodoropsis sp. 1.61 0.02 - - Ephesiella muelenhardte Sphaerodoridae 1.61 0.04 1.19 0.05 Aglaophamus ornatus Agl Nephtyidae 11.29 0.18 8.33 0.23 Nicon ehlersi Nereididae - - 1.19 0.03 Exogone heterosetosa 4.84 0.20 - - Exogone minuscula 1.61 0.04 - - Exogone heterosetoides 4.84 0.11 8.33 0.30 Exogone sp. 4.84 0.16 1.19 0.03 Syllis sp. 1.61 0.02 - - Branchiosyllis sp. 8.06 0.18 1.19 0.05 Syllides liouvillei Syllidae - - 2.38 0.05 Pettiboneia kerguelensis Pet 17.74 1.69 1.19 0.03 Ophryotrocha notialis Dorvilleidae - - 1.19 0.03 Lumbrineris kerguelensis Lum 8.06 0.11 15.48 0.35 Augeneria sp. Lumbrineridae 1.61 0.02 2.38 0.05 Apistobranchus glacirae Api Apistobranchidae 24.19 10.03 15.48 0.73 Spiophanes tcherniai 1.61 0.04 4.76 0.15 Laonice antarcticae 1.61 0.02 - - Microspio sp. 1.61 0.33 1.19 0.03 Scolelepis eltaninae 1.61 0.04 - - Pygospiopis dubia Spionidae - - 1.19 0.05 Aphelochaeta cf. cincinnata Aph 93.55 30.57 85.71 21.71 Cirratulidae gen. sp. 1 Cir 1 32.26 13.59 19.05 6.11 Cirratulidae gen. sp. 2 Cir 2 Cirratulidae 16.13 2.38 5.95 1.76 Brada villosa Bra 25.81 0.62 25.00 1.18 Pherusa kerguelarum Flabelligeridae - - 1.19 0.08 Ampharete kerguelensis 3.23 0.04 4.76 0.10 Amphicteis gunneri antarctica Amp 6.45 0.09 15.48 0.70 Anobothrus cf. patagonicus 1.61 0.04 2.38 0.05 Phyllocomus crocea Ampharetidae - - 1.19 0.03 Hauchiella tribullata 1.61 0.02 - - Proclea cf. graffii 3.23 0.09 1.19 0.03 Amphitrite kerguelensis - - 1.19 0.03 Eupolymnia sp. Eup 8.06 0.20 21.43 0.53 Terebellides stroemii kerguelensis 8.06 0.24 5.95 0.25 Pista cristata 6.45 0.13 4.76 0.13 Trichobranchus sp. - - 1.19 0.03 Terebelidae gen sp. Terebellidae 1.61 0.02 - - Euchone palida 1.61 0.02 - - Perkinsiana milae 1.61 0.02 - - Perkinsiana littoralis Sabellidae 1.61 0.02 - - Helicosiphon biscoensis Serpulidae - - 1.19 0.05  L. de S. Barbosa et al. / Natural Science 2 (2010) 1155-1163 Copyright © 2010 SciRes. OPEN ACCESS 1159 Figure 2. Mean densities (± stardard-error) of Polychaeta at the transects in early summer. Depths: 20 m = black bars; 30 m = white bars; 60 m = gray bars. Figure 3. Mean densities (± stardard-error) of Polychaeta at the transects in late summer. Depths: 20 m = black bars; 30 m = white bars; 60 m = gray bars. Figure 4. Mean biomass (± stardard-error) of Polychaeta at the transects in early summer. Depths: 20 m = black bars; 30 m = white bars; 60 m = gray bars. Figure 5. Mean biomass (± stardard-error) of Polychaeta at the transects in late summer. Depths: 20 m = black bars; 30 m = white bars; 60 m = gray bars. Table 2. Results of Two-way ANOVA in early and late sum- mer. Factors Early summer Late summer p F p F Transect 0.5990.737 0.0014.286 Depth 0.00111.716 0.5810.547 Transect * Depth 0.0151.554 0.5830.867 the density of Cirratulidae gen. sp. 1 was high, whereas both Bran chiosyllis sp. and S. arctowskyensis were ab- sent. In the second group (CFB and CFC at 20 m, HE at 30 m, and MP and HE at 60 m), both R. antarctica and A. glacirae were the most abundant. In the third group, formed by CFA at 20 meters, and CFA, CFB, CFC and BP, all at 30 meters, richness and densities were low in R. antarctica, A. amphiglypta and A. cincinnata. In the last group, A. cincinnata, L. gracilis and Cirratulidae gen. sp. 2 were abundant, and both S. inflatum and A. strelzovi present (Figure 6). The results from cluster analysis were confirmed through nMDS. In the late summer, no clear pattern of clustering, in relation to either transects or depths, was apparent. When using ANOSIM, no differences were detected in the polychaete community between the periods sam- pled (R global = 0.031; p = 20.5%), although the con- trary was the case as regards depths. In the early summer, communities at 60 meters differed from those found at 20 and 30 meters, whereas in the late season, the only difference was between 20 and 60 meters (Table 3). Results through Canonical Correlation Analysis (CCA) were rather similar, in both early and late summer. The first axis was responsible for 45.9% of the variance in early summer and 42.8% in late and was positively re- lated to gravel, coarse and fine sand and negatively so to silt, clay, carbonate, carbon and nitrogen contents. The sediment in all transects at 20 meters was coarser, whereas that at 60 meters was characterized by the dominance of silt and clay fractions, and that at 30 me- ters an intermediate pattern between the former two. The second axis accounted for 25.5% and 20.8% of the vari- ance in early and late summer, respectively (Figures 7 and 8). In both summer periods, most species appeared to be associated with gravel, and coarse and fine sand. The maldanids Maldane sarsi antarctica and Asychis amphiglypta were related to stations at 60 meters. The species L. geminus, L. kerguelensis, A. glacirae and C. brevicirratus and Cirratulidae gen sp.1 were positively related with gravel, and coarse and fine sand. 5. DISCUSSION The number of polychaete species found in the present study was higher than that presented by Sicinski & Ja- 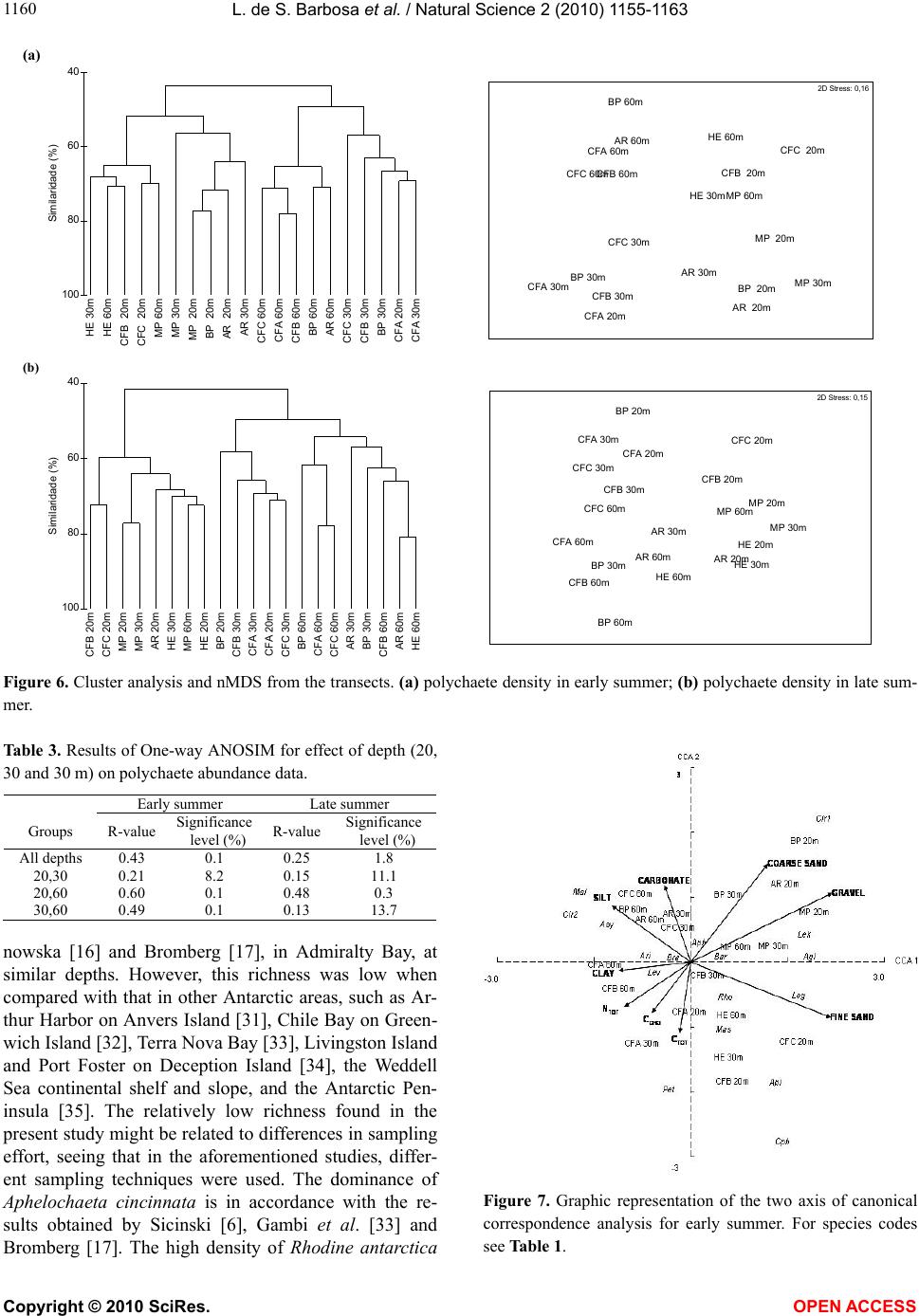 L. de S. Barbosa et al. / Natural Science 2 (2010) 1155-1163 Copyright © 2010 SciRes. OPEN ACCESS 1160 (a) HE 30m HE 60m CFB 20m CFC 20m MP 60m MP 30m MP 20m BP 20m AR 20m AR 30m CFC 60m CFA 60m CFB 60m BP 60m AR 60m CFC 30m CFB 30m BP 30m CFA 20m CFA 30m 100 80 60 40 Similaridade (%) CFA 20m CFA 30m CFA 60m CFB 20m CFB 30m CFB 60m CFC 20m CFC 30m CFC 60m MP 20m MP 30m MP 60m BP 20m BP 30m BP 60m AR 20m AR 30m AR 60m HE 30m HE 60m 2D Stress: 0,16 (b) CFB 20m CFC 20m MP 20m MP 30m AR 20m HE 30m MP 60m HE 20m BP 20m CFB 30m CFA 30m CFA 20m CFC 30m BP 60m CFA 60m CFC 60m AR 30m BP 30m CFB 60m AR 60m HE 60m 100 80 60 40 Similaridade (%) CFA 20m CFA 30m CFA 60m CFB 20m CFB 30m CFB 60m CFC 20m CFC 30m CFC 60mMP 20m MP 30m MP 60m BP 20m BP 30m BP 60m AR 20m AR 30m AR 60m HE 20m HE 30m HE 60m 2D Stress: 0,15 Figure 6. Cluster analysis and nMDS from the transects. (a) polychaete density in early summer; (b) polychaete density in late sum- mer. Table 3. Results of One-way ANOSIM for effect of depth (20, 30 and 30 m) on polychaete abundance data. Early summer Late summer Groups R-value Significance level (%) R-value Significance level (%) All depths 0.43 0.1 0.25 1.8 20,30 0.21 8.2 0.15 11.1 20,60 0.60 0.1 0.48 0.3 30,60 0.49 0.1 0.13 13.7 nowska [16] and Bromberg [17], in Admiralty Bay, at similar depths. However, this richness was low when compared with that in other Antarctic areas, such as Ar- thur Harbor on Anvers Island [31], Chile Bay on Green- wich Island [32], Terra Nova Bay [33], Livingston Island and Port Foster on Deception Island [34], the Weddell Sea continental shelf and slope, and the Antarctic Pen- insula [35]. The relatively low richness found in the present study might be related to differences in sampling effort, seeing that in the aforementioned studies, differ- ent sampling techniques were used. The dominance of Aphelochaeta cincinnata is in accordance with the re- sults obtained by Sicinski [6], Gambi et al. [33] and Bromberg [17]. The high density of Rhodine antarctica Figure 7. Graphic representation of the two axis of canonical correspondence analysis for early summer. For species codes see Table 1.  L. de S. Barbosa et al. / Natural Science 2 (2010) 1155-1163 Copyright © 2010 SciRes. OPEN ACCESS 1161 Figure 8. Graphic representation of the two axis of canonical correspondence analysis for late summer. For species codes see Table 1. at the MP transect could be related to its life cycle. Ac- cording to Dayton & Oliver [12], in McMurdo Sound, the individuals of the family Maldanidae may have evolved asexual reproduction in response to high preda- tion and juvenile mortality. The dominance of maldanids was also reported by Gallardo et al. [9], who found a benthic community dominated by Maldane sarsi antarc- tica (Maldane assembly) in Chile Bay. Jazdzewski et al. [24] and Sicinski [6,13] also observed typical maldanid communities at depths over 100 meters. The highest mean density observed in the early sum- mer was similar to that reported by Sicinski [14]. On the other hand, mean density itself, although in accordance to that observed by Sicinski & Janowska [16], was lower than that reported by other authors [17,33-35]. These differences may reflect variations in sampling design, depth and seasonality. The mean values of biomass were similar to those observed in Admiralty Bay by Sicinski & Janowska [16], and were within the range reported by Gambi et al. [33]. According to Sicinski [13], polychaete biomass at 50 m may vary from 30 to 40 g.m-2 and might be responsible for 15% of the local zoobenthic biomass itself. The species responsible for the increase in bio- mass values, Aglaophamus ornatus and Eupolymnia sp. occurred mainly at 20 and 30 meters, respectively. This may be related to the deposition of organic matter from phytoplankton bloom, which occurs in the early summer [36]. Polychaete taxocoenosis structure remained the same throughout the period under study, possibly as a result of the prevailing sedimentary conditions (grain-size per- centages) remaining invariable between transects. Nev- ertheless, certain differences were observed during the early and late summer, separately. During the early summer, polychaete mean density, biomass and richness declined at the 30 meters level and increased at the 60. An increase in density related to depth had been previ- ously reported in the Martel inlet [16]. The results in early summer may be an outcome of ice impact. Accor- ding to Sahade et al. [3], ice impacts (icebergs and an- chor-ice) seem to be the major regulating factor of ben- thic assemblages in shallow waters. Although actually not observed in this study, but based mainly on under- water observations, anchor-ice impacts have been im- puted as promoting winter structuring in benthic com- munities. The displacement of established fauna in their area of influence may be attributed to these phenomena, thereby accounting for the low diversity in early summer [37]. According to Dayton et al. [38], the influence of anchor-ice impacts extends down to 33 meters, thus con- stituting the main cause of low diversity in shallow wa- ters. Anchor-ice usually occurs during the winter, but its influence might have extended throughout the early summer of 2003/04, with consequential superficial sediment defaunation, thus making it difficult for the community to recover within a few months. The increase in temperature in the late summer pro- motes the formation of icebergs. Echeverría & Paiva [39] reported the presence of one in the summer of 2001 at the CFB 25 meter station, where it remained for over 20 days. Iceberg impacts are likely to affect benthic com- munities down to 20 meters. Below this, conditions are more stable, with higher densities, biomass and richness, the area below 30 meters thus presenting a substantial change in benthic megafauna structure, composition and diversity [37]. In both summer periods, most of the spe- cies which appear to be related to coarser sediment frac- tions are motile or discretely motile polychaetes. On the other hand, the maldanids (sessile polychaetes) related to higher percentages of silt and clay, appear mostly at 60 meters. Further analysis of polychaete feeding guilds is necessary to better evaluate their distribution in Admi- ralty Bay. The possible environmental impacts related to active- ties of the Brazilian research station (Cmte. Ferraz) were not revealed in this survey, since variability among those transects under the influence of the station itself (CFA, CFB and CFC) was higher than among all the others. Furthermore, the slight variation between early and late summer seems to be more related to natural impacts than to the more intense activities at the research station it- self.  L. de S. Barbosa et al. / Natural Science 2 (2010) 1155-1163 Copyright © 2010 SciRes. OPEN ACCESS 1162 6. ACKNOWLEDGEMENTS The authors would like to thank the staff of the Brazilian Antarctic Station “Comandante Ferraz” and the Brazilian Antarctic Programm (PROANTAR) for logistical support, Laboratorio de Ciências Ambi- entais/UENF for the abiotic data, Lucia Campos (GEAMB/UFRJ) for providing biological material, and Rafael Moura for the map. This work was supported by grant from CNPq/MCT/MMA (PROANTAR), and a MSC fellowship from Coordenadoria de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for first author and research fel- lowships from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for second and third author. REFERENCES [1] Arntz, W.E., Brey, T. and Gallardo, V.A. (1994) Antarctic zoobenthos. Oceanography and Marine Biology - An An- nual Review, 32, 241-304. [2] Clarke, A. (1996) The distribution of Antarctic marine benthic communities. In: Ross, R.M., et al. Eds., Foun- dations for Ecological Research West of the Antarctic Peninsula. Antarctic Research Series, 70, 219-230. [3] Sahade, R., Tatian, M., Kowalke, J., Kuhne, S. and Esnal, G.B. (1998) Benthic faunal associations on soft substrates at Potter Cove, King George Island, Antarctica. Polar Biology, 19(2), 85-91. [4] Parulekar, A.H., Ansari, Z.A. and Harkantra, S.N. (1983) Benthic fauna of the Antarctic Ocean: Quantitative as- pects. In: Iyenger, R.V. and Rajaram, R., Eds., Scientific Report of First Indian Expedition to Antarctica, Depart- ment of Ocean Development, New Delhi, 213-218. [5] Barnes, D.K.A. and Conlan, K.E. (2007) Disturbance, colonization and developmentof Antarctic benthic com- munities. Philosophical Transactions of the Royal Soci- ety B, 362(1477), 11-38. [6] Sicinski, J. (1986) Benthic assemblages of polychaeta in chosen regions of Admiralty Bay (King George Island, South Shetlands Island). Polish Polar Research, 7(1-2), 63-78. [7] Arnaud, P.M., Jazdzewski, K., Presler, P. and Sicinski, J. (1986) Preliminary survey of benthic invertebrates col- lected by Polish Antarctic Expeditions in Admiralty Bay, King George Island, South Shetland Islands, Antarctica. Polish Polar Research, 7(1-2), 7-24. [8] Clarke, A. and Johnston, N.M. (2003) Antarctic marine benthic diversity. Oceanography and Marine Biology: An Annual Review, 41, 47-114. [9] Gallardo, V.A., Castillo, J.C., Retamal, A.Y., Moyano, H. I. and Hermosilla, J.G. (1977) Quantitative studies on the soft-bottom macrobenthic animal communities of shal- low Antarctic Bays. In: Llano, G.A. Ed., Adaptations within Antarctic Ecosystems, Gulf Publishing, Houston, 361-387. [10] Retamal, M.A., Quintana, R. and Neira, E. (1982) Ana- lisis cualiy cuantitativo de las comunidades bentonicas en Bahia Foster (Isla Decepción) (XXXV Expedición An- tartica Chilena, enero 1981). Série Científica Instituto Antártico Chileno, 29, 5-15. [11] Lowry, J.K. (1975) Soft bottom macrobenthic commu- nity of Arthur Harbor, Antarctica. Antarctic Resesarch Series, 23(1), 1-19. [12] Dayton, P.K. and Oliver, J.S. (1977) Antarctic soft-bot- tom benthos in oligotrophic and eutrophic environments. Science, 197(4298), 55-58. [13] Sicinski, J. (1993) Polychaeta. In: Racuksa-Suszczewski, S. Ed., The Maritime Antartic Coastal Ecosystem of Ad- miralty Bay, Department of Antarctic Biology, Polish Academy of Sciences, Warsaw, 101-107. [14] Sicinski, J. (2000) Polychaeta (Annelida) of Admiralty Bay: Species richness, diversity, and abundance. Polish Polar Research, 21(3-4), 153-169. [15] Wägele, J.W. and Brito, T.A.S. (1990) Die sublitorale fauna der maritimen Antarktis, Erste unterwasserbio- bachtungen in der Admiralitasbucht. Natur und Museum, 120(7), 269-282. [16] Sicinski, J. and Janowska, E. (1993) Polychaetes of the shallow sublittoral of Admiralty Bay, King-George Island, South Shetland Islands. Antarctic Science, 5(2), 161-167. [17] Bromberg, S., Nonato, E.F., Corbisier, T.N. and Petti, M.A.V. (2000) Polychaetes distribution in the nearshore zone of Martel Inlet, Admiralty Bay (King George Island, Antarctica). Bulletin of Marine Science, 67(1), 175-188. [18] Echeverría, C.A., Paiva, P.C. and Alves, V.C. (2005) Composition and biomass of shallow benthic megafauna during an annual cycle in Admiralty Bay, King George Island, Antarctica. Antarctic Science, 17(3), 312-318. [19] Petti, M.A.V., Nonato, E.F., Skowronski, R.S.P. and Cor- bisier, T.N. (2006) Bathymetric distribution of the meio- faunal polychaetes in the nearshore zone of Martel intel, King George Island, Antarctica. Antarctic Science, 18(2), 163-170. [20] Conlan, K.E., Kim, S.L., Leninhan, H.S. and Oliver, J.S. (2004) Benthic changes during 10 years of organic en- richment by McMurdo Station, Antarctica. Marine Po- llution Bulletin, 49(1-2), 43-60. [21] Conlan, K.E., Leninham, H.S., Kvitek, R.G. and Oliver, J.S. (1998) Ice scour disturbance to benthic communities in the Canadian high arctic. Marine Ecology Progress Series, 166(1), 1-16. [22] Gerdes, D., Hilbig, B. and Montiel, A. (2003) Impact of iceberg scouring on macrobenthic communities in the high-Antarctic Weddell Sea. Polar Biology, 26(5), 295- 301. [23] Lipski, M. (1987) Variations of physical conditions, nu- trients and chlorophyll a content in Admiralty Bay (King George, South Shetland Islands, 1979). Polish Polar Re- search, 8(4), 307-332. [24] Jazdzewski, K., Jurasz, W., Kittel, W., Presler, E., Presler P. and Sicinski, J. (1986) Abundance and biomass esti- mates of the benthic fauna in Admiralty Bay, King George Island, South Shetland Islands. Polar Biology, 6(1), 5-16. [25] Santos, I.R., Silva-Filho, E.V., Schaefer, C.E.G.R., Albu- querque-Filho, M.R. and Campos, L.S. (2005) Heavy metal contamination in coastal sediments and soils near the Brazilian Antarctic Station, King George Island. Ma- rine Pollution Bulletin, 50(2), 185-194. [26] Lange, P.K., Tenenbaum, D.R., Braga, E.S. and Campos, L.S. (2007) Micro phytoplankton assemblages in shallow waters at Admiralty Bay (King George Island, Antarctica) during the summer 2002-2003. Polar Biology, 30(11),  L. de S. Barbosa et al. / Natural Science 2 (2010) 1155-1163 Copyright © 2010 SciRes. OPEN ACCESS 1163 1438-1492. [27] Suguio, K. (1973) Introdução à sedimentologia. Editora da Universidade de São Paulo, São Paulo, 317. [28] Skoog, D.A. and Leary, J.J. (1992) Principles of instru- mental analysis. 4th Edition, Saunders College Publish- ing, Forth Worth, 801. [29] Clarke, K.R. and Warwick, R.W. (1994) Change in ma- rine communities: An aproach to statistical analysis and interpretation. Plymouth Marine Laboratory, Bourne Press, Bournemouth. [30] Lipkovich, I. and Smith, E.P. (2002) Biplot and singular value decomposition macros for Excel. Journal of Statis- tical Software, 7(5), 1-15. [31] Richardson, M.D. and Hedgpeth, J.W. (1977) Antarctic soft-bottom macrobenthic community adaptations to a cold stable, highly, productive, glacially affected envi- ronment. In: Llano, G.A., Ed., Adaptations within Ant- arctic Ecosystems, Gulf Publishing, Houston, 181-196. [32] Gallardo, V., Medrano, S.A. and Carrasco, F.D. (1988) Taxonomic composition of the sublittoral soft-bottom polychaetes of Chile Bay (Greenwich Island, South Shet- land Islands, Antarctica). Serie Científica Instituto Antár- tico Chileno, 37(1), 49-67. [33] Gambi, M.C., Castelli A. and Guizzardi, M. (1997) Poly- chaete populations of the shallow soft bottoms off Terra Nova Bay (Ross Sea, Antarctica): Distribution, diversity and biomass. Polar Biology, 17(3), 199-210. [34] San Martin, G., Parapar, J., García, F.J. and Redondo, M.S. (2000) Quantitative analysis of soft-bottoms infau- nal macrobenthic polychaetes from South Shetland Is- lands (Antarctica). Bulletin of Marine Science, 67 (1) , 83- 102. [35] Hilbig, B., Verdes, D. and Montiel, A. (2006) Distribu- tion patterns and biodiversity in polychaete communities of the Weddell Sea and Antarctic Peninsula area (South- ern Ocean). Journal of Marine Biological Association of the U.K., 86(4), 711-725. [36] Mincks, S. and Smith, C.R. (2007) Recruitment patterns in Antarctic Peninsula shelf sediments: Evidence of de- coupling from seasonal phytodetritus pulses. Polar Biol- ogy, 30(5), 587-600. [37] Nonato, E.F., Brito, T.A.S., Paiva, P.C., Petti, M.A.V. and Corbisier, T.N. (2000) Benthic megafauna of the near- shore zone of Martel Inlet (King George Island, South Shetland Islands, Antarctica): Depth zonation and under- water observations. Polar Biology, 23(8), 580-588 [38] Dayton, P.K., Robilliard, G.A., Paine, R.T. and Dayton, L. B. (1974) Biological accommodation in the benthic com- munity at McMurdo Sound, Antarctica. Ecological Mo- nographs, 44(1), 105-128. [39] Echeverría, C.A and Paiva, P.C. (2006) Macrofaunal shallow benthic communities along a discontinuous an- nual cycle at Admiralty Bay, King George Island, Ant- arctica. Polar Biology, 29(3), 263-269.
|