American Journal of Plant Sciences
Vol.06 No.19(2015), Article ID:62445,11 pages
10.4236/ajps.2015.619320
Effect of Nutrient Media and KNO3 on in Vitro Plant Regeneration in Saraca asoca (Roxb.) Willd
Fatima Shirin*, Nitish Singh Parihar, Syed Naseer Shah
Forest Biodiversity Division, Institute of Forest Biodiversity, Hyderabad, India

Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 20 November 2015; accepted 27 December 2015; published 30 December 2015
ABSTRACT
Forest trees in general and those belonging to family Fabaceae in particular, have proved to be recalcitrant for propagation through tissue culture. Saraca asoca (Roxb.) Willd. (Family-Caesal- pinaceae) is one such tree which has become vulnerable in nature due to over exploitation of its bark. Four nutrient media [MS (Murashige and Skoog Medium), WPM (Woody Plant Medium), B5 (Gamborg’s Medium) and NN (Nitsch and Nitsch Medium, 1969)] and five doses of BA (N6-Benzy- ladenine) (0, 2.2, 4.4, 8.8 and 17.8 µM) and their all possible interactions were tested for shoot induction and proliferation from nodal segments of 3-year-old plants. B5 medium supplemented with 2.2 µM BA was screened out as the most suitable medium shoot induction, proliferation and elongation of regenerated shoots. In order to enhance shoot number, the nitrogen source in B5 medium was modified and five strengths of KNO3 (0.25×, 0.5×, 1.0×, 1.25× and 1.5×) were tested. The different strengths of KNO3 (Potassium nitrate) had statistically significant effect on number of shoots and on 0.25× strength of KNO3, maximum number of shoots (1.92) were obtained. The modified strengths of KNO3 did not significantly affect the elongation of shoots. Effect of 5 durations (quick dip, transfer of shoots after 1 day, after 3 days, after 5 days and after 7 days) of pulse treatment with 200 µM IBA (Indole-3-butyric acid) in 1/2 strength MS liquid medium was tested. Thereafter, the shoots were transferred to semi-solid half strength MS medium supplemented with 0.2 µM IBA and 3.96 μM phloroglucinol. Pulse treatment of 5 days duration resulted in 37.5% in vitro rooting of shoots. Plantlets were hardened in soilrite soaked with half strength MS medium in culture room and later shifted to a soil mixture in shade house.
Keywords:
KNO3, Micropropagation, Nutrient Medium, Pulse Treatment, Rooting, Saraca asoca, Shoot Induction

1. Introduction
Saraca asoca (Roxb.) Willd. (Family-Caesalpinaceae, commonly known as sita-ashoka or sorrowless tree) is a small evergreen tree found wild along streams or in the shade of evergreen forests throughout India up-to an altitude of 750 m [1] . As the name signifies, the tree is believed to be capable of relieving the sorrow of people. There is huge demand for Ashoka bark by Indian herbal industry (>2000 mt per year). Ashoka bark has been used since time immemorial in many ayurvedic preparations like Ashoka grihta, Ashoka saar and U-CAP capsules to cure internal uterine haemorrhages, colic piles, ulcers, dyspepsia and diabetes. Field studies reveal inadequate wild populations of S. asoca to cater to the needs of Indian herbal industry. This species exists only as avenue trees and not as a sizeable wild or planted population in India. Large quantities of seeds/seedlings are collected by the Forest Department for nursery raising, leaving less room for natural regeneration in the original habitat. According to field studies, there is no documentation of known plantations [2] .
The bark of the tree is very useful in gynaecological problems especially uterine bleeding associated with fibroids [3] . Recently the analgesic potential of hydrogels of silver nanoparticles using Saraca asoca bark extract has been reported [4] . It is one of the most sacred trees of the Hindus and Buddhists, the flowers being much used for religious ceremonies [5] . It is a very important forest resource of the country and a commercially important medicinal tree. But, due to un-mindful exploitation for its bark and habitat destruction, it has been declared as a vulnerable species [6] . It has also become vulnerable in nature due to increasing demand for its phytochemicals, ruthless exploitation of other plant parts like seeds and flowers and unscientific management practices. Conventionally, the tree is propagated by sowing seeds in rainy season, but seed propagation has limitations like low seed production and seed viability for a short period of only two months [7] . Vegetative propagation methods for this species have also not been reported for production of clonal planting stock. Thus, in order to rescue the species from further depletion, there is an urgent need to develop suitable micropropagation technology for its mass multiplication, which will go a long way in the conservation of this vulnerable species. In the past, very limited work has been carried out on propagation under in vitro conditions in Saraca asoca [8] [9] . As the species belongs to Family-Caesalpinaceae whose members are reported to be recalcitrant to tissue culture, efforts were made to standardize nutrient media and thereafter modify the strength of KNO3 in the medium in order to enhance shoot induction. For this purpose, four commonly used media were tried. In previous reports though MS, WPM and Gamborg’s B5 medium have been used, Nitsch and Nitsch medium has not been so commonly used. To the best of our knowledge, this study has reported modification in strength of KNO3 in the B5 medium for the first time.
2. Materials and Methods
2.1. Plant Material
Young plants (3 years old) of seed origin were collected from Jabalpur, Madhya Pradesh and Nagpur, Maharashtra, India (Figure 1(a)). Shoots were cut from the young plants and explants prepared from them. The shoots were cut into 2 - 3 cm long nodal segments, rinsed thoroughly in distilled water and swabbed with 70% ethyl alcohol soaked cotton. Cetrimide (ICI Ltd. India) solution (2%) prepared in sterile distilled water was added to these nodal segments in an Erlenmeyer flask and put for continuous agitation in rotary shaker for 30 min. Treatment with 0.2% solution of Bavistin® and 0.5% Streptomycin® was given for 30 min each. After thorough rinsing in sterile distilled water, they were surface sterilized in the laminar flow with different sterilizing agents and finally rinsed thrice with sterile distilled water. The sheath over the buds on the nodal segments was removed and they were inoculated on MS [10] semisolid medium supplemented with 10 µM BA (Figure 1(b)). The in vitro shoots so obtained were cut into nodal segments and used in further experiments.
2.2. Shoot Induction and Proliferation
A series of experiments were conducted for initiation and multiplication of shoots through nodal segments. A simple randomized block design experiment was conducted to screen various cytokinin sources for shoot initiation. A uniform dose of 20 µM of various cytokinins, viz., BA, kinetin, adenine hemisulphate, zeatin and 2-iP was tested in MS medium. MS medium supplemented with BA and zeatin resulted in 60% sprouting of nodal segments (data not shown). BA being the cheaper source of cytokinin, was used in further experiments. In a two way factorial randomized block design experiment, four nutrient media, viz., MS medium, B5 medium [11] ,



Figure 1. Shoot proliferation in Saraca asoca (a) Three year old mother plant; (b) Culture establishment from nodal segment, ((c), (d)) Shoot formation on B5 medium supplemented with 2.2 µM BA; ((e), (f)) Shoot formation and elongation of shoots on B5 medium containing modified strength of 0.25× KNO3.
Woody Plant Medium [12] and NN medium [13] along with 5 concentrations of BA (0, 2.2, 4.4, 8.8 and 17.8 µM) were tried. As an additional source of nitrogen, glutamine was tried in 4 concentrations (0, 50, 150 and 450 mg∙l−1) with B5 medium for shoot induction and proliferation in a simple randomized block design experiment. A two way factorial randomized block design experiment was conducted to screen four carbon sources, viz., sucrose, fructose and lactose in three concentrations (0.03, 0.09 and 0.15 M). A simple randomized block design experiment was conducted to test four modified strengths of KNO3 (0.25×, 0.5×, 1.0×, 1.25× and 1.5×) in B5 basal medium. The parameters recorded after four weeks were sprouted nodal segments (%), number of shoots and shoot length (cm). In the KNO3 strengths experiment the shoots were growing actively, therefore data was also recorded after eight weeks.
2.3. In Vitro Rooting of Shoots
Shoots obtained from experiments conducted on shoot formation were used for rooting experiments. The following experiments were conducted for rooting of shoots. Firstly simple randomized block design experiment was conducted for rooting of in vitro shoots on B5 medium supplemented with two auxins NAA (Napthalene acetic acid) and IBA (Indole-3-butyric acid) at a common dose of 13 µM. Secondly a simple randomized block design experiment was conducted for rooting of in vitro shoots comprising of two step culture process as follows: In the first step, the shoots were pulse-treated with IBA. Effect of 5 durations (quick dip, transfer of shoots after 1 day, after 3 days, after 5 days and after 7 days) of pulse treatment with 200 µM IBA in 1/2 strength MS liquid medium with sucrose (2%) was studied. In the second step, the pulse-treated shoots were subsequently transferred onto 0.6% agar-gelled half-strength MS medium augmented with IBA (0.2 μM) and 3.96 μM phloroglucinol. Observations for rooting experiments were recorded after six weeks.
2.4. Hardening of Plantlets
The rooted plantlets were carefully taken out of bottles and gently washed under running tap water to remove the adhering agar and dipped in a solution of 0.1% Bavistin®. The plantlets with washed and cleaned root system were transferred to root trainers consisting of 20/25 cells, each of 150 cc, filled with a mixture of autoclaved soilrite and supplied with half strength MS medium (without organics and sucrose) once a week for 15 days and kept in the culture room. After two weeks, the plantlets were transferred to polybags and pots containing a mixture of sand, farmyard manure and soil in a ratio of 1:1:1 (by volume) and shifted to a shadehouse covered with double layer of high density polyethylene black agronet.
2.5. Culture Conditions
The MS medium supplemented with 3% (w/v) sucrose (DSM, Dhampure, India), 0.8% (w/v) agar (Qualigens Ltd., India) and 0.01% (w/v) myo-inositol (SRL Ltd., India) was used in all experiments except experiments to screen nutrient medium. The pH of the medium was adjusted to 5.8, prior to autoclaving for 20 min at 15 psi. Each explant was cultured in a 25 × 150 mm culture tube containing 15 ml of sterilized semisolid medium for culture establishment and 150 ml conical flasks containing 40 ml semisolid medium for in vitro shoot multiplication and rooting experiments. The cultures were incubated at a temperature of 25˚C ± 2˚C under16 h photoperiod with 50 µmol∙m−2∙s−1 light intensity provided by white fluorescent tubes (40 W, Phillips, India) in the culture room.
2.6. Statistical Analysis
The data was analysed with SX statistical package using one-way and two-way analysis of variance. The interactions of the treatments were studied in their factorial combinations. The experiments comprised of three to four replications. Data expressed in percentage was transformed using the arc sine transformations [14] . The significance of the data was ascertained by F-test and the critical difference (CD) values at p = 0.05 computed for comparing means of various treatments.
3. Results and Discussion
3.1. Shoot Induction and Proliferation
The results obtained in various experiments are described below.
3.1.1. Effect of Nutrient Media
Significant differences were found between different concentrations of BA and different basal media for sprouting of nodal segments (Table 1). Maximum sprouting (64.6%) was obtained on 2.2 µM BA (Figure 1(e) and Figure 1(f)). The interaction between medium and BA doses did not have significant effect on sprouting of nodal segments. Basal medium had significant effect on nodal segments with regenerated elongated shoots (%).
Table 1. Effect of basal medium, BA concentrations and their interactions on sprouted nodal segments (%) in Saraca asoca after four weeks.
The values in parenthesis are arc sine transformed.
Differences were observed among various media with maximum regenerated elongated shoots (15.4%) obtained on B5 medium (Table 2). Thus, B5 medium was screened out as the most suitable medium for shoot proliferation in Saraca asoca.
Selection of medium is vital to success in tissue culture and the choice of the medium is dictated by the purpose of the tissue culture technology, which is to be employed for plant species or variety [15] [16] . There are many reports comparing different media for their effect on in vitro shoot multiplication [17] - [20] . Different plant species usually vary in their nutritional requirements, therefore they respond differently to various basal media. Similar variation was observed in our study, and out of the four culture media tested, B5 medium proved to be the best culture medium for shoot proliferation. B5 medium contains high concentration of thiamine (vitamin B1), which seems to have facilitated formation of in vitro shoots.
Furthermore, B5 medium is a low salt strength medium with only one source of nitrate as KNO3. Earlier reports on micropropagation of different leguminous species have also used B5 medium for shoot induction [21] - [23] . In contrast to our results, B5 medium was found to be less effective than MS and WP medium in leguminous species, viz., Swartzia madagascariensis [24] and Lens culinaris [25] . Our results are also in agreement with earlier workers, who have used B5 medium for in vitro shoot formation in other species. For instance, maximum number of shoots was obtained on B5 medium in Curculigo orchioides by Suri et al. [26] . Burnouf-Radosevich and Paupardin [27] also reported multiple shoot formation in Chenopodium quinoai on B5 medium.
In the leguminous tree Prosopis alba, three of the better known basal salt media for woody plants; Murashige and Skoog’s, Gamborg’s B5, and McCowen’s Woody Plant medium were compared. Overall, the full-strength media yielded a greater response than the quarter or half-strength media. Contrary to our results, while the Gamborg’s B5 medium was initially quite good, nearly all of the leaves dropped off after 55 days. The Murashige and Skoog’s medium yielded the best results overall and was adopted for routine use [28] .
3.1.2. Effect of Glutamine
Addition of glutamine to the medium as an additional source of nitrogen did not enhance sprouting in nodal segments. Glutamine treatments also did not significantly affect the percentage of elongated shoots (data not shown). The use of glutamine has been shown to increase the frequency of organogenesis and regeneration in the in vitro culture of several plants [29] - [31] . But our results are in contrast to these reports. This may be because Saraca asoca does not require an additional source of nitrogen for growth.
3.1.3. Effect of Various Carbon Sources
The three carbon sources tried did not significantly affect the shoot formation from nodal segments or the elongation of shoots (data not shown). The growth of shoots in vitro is affected by the concentration and type of exogenous carbon sources added to medium to serve as energy source and also to maintain the osmotic potential [32] . In cultured plant tissues, the normal function of chloroplast as a source of energy is reduced and a continuous supply of carbohydrates from the medium is therefore necessary. In general, sucrose is the carbohydrate
Table 2. Effect of basal medium, BA concentrations and their interactions on nodal segments with regenerated elongated shoots (%) in Saraca asoca after four weeks.
The values in parenthesis are arc sine transformed.
of choice as carbon source for in vitro plant culture, probably because it is the most common carbohydrate in the phloem sap of many plants [33] . As sucrose has been reported, as the most effective source of carbon for many other tree species [34] - [36] , it was used in all further experiments.
3.1.4. Effect of Modified Strengths of KNO3
In order to improve the shoot induction and proliferation, modifications were carried out in the nitrogen source i.e. KNO3 of the B5 medium.
The modified strengths of KNO3 in B5 medium had statistically significant effect on sprouting of nodal segments and number of shoots after four weeks of culture (Table 3). On 0.25× strength of KNO3, maximum shoots (1.12) were obtained. Significant differences were observed between treatments for number of shoots even after eight weeks of culture and maximum shoots (1.92) were obtained on 0.25× strength of KNO3, which was statistically on par with 1.36 shoots obtained with 0.50× strength of KNO3 (Table 4). The modified strengths of KNO3 did not significantly affect the elongation of shoots.
Nitrogen plays a major role in growth and differentiation such as stem elongation and leaf morphology. Both form and amount of nitrogen in culture medium have significant effects on rate of cell growth and differentiation. In tissue culture medium, nitrate, ammonium salts, amino acids and complex organic products supply nitrogen. Nitrogen is an essential element in modern mineral salt formulations and is present in the form of both nitrate
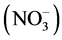 and ammonium
and ammonium
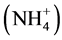 ions. Nitrate is a good source of nitrogen because it is readily taken up and metabolized by the cells and affects a number of developmental processes leading to root branching, breaking of seed and bud dormancy and release of apical dominance [37] . Decreased strength of KNO3 (0.25×) resulted in better growth of shoots, indicating that Saraca asoca, needs low levels of
ions. Nitrate is a good source of nitrogen because it is readily taken up and metabolized by the cells and affects a number of developmental processes leading to root branching, breaking of seed and bud dormancy and release of apical dominance [37] . Decreased strength of KNO3 (0.25×) resulted in better growth of shoots, indicating that Saraca asoca, needs low levels of
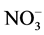 nitrogen. Saraca asoca is not found commonly in cultivation with supply of high inputs, but is found sporadically growing wild in forests. Therefore, it is adapted to grow with minimum resources available from the soil and thus under in vitro conditions also it grows better on low salt concentrations. Similarly, shoot cultures of Ceratonia siliqua, a leguminous tree could be successfully maintained on MS media with two to four times lower content of nitrogen salts [38] . Similarly, Tabone et al. reported that for Prosopis alba also nitrate as a source of nitrogen produced healthy shoots.
nitrogen. Saraca asoca is not found commonly in cultivation with supply of high inputs, but is found sporadically growing wild in forests. Therefore, it is adapted to grow with minimum resources available from the soil and thus under in vitro conditions also it grows better on low salt concentrations. Similarly, shoot cultures of Ceratonia siliqua, a leguminous tree could be successfully maintained on MS media with two to four times lower content of nitrogen salts [38] . Similarly, Tabone et al. reported that for Prosopis alba also nitrate as a source of nitrogen produced healthy shoots.
3.2. In Vitro Root Induction
Two thin and delicate roots developed from basal end of shoots on medium supplemented with 13 µM NAA and around 6% rooting was obtained (Figure 2(a)). In order to improve rooting success a two step pulse treatment method was followed. Pulse treatment of 5 days duration with 200 µM IBA and subsequent transfer to lower level of IBA along with phenolic compound phloroglucinol resulted in 37.5% in vitro rooting of shoots (Table 5, Figure 2(b)). Thick, strong brown coloured roots were obtained and the highest number of roots (2.62) with maximum root length (6.25 cm) was achieved with the above procedure. Two-step rooting procedure has been used to advantage in many leguminous species including Prosopis cineraria [39] . Auxin induces new root formation by breaking root apical dominance induced by cytokinins. IBA is reported to be more effective than other



Figure 2. In vitro rooting and hardening of Saraca asoca plantlets (a) Rooting on B5 medium supplemented with 13 µM NAA; (b) Rooting with a pulse treatment of 200 µM IBA for a duration of 5 days, ((c), (d)) Hardening in soilrite soaked with half strength MS medium in root trainers, ((e), (f)) Transfer of plantlets to mixture of sand, soil and farmyard manure (v/v, 1:1:1).
Table 3. Effect of modified strengths of KNO3 in B5 medium on shoot parameters of Saraca asoca after four weeks.
The values in parenthesis are arc sine transformed.
Table 4. Effect of modified strengths of KNO3 in B5 medium on shoot parameters of Saraca asoca after eight weeks.
The values in parenthesis are arc sine transformed.
Table 5. Effect of different durations of pulse treatment with IBA on in vitro rooting of shoots in Saraca asoca after 4 weeks.
The values in parenthesis are arc sine transformed.
auxins because it is less quickly destroyed by autoclaving or light [40] . It also exhibits long tissue half-life and thus escapes attack of endogenous degrading enzyme system like IAA oxidase [41] . The slow movement and slow degradation of IBA facilitates its localization near the site of application and thus its better function in inducing roots [42] [43] . Pulse treatment with IBA has been earlier reported for rooting of teak shoots [44] [45] . Pulse treatment with IBA in various concentrations have been found to be effective for many other species such as Picea sitchensis [46] , Wrightia tinctoria [47] , Garcinia indica [48] , Pterocarpus marsupium [49] and Erythrina variegate [50] . The auxin-phenol synergism also may be resulted in suppression of the peroxidase activity in the culture, thereby protecting the endogenous auxin from peroxidase-catalyzed oxidation [51] . In several plant species, viz., Malus pumila [52] [53] and Prunus avium [54] , the promotive effect of phloroglucinol on rooting has been reported. In all these reports, phloroglucinol enhanced rooting in the presence of an auxin, which is similar to the findings of the present study.
3.3. Hardening and Transfer of Plantlets
The two main deficiencies of in vitro grown plants are: i) poor control of water loss and ii) heterotrophic mode of nutrition. Therefore, gradual acclimatization is necessary for these plants to survive transition from culture to the field [55] . In this study a step-by-step procedure of hardening and acclimatization of the plantlets was adopted. This step-wise transfer to high temperature conditions prevented the plants from shock caused by sudden increase in temperature. The rooted plantlets were first hardened under in vitro conditions by transferring to root trainers filled with soilrite and supplied with half strength MS liquid medium lacking sucrose for 15 days (Figure 2(c) and Figure 2(d)). Hardened plants were finally transferred to polybags and clay pots containing a mixture of sand, farmyard manure and soil in a ratio of 1:1:1 (by volume) (Figure 2(e) and Figure 2(f)). All the established plants showed a high degree of uniformity without any detectable phenotypic variation.
4. Conclusion
In the present study, B5 medium was screened out as the most suitable medium for shoot induction and proliferation of Saraca asoca. An important finding reported here is that the quantity of nitrogen in B5 medium is supra-optimal for Saraca asoca shoot cultures. In conclusion, a method of in vitro clonal propagation through nodal segments has been developed which will be useful for the improvement and conservation of this economically important vulnerable leguminous tree species of medicinal importance.
Acknowledgements
The first author and Principal Investigator are grateful to the Council for Scientific and Industrial Research (CSIR), New Delhi for financial support to the project.
Cite this paper
FatimaShirin,Nitish SinghParihar,Syed NaseerShah, (2015) Effect of Nutrient Media and KNO3 on in Vitro Plant Regeneration in Saraca asoca (Roxb.) Willd. American Journal of Plant Sciences,06,3282-3292. doi: 10.4236/ajps.2015.619320
References
- 1. Troup, R.S. (1983) The Silviculture of Indian Trees: Leguminosae (Caesalpinieae) to Verbenaceae. Vol. 4, The Controller of Publications, Government of India, Delhi.
- 2. Noorunnisa Begum, S., Ravikumar, K. and Ved, D.K. (2014) “Asoka”—An Important Medicinal Plant, Its Market Scenario and Conservation Measures in India. Current Science, 107, 26-28.
- 3. Kirtikar, K.R. and Basu, B.D. (1981) Indian Medicinal Plants. Vol. 2, International Book Distribution, Dehradun.
- 4. Garg, S., Chandra, A., Mazumder, A. and Mazumder, R. (2014) Analgesic Potential of Hydrogels of Silver Nanoparticles Using Aqueous Extract of Saraca indica Bark. International Journal of Pharmaceutical Science Research, 5, 240-245.
- 5. Anon (1985) The Wealth of India, Raw Materials. Vol. 4, CSIR, New Delhi, 232-234.
- 6. IUCN (2010) IUCN Red List of Threatened Species. Version 2010.
www.iucnredlist.org - 7. Parkash, R., Choudhary, D.C. and Negi, S.S. (1991) Propagation Practices of Important Indian Trees. International Book Distributors, Dehradun.
- 8. Rama Subbu, R., Chandraprabha, A. and Sevuga, P. (2008) In Vitro Clonal Propagation of Vulnerable Medicinal Plant Saraca asoca (Roxb.) De Wilde. Natural Product Radiance, 7, 338-341.
- 9. Shirin, F., Parihar, N., Rana, P.K. and Ansari, S.A. (2011) In Vitro Shoot Regeneration from Embryonic Axis of a Multipurpose Vulnerable Leguminous Tree, Saraca indica L. Tree and Forestry Science and Biotechnology, 5, 45-48.
- 10. Murashige, T. and Skoog, F. (1962) A Revised Medium for Rapid Growth and Bioassay with Tobacco Tissue Culture. Physiologia Plantarum, 15, 473-497.
http://dx.doi.org/10.1111/j.1399-3054.1962.tb08052.x - 11. Gamborg, O.L., Miller, R.A. and Ojima, K. (1968) Nutrient Requirements of Suspension Cultures of Soyabean Root Cells. Experimental Cell Research, 50, 151-158.
http://dx.doi.org/10.1016/0014-4827(68)90403-5 - 12. Lloyd, G. and McCown, B.H. (1980) Commercially-Feasible Micropropagation of Mountain Laurel, Kalmia latifolia, by Use of Shoot-Tip Culture. Combined Proceedings-International Plant Propagator’s Society, 30, 421-427.
- 13. Nitsch, J.P. and Nitsch, C. (1969) Haploid Plants from Pollen Grains. Science, 163, 85-87.
http://dx.doi.org/10.1126/science.163.3862.85 - 14. Gomez, K.A. and Gomez, A.A. (1984) Statistical Procedures for Agricultural Research. 2nd Edition, John Willey and Sons, New York.
- 15. Gamborg, O.L. and Phillips, G.C. (1995) Media Preparation and Handling. In: Gamborg, O.L. and Phillips, G.C., Eds., Plant Cell, Tissue and Organ Culture—Fundamental Methods, Springer-Verlag, Berlin, 21-34.
http://dx.doi.org/10.1007/978-3-642-79048-5_2 - 16. Smith, R.H. (2000) Plant Tissue Culture Techniques and Experiments. Academic Press, Waltham.
- 17. Rugini, E. (1984) In Vitro Propagation of Some Olive (Olea europaea sativa L.) Cultivars with Different Root-Ability, and Medium Development Using Analytical Data from Developing Shoots and Embryos. Scientia Hotriculturae, 24, 123-134.
http://dx.doi.org/10.1016/0304-4238(84)90143-2 - 18. Mehta, U.J., Krishnamurthy, K.V. and Hazra, S. (2000) Regeneration of Plants via Adventitious Bud Formation from Mature Zygotic Embryo Axis of Tamarind (Tamarindus indica L.). Current Science, 78, 1231-1234.
- 19. Lu, M.C. (2005) Micropropagation of Vitis thunbergii Sieb. et Zucc., a Medicinal Herb, through High-Frequency Shoot Tip Culture. Scientia Hotriculturae, 107, 64-69.
http://dx.doi.org/10.1016/j.scienta.2005.05.014 - 20. Jain, N., Bairu, M.W., Stirk, W.A. and Van Staden, J. (2009) The Effect of Medium, Carbon Source and Explant on Regeneration and Control of Shoot-Tip Necrosis in Harpagophytum procumbens. South African Journal of Botany, 75, 117-121.
http://dx.doi.org/10.1016/j.sajb.2008.08.005 - 21. Vlachova, M., Metz, B.A., Schell, J. and de Bruijn, F.J. (1987) The Tropical Legume Sesbania rostrata: Tissue Culture, Plant Regeneration and Infection with Agrobacterium tumefaciens and Rhizogenes Strains. Plant Science, 50, 213-223.
http://dx.doi.org/10.1016/0168-9452(87)90076-8 - 22. Brandt, E.B. and Hess, D. (1994) In Vitro Regeneration and Propagation of Chick Pea (Cicer arietinum L.) from Meristem Tips and Cotyledonary Nodes. In Vitro Cellular and Developmental Biology-Plant, 30, 75-80.
http://dx.doi.org/10.1007/BF02632124 - 23. Diallo, M.S., Ndiaye, A., Sagna, M. and Gassama-Dia, Y.K. (2008) Plants Regeneration from African Cowpea Variety (Vigna unguiculata L. Walp.). African Journal of Biotechnology, 7, 2828-2833.
- 24. Berger, K. and Schaffner, W. (1995) In Vitro Propagation of the Leguminous Tree Swartzia madagascariensis. Plant Cell, Tissue and Organ Culture, 40, 289-291.
http://dx.doi.org/10.1007/BF00048136 - 25. Ye, G., Mcneil, D.L., Conner, A.J. and Hill, G.D. (2002) Multiple Shoot Formation in Lentil (Lens culinaris) Seeds. New Zealand Journal of Crop and Horticultural Science, 30, 1-8.
http://dx.doi.org/10.1080/01140671.2002.9514193 - 26. Suri, S.S., Jain, S. and Ramawat, K.G. (1999) Plantlet Regeneration and Bulbil Formation in Vitro from Leaf and Stem Explants of Curculigo orchioides, an Endangered Medicinal Plant. Scientia Hotriculturae, 79,127-134.
http://dx.doi.org/10.1016/S0304-4238(98)00118-6 - 27. Burnouf-Radosevich, M. and Paupardin, C. (1985) Vegetative Propagation of Chenopodium quinoa by Shoot Tip Culture. American Journal of Botany, 72, 278-283.
http://dx.doi.org/10.2307/2443555 - 28. Tabone, T.J., Felker, P., Bungham, R.L., Reyes, I. and Loughery, S. (1986) Techniques in the Shoot Multiplication of the Leguminous Tree Prosopis alba Clone B2V5o. Forest Ecology and Management, 16, 191-200.
http://dx.doi.org/10.1016/0378-1127(86)90019-8 - 29. Shrivastava, S. and Chawla, H.S. (2001) Synergistic Effect of Growth Regulators and Glutamine on Regeneration Response in High Yielding Cultivars of Wheat (Triticum aestivum L.). Indian Journal of Genetics and Plant Breeding, 61, 12-15.
- 30. Zamir, R., Ali, N., Shah, S.T., Muhammad, T. and Shah, S.A. (2007) In Vitro Re-Generation of Guava (Psidium guajava L.) from Shoot Tips of Mature Trees. Pakistan Journal of Botany, 39, 2395-2398.
- 31. Patil, G., Deokar, A., Jain, P.K., Thengane, R.J. and Srinivasan, R. (2009) Development of a Phosphomannose Isomerase-Based Agrobacterium-Mediated Transformation System for Chickpea (Cicer arietinum L.). Plant Cell Reports, 28, 1669-1676.
http://dx.doi.org/10.1007/s00299-009-0766-3 - 32. De Neto, V.B.P. and Otoni, W.C. (2003) Carbon Sources and Their Osmotic Potential in Plant Tissue Culture: Does It Matter? Scientia Hotriculturae, 97, 193-202.
http://dx.doi.org/10.1016/S0304-4238(02)00231-5 - 33. Thorpe, T.A. (1982) Carbohydrate Utilization and Metabolism. In: Bonga, J.M. and Durzan, D.J., Eds., Tissue Culture in Forestry, Martinus Nijhoff/Dr. W. Junk Publishers, The Hague, 325-368.
http://dx.doi.org/10.1007/978-94-017-3538-4_11 - 34. Lemos, E.E.P. and Baker, D.A. (1995) Shoot Regeneration in Response to Carbon Source on Internodal Explants of Annona muricata L. Plant Growth Regulation, 25, 106-112.
- 35. Ahmad, T., Abbasi, N.A., Ahmad, H.I. and Ali, A. (2007) Comparison of Sucrose and Sorbitol as Main Carbon Energy Sources in Micropropagation of Peach Rootstock GF-677. Pakistan Journal of Botany, 39, 1269-1275.
- 36. Kabir, A.H., Bari, M.A., Huda, A.K.M.N., Rezvy, M.A. and Mahfuz, I. (2007) Effect of Growth Regulators and Carbon Sources on Axillary Shoot Proliferation from Shoot-Tip Explant and Successful Transplantation of Papaya (Carica papaya L.). Biotechnology, l6, 268-272.
- 37. Shanjani, P.S. (2003) Nitrogen Effect on Callus Induction and Plant Regeneration of Juniperus excelsa. International Journal of Agriculture and Biological Engineering, 5, 419-422.
- 38. Vinterhalter, B., Ninkovic, S., Zdravkovic-Korac, S., Subotic, A. and Vinterhalter, D. (2007) Effect of Nitrogen Salts on the Growth of Ceratonia siliqua L. Shoot Cultures. Archives of Biological Science, 59, 217-222.
http://dx.doi.org/10.2298/ABS0703217V - 39. Shekhawat, N.S., Rathore, T.S., Singh, R.P., Deora, N.S. and Rao, S.R. (1993) Factors Affecting in Vitro Clonal Propagation of Prosopis cineraria. Plant Growth Regulation, 12, 273-280.
http://dx.doi.org/10.1007/BF00027208 - 40. Gaspar, T. and Coumans, M. (1987) Root Formation. In: Bonga, J.M. and Durzan, D.J., Eds., Cell and Tissue Culture in Forestry, Vol. 2, Martinus Nijhoff, Dordrecht, 202-217.
http://dx.doi.org/10.1007/978-94-009-4484-8_10 - 41. Zaerr, J.B. and Mapes, M.O. (1982) Action of Growth Regulators. In: Bonga, J.M. and Durzan, D.J., Eds., Tissue Culture in Forestry, Martinus Nijhoff/Dr. W. Junk Publishers, The Hague, 231-255.
http://dx.doi.org/10.1007/978-94-017-3538-4_9 - 42. Epstein, E. and Ludwig-Muller, J. (1993) Indole-3-Butyric Acid in Plants: Occurrence, Synthesis, Metabolism and Transport. Physiologia Plantarum, 88, 382-389.
http://dx.doi.org/10.1111/j.1399-3054.1993.tb05513.x - 43. Martin, K.P. (2002) Rapid Propagation of Holostemma ada-kodein Schult., a Rare Medicinal Plant, through Axillary Bud Multiplication and Indirect Organogenesis. Plant Cell Reports, 21, 112-117.
http://dx.doi.org/10.1007/s00299-002-0483-7 - 44. Devi, Y.S., Mukherjee, B.B. and Gupta, S. (1994) Rapid Cloning of Elite Teak (Tectona grandis Linn.) by in Vitro Multiple Shoot Production. Indian Journal of Experimental Biology, 32, 668-671.
- 45. Tiwari, S.K., Tiwari, K.P. and Siril, E.A. (2002) An Improved Micropropagation Protocol for Teak. Plant Cell Tissue and Organ Culture, 71, 1-6.
http://dx.doi.org/10.1023/A:1016570000846 - 46. Shelby, C., Kennedy, J. and Harvey, M.R. (1992) Adventitious Root Formation in Hypocotyl Cuttings of Picea sitchensis (Bong.) Carr.—The Influence of Plant Growth Regulators. New Phytologist, 120, 453-457.
http://dx.doi.org/10.1111/j.1469-8137.1992.tb01792.x - 47. Purohit, S.D. and Kudka, G. (2004) Micropropagation of an Adult Tree—Wrightia tinctoria. Indian Journal of Biotechnology, 3, 216-220.
- 48. Chabukswar, S. and Deodhar, M. (2005) Rooting and Hardening of in Vitro Plantlets of Garcinia indica Chois. Indian Journal of Biotechnology, 4, 409-413.
- 49. Husain, M.K., Anis, M. and Shahzad, A. (2007) In Vitro Propagation of Indian Kino (Pterocarpus marsupium Roxb.) Using Thidiazuron. In Vitro Cellular and Developmental Biology-Plant, 43, 59-64.
http://dx.doi.org/10.1007/s11627-006-9011-8 - 50. Shasthree, T., Imran, M.A. and Mallaiah, B. (2009) In Vitro Rooting from Callus Cultures Derived from Seedling Explants of Erythrina variegate L. Current Trends in Biotechnology and Pharmacy, 4, 447-452.
- 51. De Klerk, G.J., Vander Kreiken, W. and de Jong, J.C. (1999) The Formation of Adventitious Roots: New Concepts, New Possibilities. In Vitro Cellular and Developmental Biology-Plant, 35,189-199.
http://dx.doi.org/10.1007/s11627-999-0076-z - 52. James, D.J. (1983) Adventitious Root Formation in Vitro in Apple Rootstocks (Malus pumila) I. Factors Affecting the Length of the Auxin-Sensitive Phase in M.9. Physiologia Plantarum, 57, 149-153.
http://dx.doi.org/10.1111/j.1399-3054.1983.tb00745.x - 53. Zanol, G.C., de Fortes, G.R.L., Campos, A.D., Centellas, A.Q. and da Silva, J.B. (1998) In Vitro Rooting and Peroxidase Activity of Apple Rootstock cv, Marubakadio Treated with Indole Butyric Acid and Phloroglucinol. Revista Brasileira de Fisiologia Vegetal, 10, 65-68.
- 54. Hammatt, N. and Grant, N.J. (1997) Micropropagation of Mature British Wild Cherry. Plant Cell, Tissue and Organ Culture, 47, 103-110.
http://dx.doi.org/10.1007/BF02318945 - 55. Bhojwani, S.S. and Razdan, M.K. (1996) Plant Tissue Culture: Theory and Practice. Elsevier Science, Amsterdam.
Abbreviations
BA, 6-Benzyladenine; B5, Gamborg’s medium 1968; IBA, Indole butyric acid; MS, Murashige and Skoog (1962); WPM, Woody Plant Medium, Lloyd and McCown, 1980; NN, Nitsch and Nitsch medium, 1969; 2-iP, N6-(2-isopentenyl) adenine.
NOTES
*Corresponding author.









