 Advances in Infectious Diseases, 2013, 3, 35-43 http://dx.doi.org/10.4236/aid.2013.31004 Published Online March 2013 (http://www.scirp.org/journal/aid) 35 Considerations for Erythema Nodosum Leprosum, with Emphasis on Its Oral Manifestations Antonio Carlos Vinhas1*, Roberto Meyer Nascimento2 1Interactive Processes in Organs and Systems Program, Concentration Area in Biosafety (PPGORGSISTEM), Salvador, Brazil; 2Immunology Institute of Sciences and Health (ICS-UFBA), Salvador, Brazil. Email: *toncar2vinhas@gmail.com Received September 25th, 2012; revised October 28th, 2012; accepted November 29th, 2012 ABSTRACT Leprosy is an infectious disease caused by Mycobacterium leprae, transmitted from person to person through contact among susceptible untreated patients. It presents a broad clinical spectrum which is related to the host’s ability to mount a specific immune response. The lesions caused by the proliferation of Mycobacterium leprae (M. leprae) were sig- nificantly reduced in recent years with the early detection of new cases. Because they are less evident and/or study, maxillofacial injuries and the oral mucosa may reveal important details about the transmissibility and immuno- pathogenesis of leprosy. This article was based on a literature to verify an interrelation between oral manifestations in virchowian patients and immuno-pathological factors. Association between the infection of oral mucosa and some pathological findings as well as the participation of the local immune response in protection against the disease are research topics still not fully ex ploited. Keywords: Leprosy; Hansen Disease; Oral Manifestation 1. Introduction Leprosy is an infectious-contagious disease caused by M. leprae, transmitted from individual to individual through the contact with contagious patients without treatment. It presents a broad clinical spectrum that is related to the ability of the host to mount an immune response. The main local of entry of the Bacillus are the upper airways. There is still much to learn about the cellular and mo- lecular mechanisms responsible for the ineffectiveness of the cellular immune response in leprosy. It is known, however, that there are changes in the processing of an- tigens and the production of some interleukins. Immune response in leprosy involves all major components of the immune response, such as macrophages, various inter- leukins, T lymphocytes and their subpopulations, NK cells, B lymphocytes and antibodies. The cytokines re- leased by macrophages activated by M. leprae, perform various effects in cells of the immune system, helping to increase the effector mechanisms of the site of inflamma- tion. In leprosy, as well as in other infections where the macrophage is the main target of the parasite, the mecha- nism of immune response that determines cure or disease relates to types of cytokines produced by the immune system, where the predominance of Th1 profile deter- mines the tuberculoid form, while Th2 profile leads to lepromatous form. So a great difficulty for the control and management of the disease is the occurrence of lep- rosy reactions. The cellular responses are related to the pathogenesis of reverse reactions. Several reactional stages are produced by a variety of immune mechanisms that give rise to severe tissue damage in the course of the clinical figure of the patient. Leprosy reactions are re- lated to the exacerbation of cellular immunity, or demon- strate marked effects of immune-complex formation, called reaction type 1 and type 2, respectively [1]. The identification of specific lesions in the oral cavity in leprosy patients becomes of great importance, as well as the relevance of an imunopathology study, considering the scarcity on the subject in literature. There are many reports that discuss oral lesions, however there is a di- vergence about the region of the same. Authors report that skin lesions are concomitant with oral lesions. Indeed so far not found. Furthermore, were found a few reports about the role of cytokines expressed in the oral mucosa. 2. Series of Problem Leprosy is considered still today one of the biggest pub- lic health problems. The World Health Organization *Corresponding a uthor. Copyright © 2013 SciRes. AID 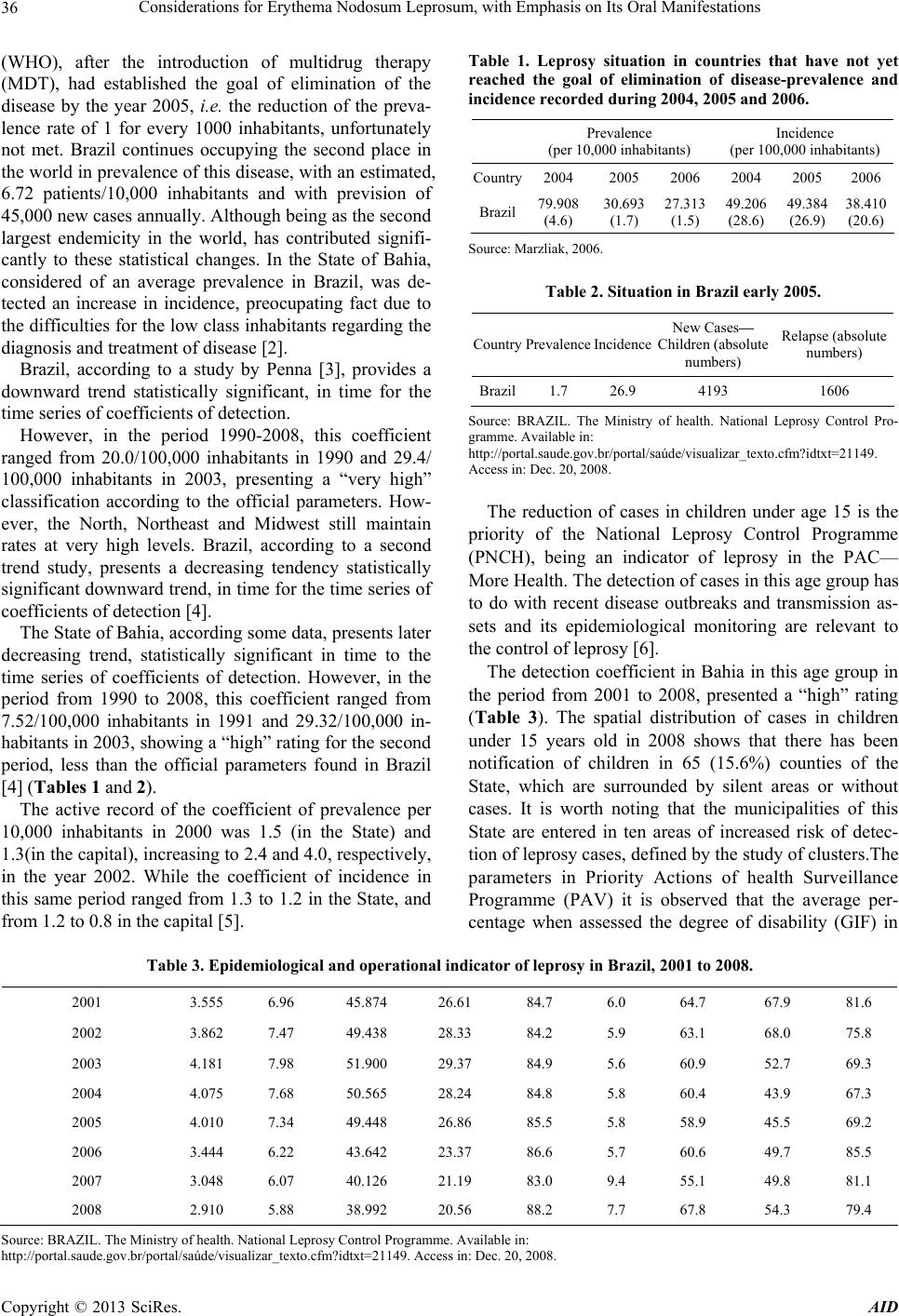 Considerations for Erythema Nodosum Leprosum, with Emphasis on Its Oral Manifestations 36 (WHO), after the introduction of multidrug therapy (MDT), had established the goal of elimination of the disease by the year 2005, i.e. the reduction of the preva- lence rate of 1 for every 1000 inhabitants, unfortunately not met. Brazil continues occupying the second place in the world in prevalence of this disease, with an estimated, 6.72 patients/10,000 inhabitants and with prevision of 45,000 new cases annually. Although b eing as the second largest endemicity in the world, has contributed signifi- cantly to these statistical changes. In the State of Bahia, considered of an average prevalence in Brazil, was de- tected an increase in incidence, preocupating fact due to the difficulties for the low class inhabitants regarding the diagnosis and treatment of disease [2]. Brazil, according to a study by Penna [3], provides a downward trend statistically significant, in time for the time series of coefficients of detection. However, in the period 1990-2008, this coefficient ranged from 20.0/100,000 inhabitants in 1990 and 29.4/ 100,000 inhabitants in 2003, presenting a “very high” classification according to the official parameters. How- ever, the North, Northeast and Midwest still maintain rates at very high levels. Brazil, according to a second trend study, presents a decreasing tendency statistically significant downward trend, in time for the time series of coefficients of detection [4]. The State of Bahia, according some data, presents later decreasing trend, statistically significant in time to the time series of coefficients of detection. However, in the period from 1990 to 2008, this coefficient ranged from 7.52/100,000 inhabitants in 1991 and 29.32/100,000 in- habitants in 2003, sho wing a “high” rating for th e second period, less than the official parameters found in Brazil [4] (Tables 1 and 2). The active record of the coefficient of prevalence per 10,000 inhabitants in 2000 was 1.5 (in the State) and 1.3(in the capital), increasing to 2.4 and 4.0 , respectively, in the year 2002. While the coefficient of incidence in this same period ranged from 1.3 to 1.2 in the State, and from 1.2 to 0.8 in the capital [5 ]. Table 1. Leprosy situation in countries that have not yet reached the goal of elimination of disease-prevalence and incidence recorded during 2004, 2005 and 2006. Prevalence (per 10,000 inhabitants) Incidence (per 100,000 inhabitants) Country2004 2005 2006 2004 2005 2006 Brazil 79.908 (4.6) 30.693 (1.7) 27.313 (1.5) 49.206 (28.6) 49.384 (26.9) 38.410 (20.6) Source: Marzliak, 2006. Table 2. Situation in Brazil early 2005. Country Prevalence IncidenceNew Cases— Children (absolute numbers) Relapse (absolute numbers) Brazil1.7 26.9 4193 1606 Source: BRAZIL. The Ministry of health. National Leprosy Control Pro- gramme. Availabl e in: http://portal.saude.gov.br/portal/saúde/visualizar_texto.cfm?idtxt=21149. Access in: Dec. 20, 2008. The reduction of cases in children under age 15 is the priority of the National Leprosy Control Programme (PNCH), being an indicator of leprosy in the PAC— More Health. The detection of cases in this age group has to do with recent disease outbreaks and transmission as- sets and its epidemiological monitoring are relevant to the control of leprosy [6]. The detection coefficient in Bahia in this age group in the period from 2001 to 2008, presented a “high” rating (Table 3). The spatial distribution of cases in children under 15 years old in 2008 shows that there has been notification of children in 65 (15.6%) counties of the State, which are surrounded by silent areas or without cases. It is worth noting that the municipalities of this State are entered in ten areas of increased risk of detec- tion of leprosy cases, defined by the study of clusters.The parameters in Priority Actions of health Surveillance Programme (PAV) it is observed that the average per- centage when assessed the degree of disability (GIF) in Table 3. Epidemiological and operational indicator of leprosy in Brazil, 2001 to 2008. 2001 3.555 6.96 45.874 26.61 84.7 6.0 64.7 67.9 81.6 2002 3.862 7.47 49.438 28.33 84.2 5.9 63.1 68.0 75.8 2003 4.181 7.98 51.900 29.37 84.9 5.6 60.9 52.7 69.3 2004 4.075 7.68 50.565 28.24 84.8 5.8 60.4 43.9 67.3 2005 4.010 7.34 49.448 26.86 85.5 5.8 58.9 45.5 69.2 2006 3.444 6.22 43.642 23.37 86.6 5.7 60.6 49.7 85.5 2007 3.048 6.07 40.126 21.19 83.0 9.4 55.1 49.8 81.1 2008 2.910 5.88 38.992 20.56 88.2 7.7 67.8 54.3 79.4 Source: B RAZIL. The Ministry of he alth. National Leprosy Control Pr o gramme. Available in : http://portal.saude.gov.br/portal/saúde/visualizar_texto.cfm?idtxt=21149. Access in: Dec. 20, 2008. Copyright © 2013 SciRes. AID 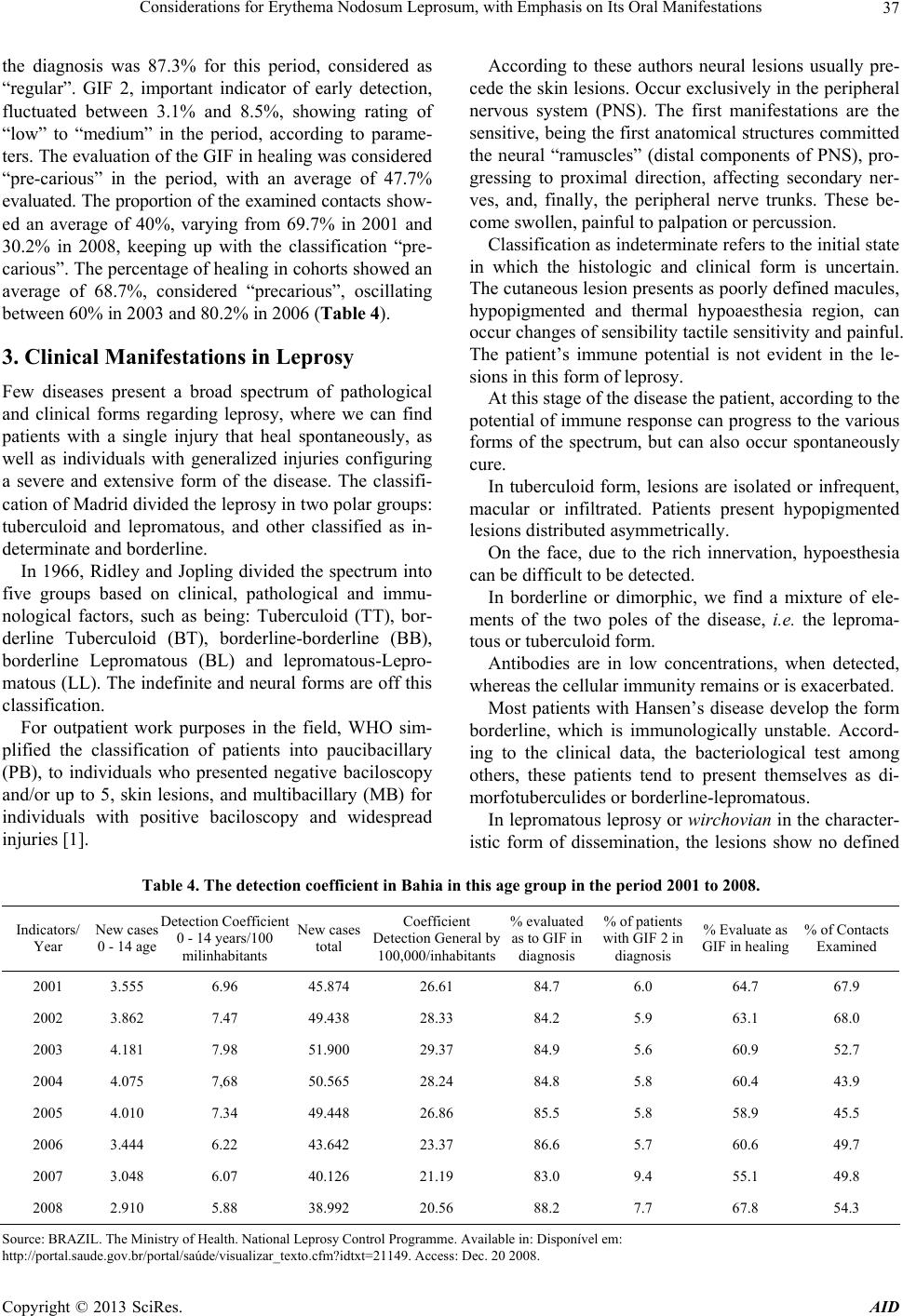 Considerations for Erythema Nodosum Leprosum, with Emphasis on Its Oral Manifestations 37 the diagnosis was 87.3% for this period, considered as “regular”. GIF 2, important indicator of early detection, fluctuated between 3.1% and 8.5%, showing rating of “low” to “medium” in the period, according to parame- ters. The evaluation of the GIF in healing was considered “pre-carious” in the period, with an average of 47.7% evaluated. The proportion of the examined contacts sho w- ed an average of 40%, varying from 69.7% in 2001 and 30.2% in 2008, keeping up with the classification “pre- carious”. The percentage of healing in cohorts showed an average of 68.7%, considered “precarious”, oscillating between 60% in 2003 and 80.2% in 2006 (Table 4). 3. Clinical Manifestations in Leprosy Few diseases present a broad spectrum of pathological and clinical forms regarding leprosy, where we can find patients with a single injury that heal spontaneously, as well as individuals with generalized injuries configuring a severe and extensive form of the disease. The classifi- cation of Madrid divided the leprosy in two polar groups: tuberculoid and lepromatous, and other classified as in- determinate and borderline. In 1966, Ridley and Jopling divided the spectrum into five groups based on clinical, pathological and immu- nological factors, such as being: Tuberculoid (TT), bor- derline Tuberculoid (BT), borderline-borderline (BB), borderline Lepromatous (BL) and lepromatous-Lepro- matous (LL). The indefinite and neural forms are off this classification. For outpatient work purposes in the field, WHO sim- plified the classification of patients into paucibacillary (PB), to individuals who presented negative baciloscopy and/or up to 5, skin lesions, and multibacillary (MB) for individuals with positive baciloscopy and widespread injuries [1]. According to these authors neural lesions usually pre- cede the skin lesions. Occur exclusively in the peripheral nervous system (PNS). The first manifestations are the sensitive, being the first anatomical structures committed the neural “ramuscles” (distal components of PNS), pro- gressing to proximal direction, affecting secondary ner- ves, and, finally, the peripheral nerve trunks. These be- come swollen, pain f ul to palpation or percu ssion. Classification as indeterminate refers to the initial state in which the histologic and clinical form is uncertain. The cutaneous lesion presents as poorly defined macules, hypopigmented and thermal hypoaesthesia region, can occur changes of sens ibility tactile sensitivity an d painful. The patient’s immune potential is not evident in the le- sions in this form of leprosy. At this stage of the disease the patient, according to the potential of immune response can progress to the various forms of the spectrum, but can also occur spontaneously cure. In tuberculoid form, lesions are isolated or infrequent, macular or infiltrated. Patients present hypopigmented lesions distributed asymmetrically. On the face, due to the rich innervation, hypoesthesia can be difficul t to be det ected. In borderline or dimorphic, we find a mixture of ele- ments of the two poles of the disease, i.e. the leproma- tous or tuberculoid form. Antibodies are in low concentrations, when detected, whereas the cellular immunity remains or is exacerbated. Most patients with Hansen’s disease develop the form borderline, which is immunologically unstable. Accord- ing to the clinical data, the bacteriological test among others, these patients tend to present themselves as di- morfotuberculi des or borderl ine-lepr o matous. In lepromatous leprosy or wirchovian in the character- istic form of dissemination, the lesions show no defined Table 4. The detection coefficient in Bahia in this age group in the period 2001 to 2008. Indicators/ Year New cases 0 - 14 age Detection Coefficient 0 - 14 years/100 milinhabitants New cases total Coefficient Detection General by 100,000/inhabitants % evaluated as to GIF in diagnosis % of patients with GIF 2 in diagnosis % Evaluate as GIF in healing % of Contacts Examined 2001 3.555 6.96 45.874 26.61 84.7 6.0 64.7 67.9 2002 3.862 7.47 49.438 28.33 84.2 5.9 63.1 68.0 2003 4.181 7.98 51.900 29.37 84.9 5.6 60.9 52.7 2004 4.075 7,68 50.565 28.24 84.8 5.8 60.4 43.9 2005 4.010 7.34 49.448 26.86 85.5 5.8 58.9 45.5 2006 3.444 6.22 43.642 23.37 86.6 5.7 60.6 49.7 2007 3.048 6.07 40.126 21.19 83.0 9.4 55.1 49.8 2008 2.910 5.88 38.992 20.56 88.2 7.7 67.8 54.3 Source: B RAZIL. The Ministry of Health. National Leprosy Control Programme. Available in: Disponível em: http://portal.saude.gov.br/portal/saúde/visualizar_texto.cfm?idtxt=21149. Access: Dec. 20 2008. Copyright © 2013 SciRes. AID  Considerations for Erythema Nodosum Leprosum, with Emphasis on Its Oral Manifestations 38 boundaries. Even in this form of the disease we can ob- serve that, in particular, rhinitis occurs early and sp ecific, by diffuse infiltration, sometimes with “hansenomas”, later, ulceration and perforation can occur and collapse of the nasal septum. 4. Oral Manifestations Little emphasis was given to Oral lesions in leprosy. A greater interest started in 1930 with Pavloff [7]. This author published papers regarding lesions in the nose and mouth, followed by other papers drew attention of loca- lized lesions in the mucous membrane of the lips, cheek and neck [8 -10]. Pavloff shows a higher frequency of lesions in the soft palate, uvula and pillars of the fauces. There were no elements of prominence observed in the cheek and gum. On the lips and tongue nodu les were detected in skin an d mucous board. This author also reported several isolated tubers at the end and sides of the tongue, and sclerotic glossitis, geographic tongue and increase in fungiform papillae. In most published papers there was always a higher incidence of injury in the hard palate, soft palate and uvula, in descending [7,9,11-16]. Most of these works refers to lepromatous patients, or patients in the intermediate form tending to lepromatous pole. The most frequent types of lesions observed in these studies were: infiltration, hansenomas and exulcerations. Some studies did not correlate these lesions with M. le- prae, and do not cite specific lesions of leprosy patients. However, there are few studies that included an im- munohistochemical study of the oral mucosa. The study of positive bacilloscopy in oral mucosa in material apparently healthy has been cited by authors in 1939, reviewing 456 lepromatous patients and other clinical forms, found an overall frequency in the lepro- matous, 19.1% of lesions in the oral cavity, and 2.09% on the lips, 1.4% tongue, 11.7% in the hard palate, 5.9% soft palate and 3.2% in the uvula. The changes that MH may present in the oral cavity were described in 1970 from the form of the disease and its time evolution. A study conducted in 1973 found the MH lesions in the oral mucosa, reporting that they only occur in later stages of the disease, inexisting in tubercu- loid and indeterminate forms. The majority of the authors mention the nasal mucosa as the main site of contamina- tion and elimination of Hansen bacilli. With the advent of specific medication initiated at the sulphonis time, has been admitted to the disappearance of the early disap- pearance of these manifestations in the duration of ther- apy. Few researchers gave emphasis on leprosy lesions in the oral cavity, a reason why, in the prophylactic sense, little has been done in the field of dentistry. Regarding the involvement of the oral mucosa in lep- rosy, few studies have been conducted on the specific lesions in this area and, although scarce, the work fo- cused on the field have attracted the attention of resear- chers. The authors call the attention of health professionals who work with leprosy, as well as dentists, for the spe- cific lesions of the disease in the oral mucosa [17]. 5. Aspects of the Immune Response in Leprosy 5.1. General Aspects Little is known of these factors that defend against infec- tion and disease after exposure to m. leprae, however, complement activation promotes phagocytosis of m. leprae [18]. When the bacillus is in contact with phago- cytic cells of the host, it is phagocytosed and initiate to conduct mechanisms of intracellular changes acting in elimination of the parasite. The nonspecific cellular immunity has been evaluated by several tests in leprosy patients, however, some authors report that these results are unconformity. Other researchers [19] by evaluating a set of tests, consider there is a nonspecific impairment of cellular immunity in lepromatous patient. Work undertaken by using parameters such as counting T and B lymphocytes in peripheral blood blast transformation of lymphocytes, macrophage inhibition test (MIF) and prolonged allograft survival, according to these author s, is not totally discor- dant, thus emphasizing the cellular immune deficiency in lepromatous patients. Evidence suggests that the system is mycobacterial oxygen-dependent, however, other me- thods of intracellular killing such as the oxygen-inde- pendent system probably involved in the killing of M. leprae in vivo [20]. There is no evidence for defects in natural barriers skin or mucous membranes in leprosy- susceptible individuals, and there is no established role for IgA in the defense against M. leprae [21]. Macrophages from tuberculoid and lepromatous pa- tients may be inefficient in the recognition and presen- tation of some mycobacterial antigens [22]. The cellular hypersensitivity depends on specific T lymphocyte asso- ciated to the macrophage. This is responsible for resis- tance to infection by M. leprae. The main parameter for the evaluation of cellular im- munity is the Mitsuda test. The author of this test (1919) found that after intradermal injection with a suspension of ground fenicated hansenomas, presented in tubercu- loid patients a positive reaction among the first three weeks, whereas it was negative in wirchovian patients. According to Modlin et al. [23], the inverse correla- tion between cellular immunity and humoral immunity was initially investigated in terms of the adaptive im- Copyright © 2013 SciRes. AID  Considerations for Erythema Nodosum Leprosum, with Emphasis on Its Oral Manifestations 39 mune response. Different sub-populations of T-cells cor- related with the response against M. leprae, including CD4 and CD8 cells and the pattern of cytokines they produce. CD4 cells produce the pattern type 1 or Th1 cytokines, including IFN-γ predominantly in tuberculoid lepromatous lesions, whereas CD8 T-cells that produce the pattern type 2 or Th2 cytokines, including IL-4, also prevalent in L-lep. In this way the leprosy provides an excellent model for the investigation of mechanisms through which the innate immune system determines, in man, the beginning of infectious disease. 5.2. Aspects of Innate Immunity The innate immune system cells are provided with a cod- ing sequence of pattern recognition receptors (PRRs), which recognizes the molecular receptors associated to the pathogen (PAMPs), which are shared between groups of pathogens. Several toll-like receptors (TLRs) mediate the innate immune recognition of M. tuberculosis and related spe- cies. Basically, the activation of the heterodimer TLR2/1 by lipopeptides M. leprae induce the production of cyto- kines such as TNF-α, as part of the acute inflammatory response and IL-12, which mediates the role of the innate immune response to instruct the adap tive type 1 response or production of Th1 cytokines [24]. A number of mechanisms that regulates the function of TLR in leprosy has been identified. Besides ability to IL-4 downregulate the expression of TLR2/1, it also in- hibits cytokine responses induced by TLR2/1. The IL-10 has no effect on the expression of TLR2/1 but inhibits, intensely, the secretion of cytokine induced by TLR2/1 [24]. The polymorphism in TLR1 and TLR2 genes have been investigated in patients with leprosy, but th ere is no convincing data to suggest that TLR1 gene polymer- phisms may contribute to the response TLR2/1 against lipopeptides, and the pathogenesis of the disease [25-28]. The ability of TLRs to indu ce an anti microbial activity is the main aspect of their role in innate immunity. There is evidence to suggest that the microbial action of vita- min D may contribute to the onset of the disease in lep- rosy. Analysis of gene expression profiling in leprosy le- sions indicated that the genes coding for the key compo- nents in microbial pathway of vitamin D were different- tially expressed in T-lep lesions co mpared to L-lep [29]. Leprosy has provided an interesting model to invest- tigate the key role of human innate immune system in host defense in relation to the susceptibility to microbial infection, the expectation that this knowledge may con- tribute to new therapeutic interventions for leprosy and other infectious diseases of connotation worldwide. 6. Cytokines and T-Cell Subsets Cytokines are mediators of mechanisms which main function is to modulate cellular interactions and which are peptides synthesized by cells with the potential re- sponse after activation. Cytokines are soluble by-pro- ducts that are T-cells which are important in mycobacte- rial infection. The immune response of T-cells plays an importa n t role in Ha nsen dise ase . Today we know that human CD4 have functionally distinct subpopulations which differ in the pattern of production of Th1 and Th2, cellular and humoral immu- nity, respectively. TNF-α is the principal mediator of host responses to Gram-bacteria, and may also have an influence on the response of other infectious organisms. Its main source is the LPS-activated mononuclear phagocytes, although the T-cell of antigen-stimulated, activated NK-cells and ac- tivated mast cells can also secrete this protein. The biological actions of TNF, such as the LPS, are best understood as a function of quantity. The main ac- tions of TNF in low concentrations range from the induc- tion of vascular endothelial cells to express new surface receptors (adhesion molecules) that causes the endothe- lial cell surface to become adhesive to leukocytes, ini- tially neutrophils, subsequently to monocytes and lym- phocytes as well as acting on neutrophils to increase its adhesion to endothelial cells. These actions contribute to the accumulation of leukocytes at sites of inflammation, and physiologically, are the most important local effects of TNF [30]. TNF activates inflammatory leukocytes to kill the mi- crobes, stimulates mononuclear phagocytes and other cells to produce cytokines, including IL-1, IL-6, more TNF and chemoki nes. Interleukin 10, produced by the T lymphocyte, Th-1 and TH-2, Langerhans cells, macrophages and keratino- cytes are immunosuppressors and immunostimulators. In lymphocyte Th-1, suppresses the synthesis of their cyto- kines, decreasing cell-mediated responses, while in B lymphocytes, increases the proliferation and antibody production. The antigen-presenting function and the pro- duction of TNF-α, IL-1, IL-6, IL-8 and GM-CSF is pre- sented decreased in macrophages. In addition to the lymphokines produced by lympho- cytes, there are many other cytokines that participate in cell interactions of immune su bstrates [31]. According to the same author, the two major activities of IL-10 is to inhibit the production of cytokines (eg. TNF, IL-1, che- mokine and IL-12) by macrophages and inhibit the fringe function of macrophages in the T-cell activation. This latter effect is due to reduced expression of MHC II CL molecules and reduced expression of certain costimula- tory (eg. B7). The net effect of these actions is to inhibit immune mediated inflammation by T-cells In addition to Copyright © 2013 SciRes. AID  Considerations for Erythema Nodosum Leprosum, with Emphasis on Its Oral Manifestations 40 its inhibitory effects on macrophages, IL-10 has a stimu- latory action on B cells. On the other hand, there are in- terleukin-4 which has as its main physiological function the regulation of immune responses mediated by IgE and mast cells/eosinophils. The main sources of IL-4 are T CD4+ lymphocytes, especially those pertaining to sub- population Th-2. Some T CD8 cells are also capable of producing IL-4 as well as activated mast cells and basophils. IL-4 is a factor of growth and differentiation for T-cells, particu- larly for cells of Th-2 subpopulation, and growth factor for mast cell, acting synergistically with IL-3 in stimu- lating the proliferation of these cells. Interferon-gamma, also called immune interferon or type II is produced by células T CD4+ aand activated CD8+ and by NK cells. IFN is a potent activator of mo- nonuclear phagocytes. Indirectly induces the synthesis of enzymes that mediate the respiratory effort, allowing hu- man macrophages kill phagocytosed microbes, it is the main activating factor of Macroph age. Among many functions performed by IFN-γ, we know that amplify the recognition phase of immune response by promoting the activation of T CD4+ helper cells re- stricted to Class II, promotes the differentiation of T lymphocytes and stimulates the cytolytic activity of NK cells. The immune system cells (eg. T-cells and monocytes) synthesize mainly TGF- 1. Both the T-cells activated by antigens such as mononuclear phagocytes activated by LPS, secrete biologically active TGF- 1. TGF- inhibits the growth of many cell types and stimulates others. Many times, it may inhibit or stimulate the growth of the same cell type. As a cytokine, TGF- is potentially important because it antagonizes many lym- phocyte responses [32]. 6.1. Cytokines of the Tuberculoid Type In tuberculoid leprosy, displays manifestations related to exacerbation of the immune response that leads to gra- nuloma formation well defined, limiting injuries and ten- dencies for the complete destruction of the bacilli. Many laboratories have analyzed cytokines produced by T-cells of tuberculous patients and healthy individuals exposed, i.e. for those with cellular immunity to M. le- prae. A consistent finding is that the T-cells reactivated from M. leprae from tuberculous patients are predomi- nantly Th-1 phenotype. They produce high levels of IFN -γ and reduced or non-detectab le levels of IL-4 [33]. Gilka Kaplin [13], 1989, injected typ e cytokines IFN- and IL-2 in lepromatous lesions and observed evident signs and significant increase in degradation of M. leprae, suggesting a possible association among the Th-1 cyto- kines and bacterial elimination. 6.2. Cytokines in Tuberculoid Type The lepromatous type is characterized by a deficiency in its imunecelular response, excessive bacillary multiplica- tion and dissemination of bacilli to the viscera and ner- vous tissue. The results are still not so evident on the release of cytokines by T-cells in this type of lepros y. The question of whether cytokines are involved in non-responsiveness in lepromatous is important and should be examined. Some researchers [23] studied cytokine patterns in situ lesions through PCR test in and found that in leproma- tous lesions for mRNAs, Th-2 cytokine were enriched by IL-4, IL-5 and IL-10, whereas IFN- and IL-12 were absent. Padmini Salgami [34] studied the production of cyto- kines by T-cells in lepromatous leprosy, as well as Tuna Mutis [35] and many other researchers, saying that there may be other sources which originated T-cells, the me- chanisms of non-responsiveness, and perhaps other un- known variables that may give rise to differences ob- served in relation to the role of certain cytokines studied. 6.3. Correlation among Cytokines and Clinical Manifestations In addition to the clinical features, lepromatous leprosy patients presented during a specific treatment, lepra reac- tions, erythematous nodules, fever, asthenia, arthralgia and other typical findings of acute inflammatory reaction [36]. The reaction type 2—ENH—common in HIV is an acute inflammatory reaction, systemic, involving the for- mation of immune complexes that circulate in the peri- pheral blood and shows its most frequent clinical mani- festation on erythema nodosum leprosum. It affects mul- tibacillary patients, becoming worse when related to leprosy, being responsible for considerable morbidity, particularly erythema nodosum recurrent. The pathology of ENL involves deposition of immune complexes and change in cell-mediated immune response. The episode of ENL is triggered by the deposition of immune com- plexes in tissues [37]. There is an increase in TNF-α which is associated with destruction of M. leprae, granuloma formation, elevated C-reactive protein (CRP), stimulation of acute inflame- matory reaction, and is involved in defense, in macro- phage activity, and the reaction of ENL, compromising the patient's general condition (FOSS, 1993). In an acute inflammatory response occurs an increase in IL-1, IL-6 and TNF-α, where IL-1 and IL-6 will act on the hepatocyte stimulating the production of proteins of acute inflammation. Levels of IL-1 and TNF-α increase in HT and DT, and decrease in HD and DV patients [1]. It was also observed an increase of IL-4 in lepromatous Copyright © 2013 SciRes. AID  Considerations for Erythema Nodosum Leprosum, with Emphasis on Its Oral Manifestations 41 patients. In bacillary forms there was an increase in the concentration of antibody anti-PGL1 [30] being associ- ated with increased IL-4 and decreased IL-1 and TNF-α. It was also observed that during the MDT decreased in IL-4 and anti-PGL1 (flow bacillar) and increase in TNF - α. Immunological changes assessed in peripheral blood, or supernatants culture compared to immunohistochemical results showed that in patients DV and VV increased TGF- 1 and CD8+ cells in the infiltrate and the absence of TGF- 1 and increase of CD4+ cells in patients with TD and TT. 6.4. Reactional States Perhaps the biggest problem in handling and controlling leprosy is the occurrence of leprosy reactions. The im- mune-cellular responses have been questioned regarding to be involved in pathogenesis of reverse reactions [38]. Robert Modlin [39] analyzed the cytokine patterns in lesions type 1 and type 2, and basically, found that the signs of cytokines, similar the type Th-1, tended to pre- dominate in reverse reactions, whereas those of type Th-2 were exacerbated in erythema nodosum leprosum. Kaplan Gilda shows that TNF-α and IL-6 increases a lot in ENL patients [35]. However, according to Otten- hoff [38], the immunopathology of the reactions type 1 and type 2 is associated with many different cytokine patterns. According to Ottenhoff studies [38], cytokines Th-1 inhibit Th-2, and vice versa. Due to cytokines are impor- tant regulators, they can provide a new form of immuno- therapy for leprosy reactions. The reactional episodes are acute intercurrences that may occur in leprosy, as manifestations of the patient’s immune system [40] and can be of two types. Reaction Type 1, also called Reverse, more frequent in HD and HT, which is characterized by erythema and edema of the lesions and/or th ickening of the nerves (neuritis). And the reaction type 2, or ENL, where the most affected pa- tients are lepromatous. They have painful erythematous nodes anywhere in the body. It can progress to neuritis. The reaction type 2 is of humoral hypersensitivity and occurs in DV and VV. It can occur in treatment-naïve patients, but usually occurs during treatment or after leaving the hosp ital [31]. In the reaction type 2 occurs: increase in the level of PCR; increase ROI (reactive intermediate of oxygen) andcytokines TNF-α, IFN- , IL-1, IL-5 and anti-PGL-1, and IL-4 and TG F- are reduced [4]. The Ministry of Health [41] calls attention to a very important issue related to immune response in leproma- tous leprosy, because it was found that patients with this type of leprosy had an extremely small amount of T-cells responsible for production and generation of anti-My- cobacterium leprae clones. This change is not yet com- pletely understood, but it is believed that there is a mal- function in the mechanism of presentation of Mycobacte- rium leprae to lymphocytes or even the absence of lym- phocytes reactive to the bacillus. 7. Final Comments Besides the epidemiological differences and knowledge still precarious, particularly on the parasite-host rela- tionship, particularly when the generation of oral mani- festations, the information presented enable you to view operational problems that reveal the need for more en- gagement of situations in the implementation of strategic actions provided in the Pact for Life, PAVS and PAC- More Health, to improve the integral attention to people with leprosy, or consequences of the disease [3]. The National Program to Combat Leprosy in Brazil was developed in response to the commitment of the country to eliminate the disease as a public health prob- lem, as explained by the Ministry of Health [6]. The pro- gram is outlined in three fundamental points: the updat- ing of data from monitoring of p atients for reliable inter- pretation of the magnitude of the problem in Brazil, the idea that reducing the prevalence rate and interrupt the chain of transmission of the disease depends on early diagnosis and treatment MDT standard and that the re- duction of social carrying depends on early detection and assessment of physical disability, and treatment of dis- abilities already. The campaign to combat leprosy is from the federal government provides equipping Brazilians with as much information so they can be active in prevention. The sooner the disease is identified, the less likely conse- quences. Every year, Brazil has 47,000 new cases of the disease. In the first half of this year were registered 201 cases in Salvador, less than the number recorded in the same [6]. “Leprosy is still a public health problem in the country, but a new survey by the Ministry of Health reveals a re- duction of 27.5% in total new cases between 2003 and 2009, going from 51,941 to 37,610. In the same period, the number of services to patients in treatment increased by 45.9%. Between days 25 and 31 January, the ministry conveys the media campaign “Health is Good to Know”, with the focus on disease. The aim is to encourage the population to find units that makes the diagnosis and treatment. The sooner you identif y leprosy, the lower the chances of sequelae” [21]. “Despite the significant reduction in the prevalence coefficient of leprosy in Bahia, which currently is 1.9 cases/10 thousand inhabitants, the state demands intensi- fied action to eliminate the disease, justified by a stan- dard medium endemicity according to the parameters of prevalence” [2]. Copyright © 2013 SciRes. AID  Considerations for Erythema Nodosum Leprosum, with Emphasis on Its Oral Manifestations 42 REFERENCES [1] W. H. Jopling and A. C. McDouglas, “Manual de Han- seníase,” 4th Edition, Atheneu, Rio de Janeiro, 1991, p. 183. [2] BRASIL, “Ministério da Saúde. Sistema Nacional de Vigilância em Saúde,” 5th Edition, Relatório de Situação, Brasilia, 2011. [3] M. L. F. Penna, “Hanseniase No Brasil: Dados e Indica- dores Selecionados,” Ministério da Saúde, Brasília, 2009. [4] BRASIL, “Ministério Da Saúde. Departamento de Vigi- lância epidemiológica,” Hanseniase No Brasil: Dados e Indicadores Selecionados, Ministério, Brasilia, 2009. [5] Secretaria da Saúde, “Saúde Intensifica Combate à Han- seníase em Salvador,” Bahia em Foco, Salvador, 2008. [6] BRASIL. Ministério da Saúde. Programa Nacional de Controle da Hanseníase. Brasilia, DF: Ministério, 2008. [7] N. Pavloff, “Leprosy in the Nose and Mouth,” Leprosy Review, Vol. 1, No. 2, 1930, pp. 21-25. [8] B. M. Prejean, “Manifestations of Leprosy of Interest to the Dentist,” Dental Survey, Vol. 19, No. 6, 1943, pp. 1152-1156. [9] L. M. Bechelli and A. Berti, “Lesões Lepróticas Damu- cosa Bucal: Estudo Clínico,” Revista Brasileira Lepro- logia, Vol. 7, No. 3, 1039, pp. 187-199. [10] H. Leloir, “Etudes Comparèes sur la Lèpre Anatomie Pathologique de la Lèpre,” Comptes Rendus Societe Biologie, Vol. 18, No. 8, 1885, pp. 479-482. [11] L. O. Silva, “Tratamento das Localizações Leprosas nas vias Aéreas Superiores e na Boca,” Revista Médica de Minas Gerais, Vol. 6, 1038, pp. 9-211. [12] B. Mela and L. Casotti, “Sulle Manifestazioni Orali e Mascellari Sulle Leppra,” Rivista italiana di stomatologia, Vol. 1, No. 26, 1939, pp. 755-763. [13] I. Lighterman and T. Hidaka, “Leprosy of Oral Cavity and Anexa,” Oral Surgery, Oral Medicine, Oral Patho- logy, Vol. 15, No. 10, 1962, pp. 1178-1194. doi:10.1016/0030-4220(62)90153-6 [14] D. Pellegrino, D. V. A. Opromolla and I. Campos, “Le- sões Lepróticas da Cavidade Oral—Sua Importância Sob O Ponto de Vista Profilático,” Estomatologia e Cultura, Vol. 4, No. 2, 1970. [15] R. C. Hastings, “Leprosy,” Medicine in the Tropics, Ed- inburgh London Melbourne and New York, Vol. 11, No. 9, 1985, pp. 245-246. [16] V. K. Sharna, et al., “Tongue Involvement in Leproma- tous Leprosy,” International Journal of Dermatology, Vol. 32, No. 1, 1993, pp. 27-29. doi:10.1111/j.1365-4362.1993.tb00957.x [17] G. G. Santos, G. Marcucci, L. M. Marchese and J. Gui- marães Jr., “Aspectos Estomatológicos das Lesões Es- pecíficas e Não-Específicas em Pacientes Portadores da Moléstia de Hansen,” Pesquisa Odontológica Brasileira, Vol. 14, No. 3, 2000, pp. 268-272. doi:10.1590/S1517-74912000000300014 [18] L. S. Schelinger and M. A. Horwitz, “Complement Re- ceptors and Complement Component C3 Mediate Phago- cytosis of Mycobacterium tuberculosis and Mycobacte- rium leprae,” International Journal of Leprosy, Vol. 58, 1990, pp. 200-201. [19] R. G. Talharis Neves, “Dermatologia Tropical: Hansenía- se,” Editora Tropical, Manaus, 1997. [20] E. O. Rojas, “Macrophages, myeloperoxidase, and Myco- bacterium lepraemurium,” Journal of Leukocyte Biology, Vol. 43, No. 5, 1998, pp. 468-470. [21] J. A. Cree, et al., “Mucosal Immunity in Leprosy,” Inter- national Journal of Leprosy, Vol. 57, No. 4, 1989, p. 318. [22] S. J. Lad and P. R. Mahadevan, “Adherence of Mycobac- terium leprae to Macrophage as an Indicator of Pathogen Induced Membrane Changes,” Indian Journal of Medical Research, Vol. 76, No. 3, 1982, pp. 804-813. [23] R. L. Modlin, et al., “The Innate Immune Response in Le- prosy,” Current Opinion in Immunology, Vol. 22, No. 1, 2009, pp. 48-54. doi:10.1016/j.coi.2009.12.001 [24] S. R. Krutizik, et al., “Activation and Regulation of Toll- Like Cels Receptors 2 and 1 in Human Leprosy,” Nature Medicine, Vol. 9, No. 5, 2003, pp. 525-532. doi:10.1038/nm864 [25] T. J. Kang, S. B. Lee and G. T. Chae, “A Polymorphism in the Toll-Like Cell Receptor 2 is Associated with IL-12 Production from Monocyte in Lepromatous Leprosy,” Cytokine, Vol. 20, No. 2, 2002, pp. 56-62. doi:10.1006/cyto.2002.1982 [26] T. J. Kang, et al., “Differential Production of Interleu- kin-10 and Interleukin-12 in Mononuclear Cells from Leprosy Patients with a Toll-Like Receptor 2 Mutation,” Immunology, Vol. 112, No. 4, 2004, pp. 674-680. doi:10.1111/j.1365-2567.2004.01926.x [27] P. Y. Bochud, T. R. Hawn and A. Aderem, “Cutting Edge: a Toll-Like Cell Receptor 2 Polymorphism that Is Asso- ciated with Lepromatous Leprosy Is Unable to Mediate Mycobacterial Signaling,” The Journal of Immunology, Vol. 170, No. 7, 2003, pp. 3451-3454. [28] N. M. Shroeder, et al., “High Frequency of Polymor- phism Arg753Gln of the Toll-Like Receptor-2 Gene De- tected by a Novel Alleles PCR,” Journal of Molecular Medicine, Vol. 81, No. 6, 2003, pp. 368-372. [29] D. Montoya, et al., “Divergence of Macrophage Phago- cytic and Antimicrobial Programs in Leprosy,” Cell Host Microbe, Vol. 6, No. 4, 2009, pp. 343-353. doi:10.1016/j.chom.2009.09.002 [30] N. T. Foss, E. B. Oliveira and C. L. Silva, “Correlation between TNF Productions, Increase of Plasma-C-Reac- tive Protein Level and Supression of T-Lymphocyte Re- sponse to Concanavalin A during Erythema Nodosum Leprosum,” Journal of International Leprosy Association, Vol. 61, No. 1, 1993, pp. 218-226. [31] E. A. Rivitti and S. A. P. Sampaio, “Dermatologia: Han- seníase,” 1st Edition, Artes Médicas, São Paulo, 1998. [32] ABUL & ABBAS, A. H. Lichtman and S. P. Jordan, “Imunologia Celular e Molecular Citocinas,” 3rd Edition, Livraria e Editora Revinter, 2000, pp. 256-283. [33] T. H. Ottenhoff, “Immunology of Leprosy: Lessons from and for Leprosy,” International Journal of Leprosy and Copyright © 2013 SciRes. AID  Considerations for Erythema Nodosum Leprosum, with Emphasis on Its Oral Manifestations Copyright © 2013 SciRes. AID 43 Other Mycobacterial Diseases, Vol. 62, No. 1, 1993, pp. 108-121. [34] P. Salgame, et al., “Differing Lymphokine Profiles of Functional Subsets of Human CD 4 and CD 8 T Cell Clones,” Science, Vol. 254, No. 5029, 1991, pp. 279-282. doi:10.1126/science.1681588 [35] A. L. Moreira, et al., “Thalidomide Exerts Its Inhibitory Action on Tumor Necrosis Factor Alpha by Enhancing mRNA Degration,” The Journal of Experimental Medi- cine, Vol. 177, No. 6, 1993, pp. 1675-1680. doi:10.1084/jem.177.6.1675 [36] N. T. Foss, “Aspectos Imunológicos da Hanseníase,” Me- dicina, Ribeirão Preto, Vol. 30, No. 3, 1997, pp. 335-339. [37] S. N. Wemambu, et al., “Erythema Nodosum Leprosum: Clinical Manifestation of the Arthus Phenomenon,” Lan- cet, Vol. 2, No. 7627, 1969, pp. 933-935. [38] T. H. M. Ottenhoff and T. Mutis, “Specific Killing of Cytotoxic T Cells and Antigen-Presenting Cells by CD4+ Cytotoxic T Cell Clones. A Novel Potentially Immu- noregulatory T-T Cell Interaction in Man,” The Journal of Experimental Medicine, Vol. 171, No. 6, 1990, pp. 2011-2024. doi:10.1084/jem.171.6.2011 [39] R. L. Modlin, et al., “Lymphocytes Bearing Antigen Spe- cific / Receptors Accumulate in Human Infectious Dis- ease Lesions,” Nature, Vol. 339, No. 3, 1989, pp. 544- 548. doi:10.1038/339544a0 [40] G. R. G. Dirksen, H. D. Gründer and M. Stöber, “Guia Brasileiro de Vigilância Epidemiológica,” 4th Edition, Ministério de Saúde, Brasília, 1988, pp. 1-11. [41] BRASIL, “Ministério da Saúde. Guia Para o Controle da Hanseníase,” 2nd Edition, Ministério, Brasilia, 1994, p. 156.
|