 Advances in Infectious Diseases, 2013, 3, 17-34 http://dx.doi.org/10.4236/aid.2013.31003 Published Online March 2013 (http://www.scirp.org/journal/aid) 17 The Relationship between Human Papillomavirus and Oesophageal Squamous Cell Carcinoma in China—A Review of the Evidence* Surabhi S. Liyanage1#, Qian Li2, Yang Zheng3, Holly Seale1, Philip J. Crowe4, Anthony T. Newall1, Bayzidur Rahman1, Eva Segelov5, Chenxu Qu6, Fanghui Zhao6, Junfeng Liu2, Zhanhai Gao1, Weixian Shi3, Peng Yang3, Aye Moa1, Chandini Raina MacIntyre1 1School of Public Health and Community Medicine, The University of New South Wales Medicine, The University of New South Wales, Sydney, Australia; 2Department of Surgery, Fourth Hospital, Shijiazhuang, China; 3Centre for Disease Control and Prevention, Beijing, China; 4Department of Surgery, Prince of Wales Hospital Clinical School, The University of New South Wales, Sydney, Australia; 5Department of Medicine, St. Vincent’s Hospital Clinical School, The University of New South Wales, Sydney, Australia; 6Department of Cancer Epidemiology, Cancer Institute, Chinese Academy of Medical Sciences, Peking Union Medical College, Beijing, China. Email: #Surabhi_liyanage@hotmail.com Received December 6th, 2012; revised January 7th, 2013; accepted February 9th, 2013 ABSTRACT Background: China has one of the highest incidence rates of oesophageal cancer in the world. The role of human papillomavirus (HPV) has been extensively researched in oesophageal squamous cell carcinoma (OSCC) with indeter- minate results. The majority of these studies have been conducted in the Chinese population. Evidence for a definitive HPV-OSCC association could potentially support prophylactic vaccination in target populations, highlighting the need for ongoing investigation. The aim of this review is to summarise the findings of HPV DNA in OSCC tissue in Chinese subjects, with a view to informing further research in this area. Methods: A systematic literature search of the Chinese National Knowledge Infrastructure (CNKI) database, Medline, Embase and PubMed was conducted for all studies in English and Chinese language, examining OSCC tissue for HPV DNA in China. Reference lists of retrieved articles were reviewed and hand searches of relevant, key journals were conducted, to source articles wh ich were not electroni- cally indexed. Sixty-four studies met our selection criteria. Data from case-control and cross-sectional studies were analysed separately for any HPV-OSCC association, using the Epi Info™ 3.5.3 software program. Results: From all studies conducted in the Chinese population, 2166 /5953 (36%) of all OSCC tissue and 478 /1684 (28%) of healthy con- trol tissue, tested p ositive for HPV. We found that 11/16 case-contro l and cross-sectional studies had a statistically sig- nificant crude odds ratio, which supported a potential HPV-OSCC association. The largest study, carried out in the high incidence County of Anyan g in Henan Province, reported 207/265 (78%) OSCC tissues testing positive for HPV DNA against 203/357 (57%) controls and had an unadjusted odds ratio of 2.71 (p-value < 0.0001). Conclusion: A rigorous meta-analysis would improve interpretation of the d ata and a well-d esigned large-scale case-control study is warranted. If a link is found between HPV and OSCC, prophylactic HPV vaccines could be of significant benefit in China. Keywords: Human Papillomavirus; Oesophageal Carcinoma; Squamous Cell Carcinoma; HPV Vaccine; China 1. Background The role of human papillomavirus (HPV) as a potential aetiological factor in oesophageal squamous cell carci- noma (OSCC) has been debated for the last three decades [1]. The pathogenesis of HPV in cervical cancer is well established and th e International Agency on Research on Cancer (IARC) has accepted the role of HPV in several head and neck cancers [2]. However, evidence for a de- finitive link between HPV and OSCC remains controver- sial. *HS currently holds a National Health and Medical Research Council (NHMRC) Training Fellowship (1012631)—Australian Based Public Health Fellowship). ATN currently holds a National Health and Medi- cal Research Council (NHMRC) Training Fellowship (630724—Aus- tralian Based Public Health Fellowship). ATN has previously received research funding for other projects from a manufacturer of HPV vac- cine. #Corresponding author. The development of prophylactic HPV vaccines is predicted to have a major public health impact in the field of cervical cancer. If HPV is established as an aeti- ologic factor in OSCC, the prophylactic HPV vaccines may play an important part in reducing mortality from Copyright © 2013 SciRes. AID  The Relationship between Human Papillomavirus and Oesophageal Squamous Cell Carcinoma in China—A Review of the Evidence 18 OSCC, particularly in a country such as China where oesophageal cancer (OC) contributes significantly to the nation’s cancer burden. 1.1. HPV Papillomaviruses have non-capsulated icosahedral viri- ons which are approximately 55nm in diameter and con- tain a genome of approximately 8000 base pairs [3]. The genome is surrounded by 72 capsomeres. The outer coat of the virus is comprised of a major and minor capsid protein. The HPV genome is comprised of three major regions and consists of circular double-stranded DNA which codes for 8 proteins. The Early region (E1-8) co- des for genes associated with transcription, plasmid rep- lication and transformation. The Late region consists of genes which code for the major (L1) and minor (L2) cap- sid proteins. The control region is responsible for pro- ducing the vital factors in the regulation of transcription and replication [4]. HPV infections have been linked to a broad range of mucocutaneous diseases, from benign skin warts to pre- malignant lesions and invasive carcinoma. Of the cur- rently characterized HPV types, infection has been de- scribed in epithelial layers of the skin, the anogenital region and the oropharyngeal mucosa [5]. 1.2. Human Papillomaviruses in Cancer Currently, it has been estimated that HPV is responsible for 5.1 percent of the global cancer burden [6,7]. The mechanism of oncogenesis of HPV in cervical cancer has been well documented and may also be applicable to oesophageal mucosa if HPV is an aetiologic factor in OSCC. The integration of viral DNA into the host ge- nome appears to be an important step in establishing the pathway to carcinogenesis [8]. Integration of HPV dis- rupts the viral E2 gene, which acts as a negative regulator of the E6/E7, the main viral genes responsible for im- mortalization and malignant transformation of the in- fected host cell. With loss of E2 control, unregulated ex- pression of the E6 and E7 oncopro teins cause proteolytic degradation of the p53 and retinoblastoma (pRb) tumor suppressor genes respectively, effectively establishing malignancy [8,9]. To date, there has been no definitive description of how HPV could infect the oesophagus. However, as the oesophageal mucosa is continuous with that of the oro- pharynx, hypotheses related to transmission in HPV re- lated oropharyngeal cancers have also been extended to OCs. Consequently, higher numbers of sexual partners, increasing practice of oral sex and initiation of sexual encounters at an earlier age have been associated with HPV-related oropharyngeal malignancies and could simi- larly be one of the risk factors for OCs in which HPV is isolated [10-13]. Some reports have also suggested a transplacental mode of transmission of HPV from infected mothers to their babies in utero as well as during passage of the in- fant through the birth canal. [14] This is supported by findings of genital tract HPV types 6, 11, 16 and 18 (usually found in the genital tract), in oesophageal tissue of newborns [15]. 1.3. Oesophageal Squamous Cell Carcinoma Of the main histologic subtypes of OC, OSCC accounts for the majority of oesophageal malignancies worldwide and is the predominant fo rm of OC diagnosed in African and Asian countries [16,17]. The main aetiologic factors for OSCC are discussed below. The onset and progress of oesophageal cancer is insidious with few early symptoms, resulting in advanced disease at time of diagnosis for many patients. Endo- scopy and barium swallow are the mainstay of OSCC diagnosis, with follow up endosonography and chest and abdominal computer tomography scans used for staging [18]. Dysphagia, odynophagia, dyspnoea, significant wei- ght loss and other symptoms and clinical signs related to disseminated disease are generally reported and observed in patients with advanced OSCC. Prognosis is often poor and the five-year survival rate in most cases, is less than 10% [18]. 1.4. Epidemiology of Oesophageal Cancer—How China Compares to the Rest of the Globe OC is the eighth most common malignancy worldwide with an incidence of an estimated 500,000 new cases annually [6]. Approximately half of the world’s OC cases occur in China where a reported annual incidence of 250,000 cases makes OC the nation’s second most com- mon malignancy, after lung cancer [19,20]. OC has a poor prognosis as it is usually d iagnosed late, with a five year survival of less than 5% [21]. In 2008, oesophageal malignancy was responsible for 406,000 deaths globally, making it the sixth highest cause of cancer-related deaths [22] while in China, with an an nual mortality of 150,0 00, it is the fourth leading cause of death from malignancy [23,24]. It is the second most common cause of can- cer-related death in Chinese males [25]. Figure 1 depicts the age-standardized mortality rates per 100,000 for oe- sophageal cancer in China, from 1990-1992. The most recent survey on oesophageal cancer incidence and mor- tality in China was carried out from 1998 to 2002 (Table 1). From the 30 cities and counties included, Ci County in Hebei Province had the highest incidence and mortal- ity rates in age-standardized calculations for both men Copyright © 2013 SciRes. AID  The Relationship between Human Papillomavirus and Oesophageal Squamous Cell Carcinoma in China—A Review of the Evidence Copyright © 2013 SciRes. AID 19 Figure 1. Estimated oesophageal cancer incidence per 100,000 in China, 1990-1992. and women. 1.5. Epidemiology of OSCC in China OSCC is predominantly a disease of developing nations and is the principal histologic type of OC in the Central Asian OC belt, which includes high-incidence countries such as China [26]. The average incidence rate for OC in the Chinese population is 13 per 100,000 [19] with OS CC representing more than 99% of all OC cases in China [27]. The variation in geographic incidence of OSCC internationally as well as within the same country, is well documented [28,29]. The major endemic regions within China are the northern Jiangsu province and the Linxian and Anyang counties in the eastern province of Henan [19,30], with mortality rates as high as 161/100,000 for males and 103/100,000 for females, in Linxian [31]. Sig- nificant differences in the incidence of OSCC also exist between regions of the same province in China, an in- triguing and unexplained phenomenon. For instance, counties within the province of Hebei in the north of China, have reported incidence rates varying from 1.4 to 118.2 per 100,000 [32]. The broad range of incidence rates both regionally and globally has been ascribed to the complex, multifactorial aetiology of OSCC. In developed countries, tobacco use and excessive alcohol consumption are thought to be the two most important causative factors, responsible for 90% of OSCC cases [7,33-35]. However, in developing nations such as China, only a small percentage of OSCC cases can be attributed to alcohol and smoking [7,34,35]. In these high incidence areas, opium abuse, nutritional deficiencies [36,37], ingestion of hot food and beverages [38,39], exposure to nitrosamines, industrial chemicals, and certain viruses [40-45] such as HPV have also been implicated. 1.6. Evidence for Involvement of HPV in OSCC I n 1982, the carcinogenic potential of HPV in OC was 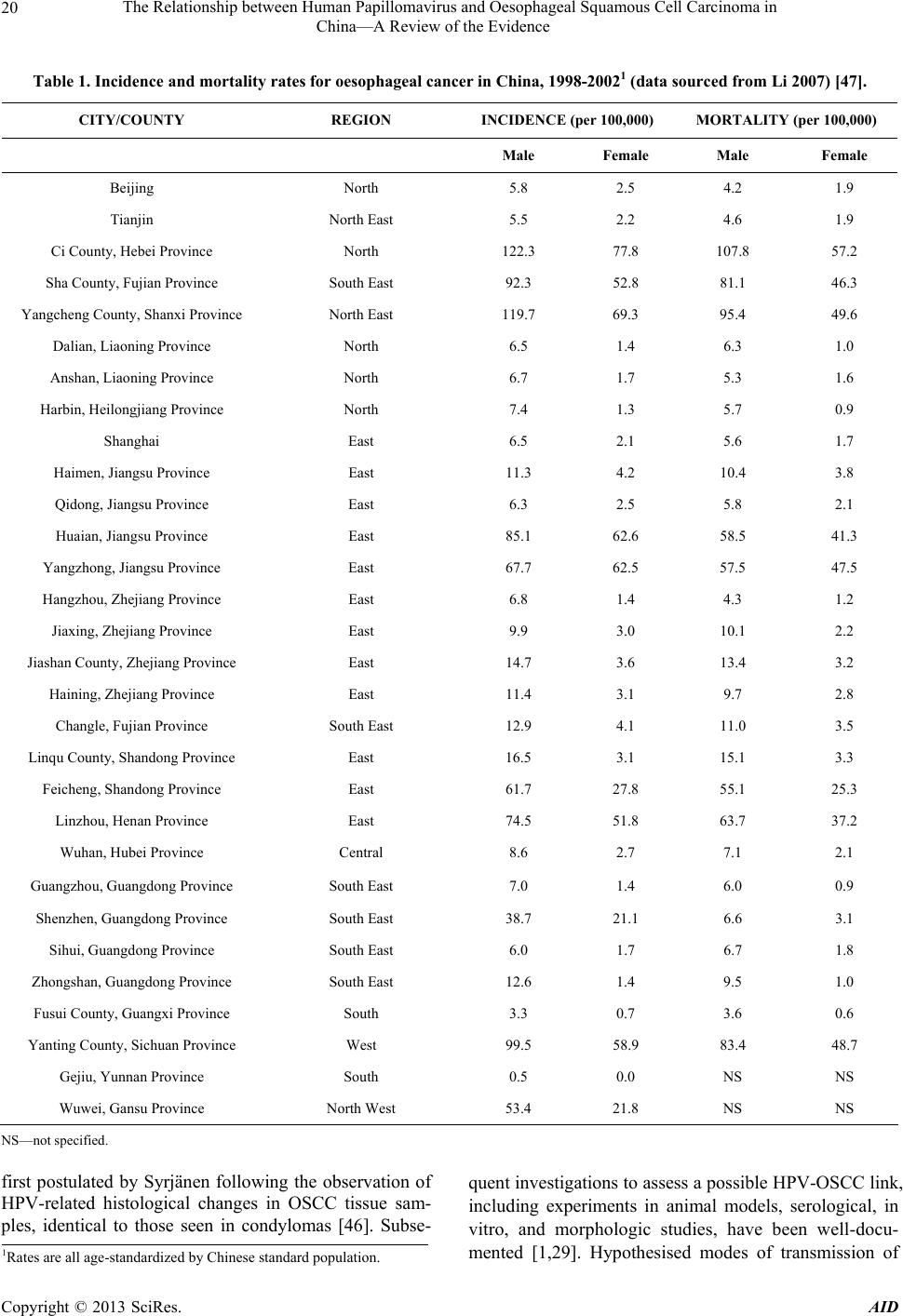 The Relationship between Human Papillomavirus and Oesophageal Squamous Cell Carcinoma in China—A Review of the Evidence 20 Table 1. Incidence and mortality rates for oesophageal cancer in China, 1998-20021 (data sourced from Li 2007) [47]. CITY/COUNTY REGION INCIDENCE (per 100,000) MORTALITY (per 100,000) Male Female Male Female Beijing North 5.8 2.5 4.2 1.9 Tianjin North East 5.5 2.2 4.6 1.9 Ci County, Hebei Province North 122.3 77.8 107.8 57.2 Sha County, Fujian Province South East 92.3 52.8 81.1 46.3 Yangcheng Count y, Shanxi Province North East 119.7 69.3 95.4 49.6 Dalian, Liaoning Pr ovince North 6.5 1.4 6.3 1.0 Anshan, Liaoning Province North 6.7 1.7 5.3 1.6 Harbin, Heilongjiang Province North 7.4 1.3 5.7 0.9 Shanghai East 6.5 2.1 5.6 1.7 Haimen, Jiangsu Province East 11.3 4.2 10.4 3.8 Qidong, Jiangsu Province East 6.3 2.5 5.8 2.1 Huaian, Jiangsu Province East 85.1 62.6 58.5 41.3 Yangzhong, Jiangs u Province East 67.7 62.5 57 .5 47.5 Hangzhou, Zhejiang Province East 6.8 1.4 4.3 1.2 Jiaxing, Zhejiang Province East 9.9 3.0 10.1 2.2 Jiashan County, Zh ejiang Province East 14.7 3.6 13.4 3.2 Haining, Zhejiang Province East 11.4 3.1 9.7 2.8 Changle, Fujian Provinc e South East 12.9 4.1 11.0 3.5 Linqu County, S h a nd o ng Province East 16.5 3.1 1 5 .1 3.3 Feicheng, Shandong Province East 61.7 27.8 55.1 25.3 Linzhou, Henan Provinc e East 74.5 51.8 63.7 37.2 Wuhan, Hubei Province Central 8.6 2.7 7.1 2.1 Guangzhou, Guangdong Province South East 7.0 1.4 6.0 0.9 Shenzhen, Guangdong Province South East 38.7 21.1 6.6 3.1 Sihui, Guangdong Province South East 6.0 1.7 6.7 1.8 Zhongshan, Gu a n g d on g P rovince South East 12.6 1.4 9.5 1.0 Fusui County, Guan gxi Province South 3.3 0.7 3.6 0.6 Yanting County, Si chuan Province West 99.5 58.9 83.4 48.7 Gejiu, Yunnan Province South 0.5 0.0 NS NS Wuwei, Gansu Province North West 53.4 21.8 NS NS NS—not specified. first postulated by Syrjänen following the observation of HPV-related histological changes in OSCC tissue sam- ples, identical to those seen in condylomas [46]. Subse- quent investigations to assess a possible HPV-OSCC link, including experiments in animal models, serological, in vitro, and morphologic studies, have been well-docu- mented [1,29]. Hypothesised modes of transmission of 1Rates are all age-standardized by Chinese standard population. Copyright © 2013 SciRes. AID  The Relationship between Human Papillomavirus and Oesophageal Squamous Cell Carcinoma in China—A Review of the Evidence 21 HPV in OSCC and mechanisms of oncogenesis based on a cervical cancer model, have also been previously sum- marized [28]. The most convincing studies have demonstrated the presence of HPV DNA sequences in OSCC tissue using techniques varying from Southern Blot to polymerase chain reaction (PCR), in situ hybridization (ISH) and immunohistochemistry (IHC). To date, the largest num- ber of studies investigating the role of HPV in OSCC have been carried out in China, with some of these stud- ies being published only in the Chinese language litera- ture. To the best of our knowledge there have been no previous reports on this topic, which assess papers from both the English and Chinese language. We aim to re- view all studies conducted in China, in English and Chi- nese, with a view to informing prevention of OSCC in China through the use of prophylactic HPV vaccines, should an aetiological link to HPV be confirmed. 2. METHODS 2.1. Search Strategy English and Chinese language papers included in this review were identified by searching the CNKI database as well as Medline, Embase and PubMed. Search terms included “human papillomavirus”, “HPV”, “oesophageal cancer”, “squamous cell carcinoma” and “China”. In ad- dition, reference lists of retrieved articles were reviewed and hand searches of key journals including Annals of Oncology, Lancet Oncology, Anticancer Research, Gas- troenterology, International Journal of Cancer, BMC Cancer, Diseases of the Esophagus, World Journal of Gastroenterology, Cancer Epidemiology Biomarkers & Prevention and Journal of Clinical Pathology, were con- ducted to source any articles which were not electroni- cally indexed. There were no limitations to date of pub- lication for either English or Chinese language studies and papers were sourced from the date when the data- bases started until February 2012. 2.2. Data Extraction Articles met the selection criteria if they investigated the presence of HPV DNA in OSCC tissue in a Chinese co- hort. All study types which included case series, cross- sectional and case-control studies, were accepted. Papers were searched and data were extracted by one author (SSL). All studies which met our search criteria were tabulated in chronological order (Table 2). For each paper, data extraction included: 1) the year in which the study w as conducted; 2) the geogra phic region of China from which subjects were recruited (Tables 2 and 3, Figure 2); 3) the testing methodology; 4) HPV types detected; 5) number of HPV positive OSCC sam- ples compared to total number of OSCC samples tested; 6) if applicable, number of HPV positive controls com- pared to total number of control specimens tested; 7) the type of study; and 8) specimen retrieval method. Recording of the specimen retrieval method is in- tended to assess whether HPV detection rates differ be- tween deep and superficial OSCC test specimens, the sample retrieval method was recorded for all studies. Deep tissue was classified as surgical resections, diag- nostic biopsies and formalin fixed and paraffin embedded samples; while superficial specimens included cell brush- ings and balloon cytology samples (Table 2). The Chinese literature was also searched for the most recent epidemiological data on oesophageal cancer inci- dence and mortality and a summary of the results ob- tained from the source [47] are presented in Table 1. In addition, authors of this review, based at the Beijing Cancer Institute & Hospital Chinese Academy of Medi- cal Sciences (CICAMS), generated a map (Figure 1) of oesophageal cancer mortality using data collected by CICAMS from 1990-19 92, on 10% of the Chinese popu- lation. Based on OC mortality data collected for various counties in China, predicted mortality rates have been projected for surrounding regions (Figure 1 ). 2.3. Analysis of Case-Control and Cross-Sectional Studies The case-control study design allows the investigator to estimate the odds of an outcome, such as OSCC, occur- ring when exposure to a potential risk factor such as HPV, has taken place. It is particularly useful as an initial study to determine causality, if a link between the expo- sure and outcome of interest, has not been previously established [48]. The case-control methodology is both time and cost-effective when investigating diseases with long latency periods, such as OSCC, because the disease state already exists at the start of the investigation [48]. Furthermore, case-control study design allows the simul- taneous assessment of multiple risk factors, which is useful in diseases such as OSCC, which have a multifac- torial aetiology [49]. Thus case-control studies are the most practical study design for examining the research question of whether HPV is an aetiological factor in OSCC. This review defines cases as patients with OSCC and controls as healthy subjects from whom macroscopically normal oesophageal biopsy samples have been obtained. Papers which identify paraoesophageal tissue from OC patients, as controls, were not acknowledged in the con- trol column of Table 2 and were not classified as case- control studies in Table 4, as there is a significant possi- bility of cross-contamination and spread of HPV from the tumour into adjacent tissue, resulting in false-positive Copyright © 2013 SciRes. AID 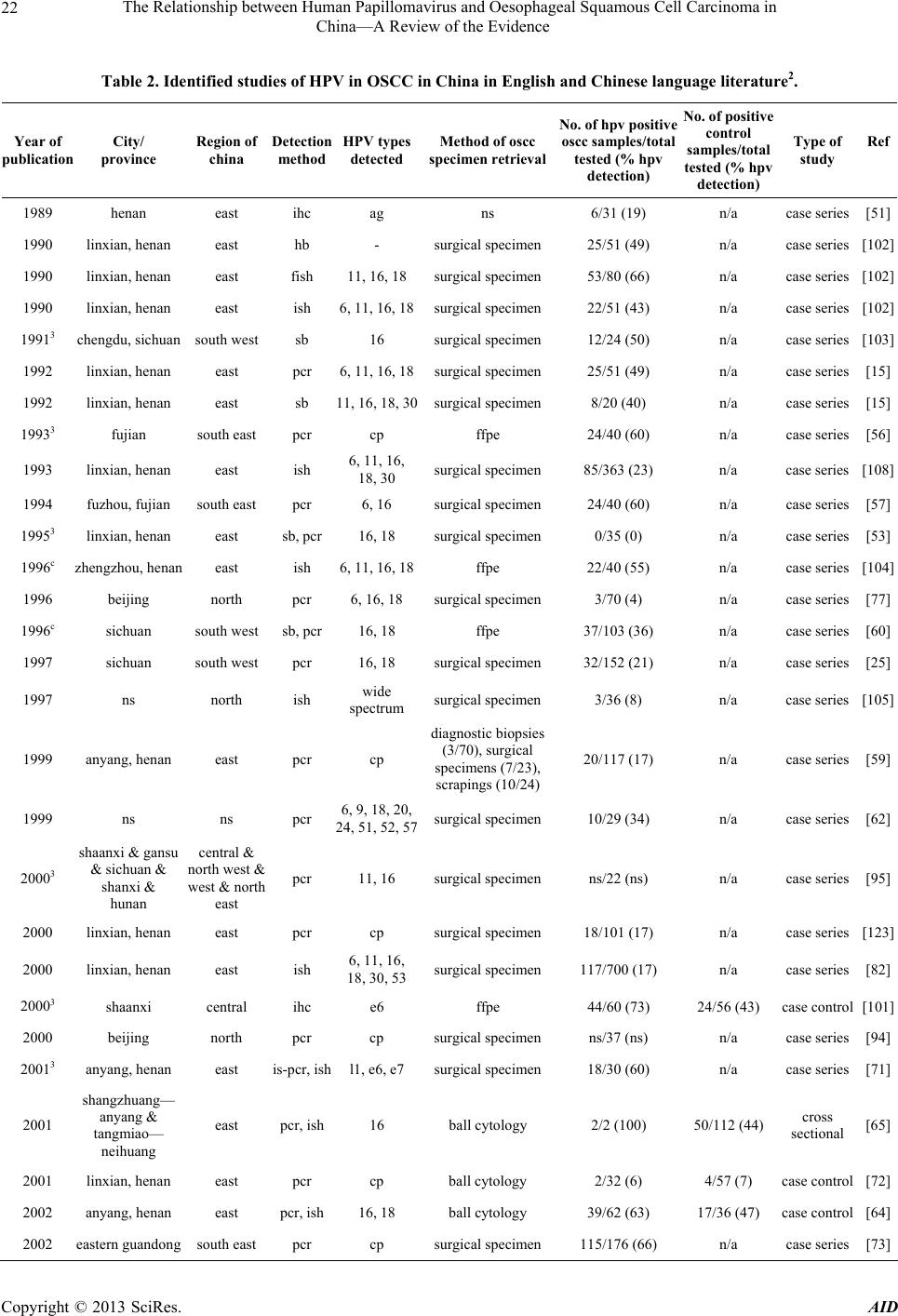 The Relationship between Human Papillomavirus and Oesophageal Squamous Cell Carcinoma in China—A Review of the Evidence Copyright © 2013 SciRes. AID 22 Table 2. Identified studies of HPV in OSCC in China in English and Chinese language literature2. Year of publication City/ province Region of china Detection method HPV types detected Method of oscc specimen retrieval No.of hpv positive oscc samples/total tested (% hpv detection) No. of positive control samples/total tested (% hpv detection) Type of study Ref 1989 henan east ihc ag ns 6/ 31 (19) n/a case series[51] 1990 linxian, henan east hb - surgical specimen 25/51 (49) n/a case series[102] 1990 linxian, henan east fish 11, 16, 18surgical specimen 53/80 (66) n/a case series[10 2] 1990 linxian, henan east ish 6, 11, 16, 18surgical specimen 22/51 (43) n/ a case series[102] 19913 chengdu, sichuan south west sb 16 surgical specimen 12/24 (50) n/a case s eries[103] 1992 linxian, henan east pcr 6, 11, 16, 18surgical specimen 25/51 (49) n/a c ase series[15] 1992 linxian, henan east sb 11, 16, 18, 30surgical specimen 8/20 (40) n/a case series[15] 19933 fujian south east pcr cp ffpe 24/40 (60) n/a case series[56] 1993 linxian, henan east ish 6, 11, 16, 18, 30 surgical specimen 85/363 (23) n/a case series[108] 1994 fuzhou, fujian south east pcr 6, 16 surgical specimen 24/40 (60) n/a case series[57] 19953 linxian, henan east sb, pcr 16, 18 surgical specimen 0/35 (0) n/a case series[53] 1996c zhengzhou, henan east ish 6, 11, 16, 18ffpe 22/40 (55) n/a case series[104] 1996 beijing north pcr 6, 16, 18 surgical specimen 3/70 (4) n/a case series[77] 1996c sichuan south wes t sb, pcr 16, 18 ff pe 37/103 (36) n/a case series[60] 1997 sichuan south west pcr 16, 18 surgical specimen 32/152 ( 21) n/a case series[25] 1997 ns north ish wide spectrum surgical specimen 3/36 (8) n/a case series[105] 1999 anyang, henan east pcr cp diagnostic biopsies (3/70), surgical specimens (7/23), scrapings (10/24) 20/117 (17) n/a case series[59] 1999 ns ns pcr 6, 9, 18, 20, 24, 51, 52, 57surgical specimen 10/29 (34) n/ a case series[62] 20003 shaanxi & gansu & sichuan & shanxi & hunan central & north west & west & north east pcr 11, 16 surgical specime n ns/22 (ns) n/a case series[95] 2000 linxian, henan east pcr cp surgical specimen 18/101 (17) n/a case series[123] 2000 linxian, henan east ish 6, 11, 16, 18, 30, 53surgical specimen 117/700 (17) n/a case series[82] 20003 shaanxi central ihc e6 ffpe 44/60 (73) 24/56 (43) case control[101] 2000 beijing north pcr cp surgical specimen ns/37 (ns) n/a case series[94] 20013 anyang, henan east is-pcr, ish l1, e6, e7 surgical specimen 18/30 (60) n/a case series[71] 2001 shangzhuang— anyang & tangmiao— neihuang east pcr, ish 16 ball cytology 2/2 (100) 50/112 (44) cross sectional [65] 2001 linxian, henan east p cr cp ball cytology 2/32 (6) 4/57 (7) case control[72] 2002 anyang, henan east pcr, ish 16, 18 ball cytology 39/62 (63) 17/36 (47) case control[64] 2002 eastern guandong south east pcr cp surgical specimen 115/176 (66) n/a case series[73] 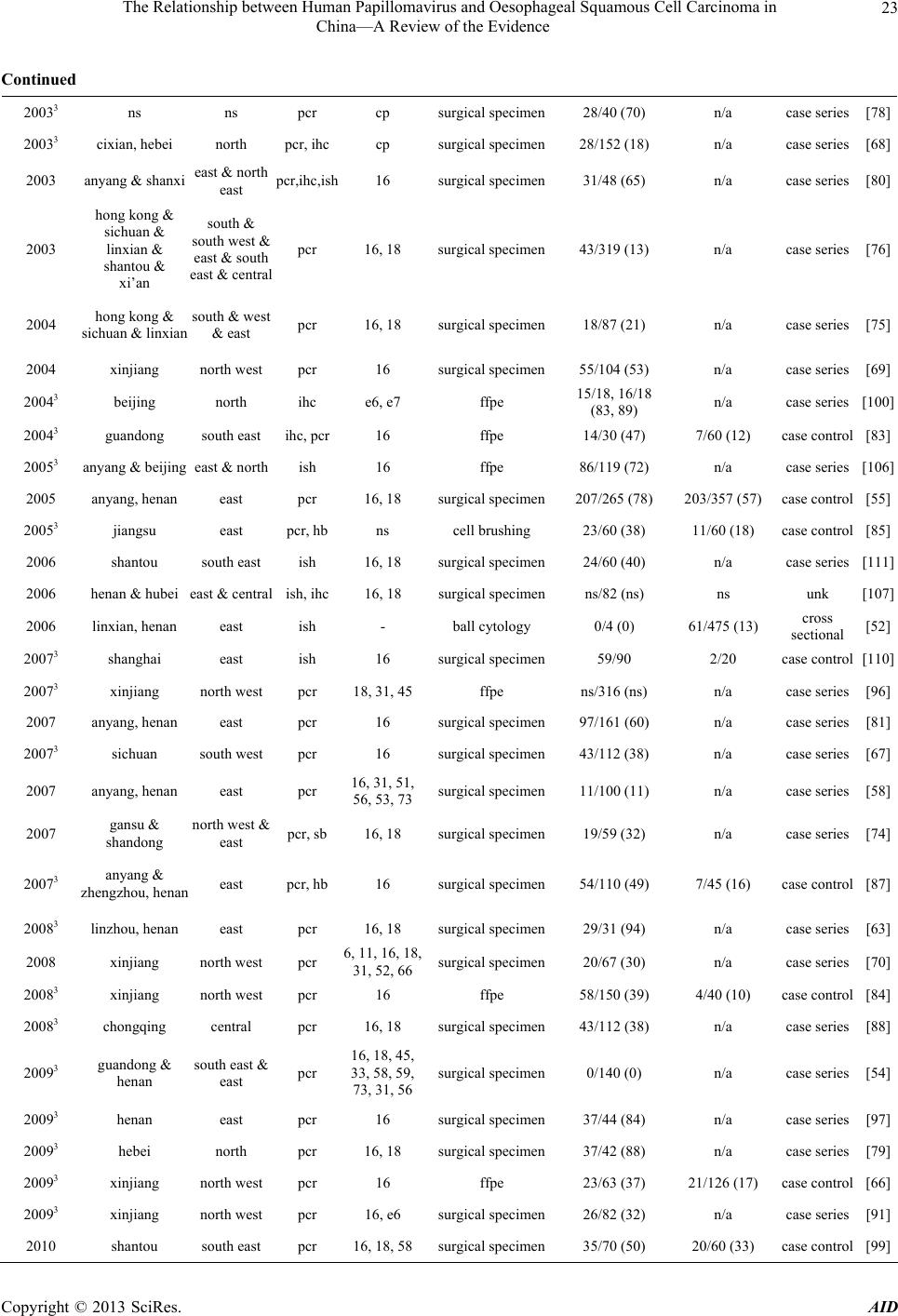 The Relationship between Human Papillomavirus and Oesophageal Squamous Cell Carcinoma in China—A Review of the Evidence 23 Continued 20033 ns ns pcr cp surgical specimen28/40 (70) n/a case series[78] 20033 cixian, hebei north pcr, ihc cp surgical specimen28/152 (18) n/a case series[68] 2003 anyang & shanxi east & north east p cr,ihc,ish 16 surgical specimen31/48 (65) n/a case series[80] 2003 hong kong & sichuan & linxian & shantou & xi’an south & south west & east & south east & central pcr 16, 18 surgical specimen43/319 (13) n/ a case series[76] 2004 hong kong & sichuan & linxian south & west & east pcr 16, 18 surgical specimen18/87 (21) n/a case series[75] 2004 xinjiang north west pcr 16 surgical specimen55/104 (53) n/a case series[69] 20043 beijing north ihc e6, e7 ffpe 15/18, 16/18 (83, 89) n/a case series[100] 20043 guandong south east ihc, pcr 16 ffpe 14/30 (47) 7/60 (12) case control[83] 20053 anyang & beijing east & north ish 16 ffpe 86/11 9 ( 7 2) n/a case series[106] 2005 anyang, henan east pcr 16, 18 surgical specimen207/265 (78) 203/357 (57) case control[55] 20053 jiangsu east pcr, hb ns cell brushing 23/60 (38) 11 / 6 0 (18) case control[85] 2006 shantou south east ish 16, 18 surgical specimen24/60 (40) n/a case series[111] 2006 henan & hubei east & central ish, ihc 16, 18 surgical specimenns/82 (ns) ns unk [107] 2006 linxian, henan east ish - ball cytology 0/4 (0) 61/475 (13) cross sectional [52] 20073 shanghai east ish 16 sur gical specimen59/90 2/20 case control[110] 20073 xinjiang nor th west pcr 18, 31, 45ffpe ns/316 (ns) n/a case series[96] 2007 anyang, henan east pcr 16 surgical specimen97/161 (60) n/a case series[81] 20073 sichuan south west pcr 16 surgical specimen43/112 (38) n/a case series[67] 2007 anyang, henan east pcr 16, 31, 51, 56, 53, 73surgical specimen1 1 /100 (11) n/a case series[58] 2007 gansu & shandong north west & east pcr, sb 16, 18 surgical specimen19/59 (32) n/a case series[74] 20073 anyang & zhengzhou, henan east pcr, hb 16 surgical specimen54/110 (49 ) 7/45 (16) case control[87] 20083 linzhou, henan east pcr 16, 18 surgical specimen29/31 (94) n/a case series[63] 2008 xinjiang north west pcr 6, 11, 16, 18, 31, 52, 66surgical specimen20/67 (30) n/a case series[70] 20083 xinjiang north west pcr 16 ffpe 58/150 (39) 4/40 (10) case c o n trol[84] 20083 chongqing central pcr 16, 18 surgical specimen43/112 (38) n/a case series[88] 20093 guando ng & henan south east & east pcr 16, 18, 45, 33, 58, 59, 73, 31, 56surgical specimen0/140 (0) n/a case series[54] 20093 henan east pcr 16 surgical specimen37/44 (84) n/a case series[97] 20093 hebei north pcr 16, 18 surgical specimen37/42 (88) n/a case series[79] 20093 xinjiang north west pcr 16 ffpe 23/63 (37) 21/126 (17) case control[66] 20093 xinjiang north west pcr 16, e6 surgical specimen26/82 (32) n/a case series[91] 2010 shantou south east pcr 16, 18, 58surgic al specime n35/70 (50) 20/60 (33) case control[99] Copyright © 2013 SciRes. AID 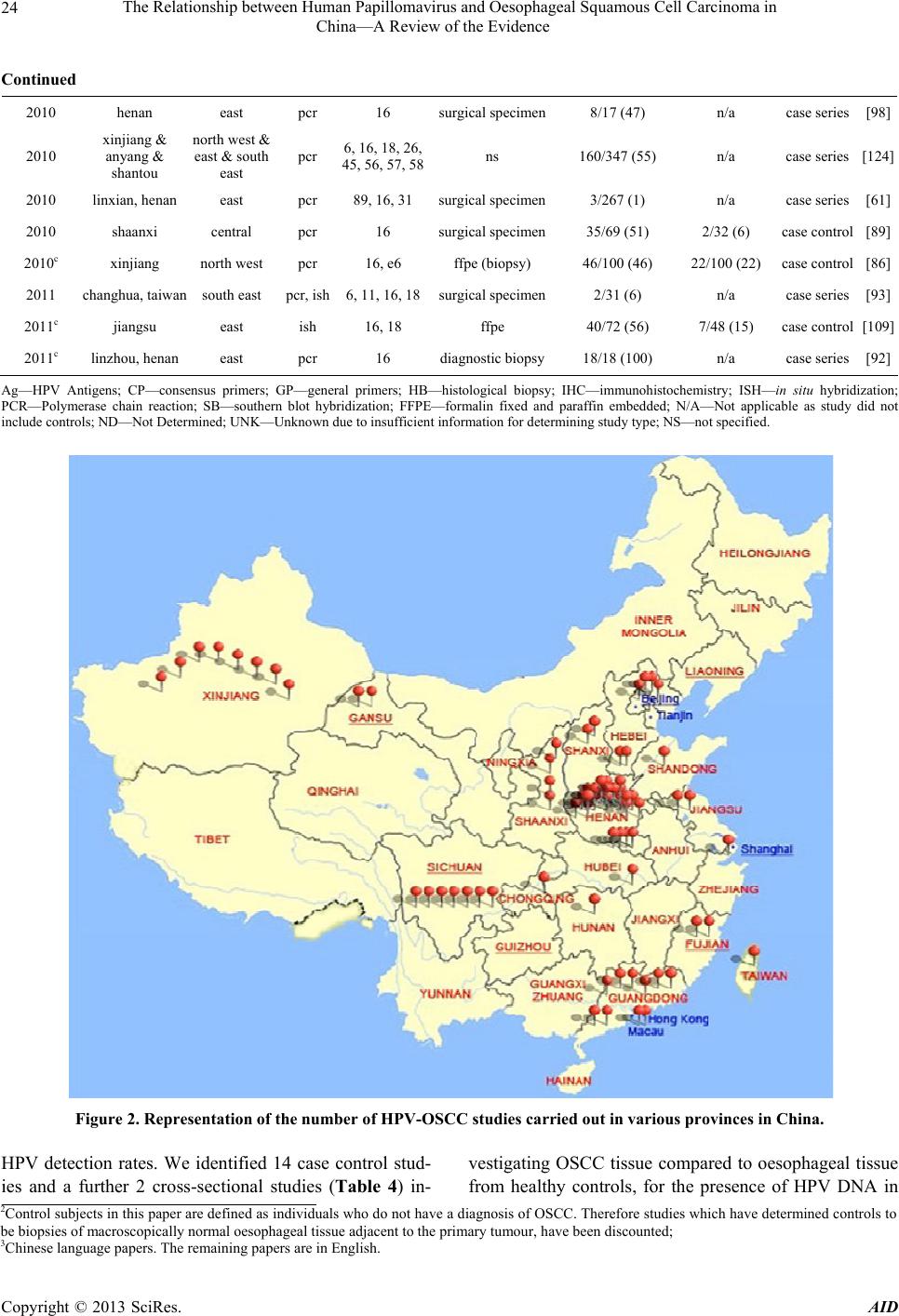 The Relationship between Human Papillomavirus and Oesophageal Squamous Cell Carcinoma in China—A Review of the Evidence Copyright © 2013 SciRes. AID 24 Continued 2010 henan east pcr 16 surgic al specimen8/17 (47) n/a case series[98] 2010 xinjiang & anyang & shantou north west & east & south east pcr 6, 16, 18, 26, 45, 56, 57, 58ns 160/347 (55) n/a case series[124] 2010 linxian, henan east pcr 89, 16, 31surgical specimen3/267 (1) n/a case series[61] 2010 shaanxi central pcr 16 surgical specimen35/69 (51) 2/32 (6) case control[89] 2010c xinjiang north west pcr 16, e6 ffpe (biopsy) 46/100 (46) 22/100 (22) case control[86] 2011 changhua, taiwan south east pcr, ish 6, 11, 16, 18surgical specimen2/31 (6) n/a case series[93] 2011c jiangsu east ish 16, 18 ffpe 40/72 (56) 7/48 (15) case control[109] 2011c linzhou, henan east pcr 16 diagnostic biopsy 18/18 (100) n/a case series[92] Ag—HPV Antigens; CP—consensus primers; GP—general primers; HB—histological biopsy; IHC—immunohistochemistry; ISH—in situ hybridization; PCR—Polymerase chain reaction; SB—southern blot hybridization; FFPE—formalin fixed and paraffin embedded; N/A—Not applicable as study did not include controls; ND—Not Determined; UNK—Unknown d ue to insufficient information for determining study type; NS—not specified. Figure 2. Representation of the number of HPV-OSCC studies carried out in various provinces in China. HPV detection rates. We identified 14 case control stud- ies and a further 2 cross-sectional studies (Table 4) in- vestigating OSCC tissue compared to oesophageal tissue from healthy controls, for the presence of HPV DNA in 2Control subjects in this paper are defined as individuals who do not have a diagnosis of OSCC. Therefore studies which have determined controls to be biopsies of macroscopically normal oesophageal tissue adjacent to the primary tumour, have been discounted; 3Chinese language papers. The remaining papers are in English.  The Relationship between Human Papillomavirus and Oesophageal Squamous Cell Carcinoma in China—A Review of the Evidence 25 Table 3. HPV Detection Rate by Region in China, based on 64 identified studies from the English and Chinese Litera- tured. REGION NO OF OSCC POSITIVE SAMPLES/TOTAL TESTED (% HPV DETECTION) North 154/447 (34) North West 228/566 (40) South East 300/643 (47) South West 124/391 (32) South 16/123 (13) East 1173/3415 (34) Central 132/298 (44) Unspecified Regions 38/69 (55) China (Total) 2165/5952 (36) the Chinese population . Epi Info™ 3.5.3 [50] wa s used to calculate odds ratios (OR) with 95% confid ence intervals, for the association of HPV with OSCC, by cross-tabu- lating the summary data presented in the papers for case-control and cross-sectional studies. P-values for the significance of the ORs were calculated from chi-squared test. Only two of the identified studies presented calcula- tions of ORs. Few studies adjusted for confounding fac- tors and our calculation of unadjusted odds ratios for the association of HPV with OSCC from the summary data provided in the papers, must therefore be interpreted in this context. 3. Results The first study looking for an aetiological link between HPV and OSCC in China was carried out in 1989 using immunohistochemistry (IHC) [51]. In total, 64 studies Table 4. Case-Control and Cross-Sectional Studies Examining HPV DNA in OSCC in China, from English and Chinese lan- guage literature4. YEAR REGION METHOD HPV TYPES FOUND POSITIVE NUMBER CASES (%) POSITIVE NUMBER CONTROLS (%) CRUDE ODDS RATIO5 P-VALUE REF 2000 Shaanxi (Centr a l) IHC E6 44/60 (73) 24/56 (43) 3.67 (1.57 - 8.65) 0.0009 [101] 2001 Shangzhua ng - Anyang & Tangmiao - Neihuang (East) PCR, ISH 16 2/2 (100) 50/112 (44) Incalculabl e 0.1192 [65] 2001 Linxian, Henan (East) PCR CP 2/32 (6) 4/57 (7) 0.88 (0.1 - 6.13) 0.8898 [72] 2002 Anyang, Henan (East) PCR, ISH 16,18 39/62 (63) 17/36 (47) 1.90 (0.76 - 4.75 ) 0.1305 [64] 2004 Guandong (SE) IHC, PCR 16 14/30 (47) 7/60 (12) 6.63 (2.04 - 22.23) 0.0002 [83] 2005 Anyang, Henan (East) PCR 16,18 207/265 (78) 203/357 (57) 2.71 (1.86 - 3.94) <0.0001[55] 2005 Ji an g su (East) PCR, HB NS 23/60 (38) 11/60 (18) 2.77 (1.12 - 6.97) 0.0151 [85] 2006 Linxian, Henan (East) ISH Nil 0/4 (0) 61/475 (13) 0.00 (0 - 10.6 8) 0.4429 [52] 2007 Anyang & Zhengzhou, Henan (East) PCR, HB 16 54/110 (49) 7/45 (16) 5.23 (2.02 - 14.12) 0.0001 [87] 2007 Shanghai (East) ISH 16 59/90 (66) 2/20 (10) 17.13 (3.46 - 114 .6) <0.0001[11 0] 2008 Xinjiang (NW) PCR 16 58/150 (39) 4/40 (10) 5.67 (1.8 - 19.87) 0.0006 [84] 20096 Xinjiang (NW) PCR 16 23/63 (37) 21/126 (17) 2.88 (1.36 - 6.11) 0.0023 [66] 2010 Shaanxi (Central) PCR 16 35/69 (51) 2/32 (6) 15.44 (3.20 - 101.46) <0.0001[89] 2010 Shantou (SE) PCR 16,18,58 35/70 (50) 20/60 (33) 2.00 (0.92 - 4.35) 0.0552 [99] 20107 Xinjiang (NW) PCR 16, E6 46/100 (46) 22/100 (22) 3.02 (1.56 - 5.86) 0.0003 [86] 2011 Jian gs u (East) ISH 16,18 40/72 (56) 7/48 (15) 7.32 (2.69 - 20. 7 1) <0.0001[109] Total 681/1239 (55) 478/1684(28) CP—consensus primers; HCII—Hybrid Capture 2; IHC—immunohistochemistry; ISH—in situ hybridization; LR—Low-risk HPV types; HR—High risk HPV pes; PCR—Polymerase chain reaction. ty 4Table excludes studies which did not report t he number of HPV positive OSCC samples from the tota l tested. 5Unadjusted ORs calculated using Epi Info™ 3.5.3, from summary data presented in the papers. As adjustments for confounding factors have not been carried out in most studies, it is important to in terpret these ORs with caution. Only two studies calculate d ORs as highlighted below. 6OR calculated by authors of study: 2.67 ( 1.38 - 5.17); P < 0.05. 7OR calculated by authors of study: 3.020; P < 0.001. Copyright © 2013 SciRes. AID  The Relationship between Human Papillomavirus and Oesophageal Squamous Cell Carcinoma in China—A Review of the Evidence 26 have been conducted in China to date, which include a total of 6409 OSCC samples as summarised in Table 2. Of these, 36 were in the English language and 29 were in Chinese. Of the 64 studies investigating the role of HPV in OSCC in China, a majority of 47 were case series, with 14 case-control studies, 2 cross-sectional studies and 1 report of indeterminable study design (Table 2). From all studies conducted in the Chinese population, 2166/5953 (36%) of all OSCC tissue and 478/1684 (28%) of all healthy control tissu e, tested positive for HPV ( Ta- ble 2). 3.1. Regions of China To date, the Chinese population has contributed the lar- gest number of OSCC specimens for HPV analysis, com- pared to any other country [28]. Henan province in East China is the site of over 50% of all na tional studies (Fig- ure 2). Of all OSCC samples tested in this high incidence area, 34% yielded positive HPV results (Table 3). Other high-incidence areas within China from the north-west, south-east, and the central regions have reported even higher rates of HPV DNA detection in OSCC tissue, ranging from 40% - 47 % (Table 3). 3.2. Testing Methods As summarized in Table 2, a variety of techniques have been used in these studies to detect HPV. The detection rate of HPV in OSCC tissue varied from 0 to 100%. Only three studies did not detect HPV in any of the OSCC samples tested [52-54]. Forty-nine of 64 studies utilized PCR with HPV detection rates varying widely from 0% - 100% [15,25,53-99]. PCR yield ed a HPV positive rate in 37% of all OSCC tissue tested using this method. Ap- proximately one quarter of all OSCC specimens were analysed by ISH, with HPV detected in 30% of these samples. Six studies have used IHC to test OSCC tissues samples with HPV detection rates varying from 18% - 89% [51,68,80,83,100,101]. One study reported a 66% HPV detection rate usi ng filter in situ hybridisation (FISH) [102], five studies used southern blot hybridization [15, 53,60,74,103] and a further three studies examined his- tological biopsies of oesophageal lesions [85,87,102]. Sixteen studies used ISH with percentage of OSCC tissues testing positive fo r HPV, varying from 0% - 72% [52,64,65,71,80,82,93,102,104-111]. ISH was the meth- odology used in the largest study to be carried out on this topic, analyzing a total of 700 OSCC samples from the Henan Province, with an HPV detection rate of 17% [82]. High-risk HPV types 16 and 18 were the most commonly detected genotypes in this study, as well as in all other investigations from the Chinese cohort. 3.3. Site of Specimen Retrieval We also examined studies for any potential relationship between HPV detection rates and whether OSCC test specimens were superficial cell scrapings or deep tissue biopsies. Of the 60 studies which had sufficient informa- tion for analysis, one tested both superficial and deep oesophageal tissue specimens, 54 tested only deep tissue and the remaining 5 tested superficial specimens (Table 2). We found that 76/184 (41%) of superficial OSCC samples and 1990/5607 (35%) of deep OSCC tissue samples tested positive for HPV DNA. 3.4. Results of Case-Control and Cross-Sectional Studies As highlighted in Table 4, we identified 14 case-control studies and 2 cross-sectional studies which investigate the association of HPV DNA in OSCC tissue compared to normal control tissue in the Chinese population. From these studies, 681/1239 (55%) of all OSCC samples and 478/1684 (28%) of all healthy control tissue tested posi- tive for HPV. Only two of these studies calculated an odds ratio in the published papers [66,86]. Table 4 pre- sents these results as well as our unadjusted, crude odds ratios which were calcula ted using Epi Info™ 3.5.3 (CDC) [50]. Our analysis demonstrates that 11/16 studies had statistically significant odds ratios which support a po- tential HPV-OSCC link. The largest stud y, carried out in the high incidence County of Anyang in Henan Province [55], reported 207/265 (78%) OSCC tissues testing posi- tive for HPV DNA against 203/357 (57%) controls and had an unadjusted odds ratio of 2.71 (p-value < 0.0001). 4. Discussion Our findings of a statistically significant association of HPV with OSCC in 11/16 case-control and cross-sec- tional Chinese studies, suggest that HPV may be a poten- tial aetiological factor in OSCC, in the Chinese popula- tion. However, as our unadjusted ORs have been calcu- lated from summary data in papers, many of which have not adjusted for important confounding factors, it is im- portant to interpret our results accordingly. In the 16 studies in China which have tested control subjects for HPV [52,55,64-66,72,83-87,89,99,101,109, 110], a number of controls have tested positive for HPV, including two studies in which HPV was isolated from a greater percentage of controls than cases [52,72]. Inter- estingly, both of these studies were carried out in Linxian within Henan province, a region which has one of the highest incidence rates of OC in the world [30]. Fidalgo et al. also reported 100% of controls testing positive for HPV DNA in a Portuguese cohort [112]. This trend may be suggestive of an early role fo r HPV in the aetiology of Copyright © 2013 SciRes. AID  The Relationship between Human Papillomavirus and Oesophageal Squamous Cell Carcinoma in China—A Review of the Evidence 27 OSCC, in which normal oesophageal mucosa infected by HPV may undergo malignant transformation following expression of the E6/E7 viral oncogenes [77]. We found that HPV types 16 and 18 are the most com- monly detected genotypes within oesophageal tissue in China. However, it is also important to note that a ma- jority of studies to date test only for the main oncogenic genotypes of HPV, namely types 16 and 18, thereby raising the possibility that less common HPV types are missed in the testing process. Nevertheless, some HPV types, which have not previously been isolated from oe- sophageal tissue, have been described in some studies which have tested for a broader range of HPV types. Of note are types 30, 53, 56, 66, 73 and 89 in the Chinese cohort (Table 2). In particular, Chang et al., reported 8 out of 85 HPV-positive OSCC samples with HPV 30 [108]. As HPV 30 has only previously been identified in two genital condylomas [113], and one malignant laryn- geal lesion, the finding of this HPV genotype in eight OSCC samples has led to proposals that HPV 30 may have a proclivity to infect oesophageal mucosa [108]. This review underscores the highly variable results of HPV detection in OSCC, between different regions of China, for which diverse testing methodology may be a contributing factor. It is difficult to draw conclusions on which testing methods yield the highest and lowest rates of HPV detection since certain techniques such as FISH, SB and HB have been employed in very few studies and PCR which has been used in 76% of studies has also shown variable results between studies. While general trends have reported higher HPV DNA detection rates in OSCC tissue from high incidence OSCC regions, the results of our review did not demon- strate this pattern. Thirty-four percent of all OSCC sam- ples, sourced predominantly from the highest OSCC in- cidence region of Henan Province in eastern China, were positive for HPV DNA. However, we found that other high-incidence areas within China from the north-west- ern counties in Xinjiang, to Guandong in the south-east, Hebei in the north and the central Province of Shaanxi have reported even higher rates of HPV DNA detection in OSCC tissue. Of note is a study carried out by Ko shiol where only 3/267 OSCC samples tested using PCR, were positive for HPV [61]. This is one of the largest studies to be carried out and th eir result is particu larly interestin g as it has been conducted in Linxian, Henan province [30]. This result is not in keeping with the generally observed trends of high HPV detection rates in high-risk OSCC populations. Furthermore, the three studies in China which did not isolate HPV from OSCC, also recruited subjects residing in Henan province [52-54]. A recent study by Furrer et al. reported higher rates of HPV DNA detection in superficial oral scrapes compared to deep tissue biopsies from patients with oral cancer, in an Argentinian cohort [114]. They suggest that th e site of specimen sampling is important in obtaining an accurate epidemiological picture on the HPV link to carcinogene- sis. This hypothesis may be extended to oesophageal cancer and we therefore aimed to examine whether the location from which the oesophageal specimen was taken in OSCC patients, i.e. superficial scrapings or deep tissue biopsy, may have any correlation with HPV detection rate. We found that 35 % of deep tissue biopsies and 41% of superficial scrapings from OSCC patients were HPV positive. This finding is consistent with results reported by Furrer et al. With the growing evidence that the spectrum of HPV- related malignancies may spread beyond cancers of the anogenital tract [1], the global health burden attributable to HPV continues to increase. As a result, there has been increasing pressure to make the HPV vaccines more widely available for males, thereby immunizing entire cohorts against the effects of this virus. If HPV plays a significant role in the aetiology of OSCC, the introduc- tion of prophylactic HPV vaccines could have a public health impact in a nation such as China where OC is one of the leading causes of malignancy-related mortality. In many developed countries public health funding for the prophylactic HPV vaccines is available for girls and young women prior to their sexual debut [115]. However, in China, there are currently no national programs for cervical cancer screening and a majority of women have never been screened. Consequently, at present, the pro- phylactic HPV vaccines have not been licensed for dis- tribution in China. Population-based surveys of Chinese women have been carried out recently to identify potential difficulties in the implementation of a prophylactic vaccination pro- gram in China [116,117]. One of the largest obstacles is the price of the vaccination, should government funding be insufficient to cover costs [116,117]. Prophylactic HPV vaccines are to date the most expensive vaccine developed with a retail price of US $120 per dose of Gardasil® (US $360 for the complete course) excluding administrative costs [118]. Other problems include rural habitation with poor access to health services, cultural and religious barriers, personal attitudes and beliefs as well as limited knowledge of HPV and vaccination [116, 117]. However, the introduction of the Hepatitis B vac- cine (HBV) to protect against hepatocellular carcinoma (HCC) in China may provide a template upon which the prophylactic HPV vaccination program can be modeled [117]. The first national HBV program was instigated in Taiwan in 1984 [119]. Over a 10 year period from 1984 to 1994, follow-up studies in ch ild ren und er the ag e of 15, demonstrated a reduction in HBsAg prevalence rates Copyright © 2013 SciRes. AID  The Relationship between Human Papillomavirus and Oesophageal Squamous Cell Carcinoma in China—A Review of the Evidence 28 from 9·8% to 1.3% [120]. A recent cross-sectional sero- prevalence study by Chang et al. also reported a statisti- cally significant reduction in incidence of HCC in a co- hort of vaccinated children aged 6-19 years, compared to a comparable unvaccinated group [119]. From the results of this review, we determine that HPV DNA was found in over one third of OSCC tissue samples, compared to cervical cancer where HPV is re- sponsible for the pathogenesis of 100% of lesions. Thus the impact of prophylactic vaccination would be consid- erably higher in cervical cancers than in OSCC, if a link exists. However, it is important to clarify any HPV- OSCC association as even a 20% - 30% rate of HPV in- fection as a causal co-factor would be significantly im- pacted by vaccination, particularly in a geographically targeted vaccination program. 5. Conclusions We found that 36% of all OSCC samples and 28% of all healthy control samples tested from the Chinese popula- tion were positive for HPV DNA and the majority (11/16) of case-control and cross-sectional studies found a statis- tically significant association between HPV and OSCC. The findings of this review are in line with the hy- pothesis that HPV detection rates are higher in superfi- cial oesophageal cancer samples compared deep tissue specimens [114]. It may therefore be important to con- sider depth of tissue biopsy when interpreting epidemi- ological studies assessing HPV aetiology in malignancy. Research carried out over the last 30 years has neither precluded nor established HPV as an aetiological factor in OSCC. The difficulty in determining a link may be due to several factors including 1) the poor methodolo- gical design and generally smaller sample sizes in a ma- jority of studies. Only few case-contro l studies have ever been done, with the vast majority of studies on the sub- ject being case-series, which are unable to adequately address the question of aetiology or risk factors. The fact that none of the identified case-control studies included statistical measures of association, even when data were collected to enable these measures, indicates the problem of poor study design; 2) the utilization of many different HPV detection methods with varying specificity and sen- sitivity ranges i.e. PCR with either general or consensus primers which identify different HPV genotypes, histo- logical biopsy (HB), IHC, ISH, FISH, general primer (GP), consensus primer (CP), serological testing, hybrid capture; 3) inter-laboratory deviation on similar testing methodology; 4) utilization of various types of specimens i.e. balloon cell samples, OSCC tissue from resections or biopsies which may be either fresh or archival; 5) varia- tion within tissue samples examined; 6) differences in histopathological classification and tissue storage i.e. Iodine staining, paraffin samples; 7) the presence of po- tential co-factors (e.g. smoking, opium abuse, nutritional deficiencies, ingestion of nitrosamines and exposure to other industrial chemicals) which may be more important depending on geographical location, could act synergis- tically with HPV to promote infection of oesophageal tissue; 8) the possible “hit and run” mechanism proposed by Campo and modeled on observations of bovine papil- lomavirus type 4 (BPV-4) infection of bovine oesophag- eal tissue [121]; 9) genetic polymorphisms facilitating malignant transformation [122]. Despite the many factors which could be responsible for the high variability of results reported, it remains that an equal potential for inconsistency with similar vari- ables existed in investigations carried ou t to establish the role of HPV in cervical and other HPV-related cancers, which have yielded more convincing results. Therefore, it may be inferred that if a link does exist between HPV and OSCC, it may be weaker than in other HPV-related cancer, or geographically varied and related to other co- factors [52]. A meta-analysis of existing case-control studies as well as further large-scale case-control studies with ade- quate statistical power are required to more meanin gfully address whether a causal relationship between HPV and OSCC exists. The introduction of the prophylactic HPV vaccines has made it even more important to definitively determine the answer to this research question, particu- larly for countries such as China, where there is a sig- nificant cancer burden from oesophageal malignancy. REFERENCES [1] K. Syrjänen, “HPV and Oesophageal Carcinoma,” In: Papillomavirus Research: From Natural History to Vac- cines and Beyond,” Caister Academic Press, Norfolk, 2006, pp. 229-253. [2] “Smokeless Tobacco and Some Tobacco-Specific N-Ni- trosamines,” IARC Monographs on the Evaluation of Ca r- cinogenic Risks to Humans, Vol. 89, World Health Or- ganisation and International Agency for Research on Cancer, Lyon, 2007. [3] G. Brooks, K. Carroll, J. Butel, S. Morse and T. Mietzner, “Human Cance r Viruses,” In: M. A. Jawetz, Ed., Medical Microbiology, 24th Edition, The McGraw-Hill Compa- nies, New York, 2007, pp. 597-600. [4] P. Day and J. Schiller, “Early Events in the Papillomavi- ral Lifecycle,” In: C. M. Norfolk, Ed., Papillomavirus Research, Caister Academic Press, Norfolk, 2006, pp. 175-192. [5] E. de Villiers, C. Fauquet, T. Broker, B. Hu and H. zur Hausen, “Classification of Papillomaviruses,” Virology, Vol. 324, No. 1, 2004, pp. 17-27. doi:10.1016/j.virol.2004.03.033 [6] J. Ferlay, H. Shin, F. Bray , D. Forman, C. Ma thers and D. Copyright © 2013 SciRes. AID  The Relationship between Human Papillomavirus and Oesophageal Squamous Cell Carcinoma in China—A Review of the Evidence 29 Parkin, “GLOBOCAN 2008, Cancer Incidence and Mor- tality Worldwide: IARC Cancer Base,” No. 10, Interna- tional Agency for Research on Cancer, Lyon, 2010. [7] D. M. Parkin, F. Bray, J. Ferlay and P. Pisani, “Global Cancer Statistics, 2002,” CA: A Cancer Journal for Clini- cians, Vol. 55, No. 2, 2005, pp. 74-108. doi:10.3322/canjclin.55.2.74 [8] H. zur Hausen, “Human Papillomaviruses in the Patho- genesis of Anogenital Cancer,” Virology, Vol. 184, No. 1, 1991, pp. 9-13. doi:10.1016/0042-6822(91)90816-T [9] H. Romanczuk and P. Howley, “Disruption of Either the E1 or the E2 Regulatory Gene of Human Papillomavirus Type 16 Increases Viral Immortalization Capacity,” Pro- ceedings of the National Academy of Sciences, Vol. 89, No. 7, 1992, pp. 3159-3163. [10] L. F. Garrote, R. Herrero, R. M. O. Reyes, S. Vaccarella, J. L. Anta, L. Ferbeye, N. Munoz and S. France schi, “R isk Factors for Cancer of the Oral Cavity and Oro-Pharynx in Cuba,” British Journal of Cancer, Vol. 85, No. 1, 2001, pp. 46-54. doi:10.1054/bjoc.2000.1825 [11] A. M. Hong, A. E. Grulich, D. Jones, C. S. Lee, S. M. Garland, T. A. Dobbins, J. R. Clark, G. B. Harnett, C. G. Milross, C. J. O’Brien, et al., “Squamous Cell Carcinoma of the Oropharynx in Australian Males Induced by Hu- man Papillomavirus Vaccine Targets,” Vaccine, Vol. 28, No. 19, 2010, pp. 3269-3272. doi:10.1016/j.vaccine.2010.02.098 [12] R. K. Aimee, J. A. Anthony, R. Daniel, E. G. Patti, R. Viscidi, S. G. Elizabeth, V. S. Keerti and L. Gillison Maura, “Oral Human Papillomavirus Infection in Adults Is Associated with Sexual Behavior and HIV Serostatus,” The Journal of Infectious Diseases, Vol. 189, No. 4, 2004, pp. 686-698. doi:10.1086/381504 [13] C. H. Shiboski, B. L. Schmidt and R. C. K. Jordan, “Ton- gue and Tonsil Carcinoma,” Cancer, Vol. 103, No. 9, 2005, pp. 1843-1849. doi:10.1002/cncr.20998 [14] E. Smith, S. Johnson, T. Cripe, S. Pignatari and L. Turek, “Perinatal Vertical Transmission of Human Papillomavi- rus and Subsequent Development of Respiratory Tract Papillomatosis,” Annals of Otology, Rhinology and Lar- yngology, Vol. 100, No. 6, 1991, pp. 478-483. [15] F. Chang, S. Syrjanen, Q. Shen, L. Wang, D. Wang and K. Syrjänen, “Human Papillomavirus (HPV) Involvement in Esophageal Precancerous Lesions and Squamous Cell Carcinomas as Evidenced by Microscopy and Different DNA Techniques,” Scandinavian Journal of Gastroen- terology, Vol. 27, No. 7, 1992, pp. 553-563. doi:10.3109/00365529209000119 [16] M. Curado, B. Edwards, H. Shin, H. Storm, J. Ferlay, M. Heanue and P. Boyle, “Cancer Incidence in Five Conti- nents,” International Agency for Research on Cancer, Lyon, 2007. [17] S. Mudan and J. Kang, “Epidemiology and Clinical Pres- entation in Esophageal Cancer,” In: S. Rankin, Ed., Car- cinoma of the Esophagus, Cambridge University Press, New York, 2008, pp. 1-10. [18] S. J. Spechler, “Squamous Cell Carcinoma of the Esopha- gus,” In: W. Scheppach, R. Bresalier and G. Tytgat, Eds., Gastrointestinal and Liver Tumors, Springer-Verlag, Ber- lin, 2004, pp. 5-11. doi:10.1007/978-3-642-18629-5_1 [19] Z. Wang, L. Tang, G. Sun, Y. Tang, Y. Xie, S. Wang, X. Hu, W. Gao, S. Cox and J.-S. Wang, “Etiological Study of Esophageal Squamous Cell Carcinoma in an Endemic Region: A Population-Based Case Cont rol Study in H ua i an, China,” BMC Cancer, Vol. 6, No. 1, 2006, p. 287. doi:10.1186/1471-2407-6-287 [20] D. Parkin, P. Pisani and J. Ferlay, “Estimates of World- wide Incidence of 25 Major Cancers in 1990,” Interna- tional Journal of Cancer, Vol. 80, No. 6, 1990, pp. 827- 841. doi:10.1002/(SICI)1097-0215(19990315)80:6<827::AID- IJC6>3.0.CO;2-P [21] Esophageal Cancer. http://www.merckmanuals.com/home/sec09/ch131/ch131 c.html [22] J. Ferlay, H. R. Shin, F. Bray , D. Forman, C. Mathers and D. M. Parkin, “Estimates of Worldwide Burden of Cancer in 2008: GLOBOCAN 2008,” International Journal of Cancer, Vol. 127, No. 12, 2010, pp. 2893-2917. doi:10.1002/ijc.25516 [23] P. Zhao, M. Dai, W. Chen and N. Li, “Cancer Trends in China,” Japanese Journal of Clinical Oncology, Vol. 40, No. 4, 2010, pp. 281-285. doi:10.1093/jjco/hyp187 [24] H. Zhang, Z. Chen, J. Cheng, X. Zhu, W. Guo, A. Hu, Y. Du, Y. Zhou and Y. Wang, “The High Incidence of Eso- phageal Cancer in Parts of China May Result Primarily from Genetic Rather than Environmental Factors,” Dis- eases of the Esophagus, Vol. 23, No. 5, 2010, pp. 392- 397. [25] D. He, D.-K. Zhang, K.-Y. Lam, L. Ma, H. Y.-S. Ngan, S. S. Liu and S.-W. Tsao, “Prevalence of HPV Infection in Esophageal Squamous Cell Carcinoma in Chinese Pa- tients and Its Relationship to the p53 Gene Mutation,” In- ternational Journal of Cancer, Vol. 72, No. 6, 1997, pp. 959-964. doi:10.1002/(SICI)1097-0215(19970917)72:6<959::AID- IJC7>3.0.CO;2-O [26] F. Kamangar, R. Malekzadeh, S. Dawsey and F. Saidi, “Esophageal Cancer in Northeastern Iran: A Review,” Ar- chives of Iranian Medicine, Vol. 10, No. 1, 2007, pp. 70- 82. [27] S. Bai, S. Zhang and B. Li, “Primary Esophageal Adeno- carcinoma,” Chinese Journal of Oncology, Vol. 11, No. 5, 1989, pp. 383-385. [28] S. S. Liyanage, E. Segelov, S. M. Garland, S. N. Tabrizi, H. Seale, P. J. Crowe, D. E. Dwyer, A. Barbour, A. T. Newall, A. Malik, et al., “The Role of Human Papillo- maviruses in Esophageal Squamous Cell Carcinoma,” Asia-Pacific Journal of Clinical Oncology, Vol. 9, No. 1, 2013, pp. 12-28. [29] K. Syrjänen, “HPV Infections and Oesophageal Cancer,” Journal of Clinical Pathology, Vol. 55, No. 10, 2002, pp. 721-728. doi:10.1136/jcp.55.10.721 [30] L. Ke, “Mortality and Incidence Trends from Esophagus Cancer in Selected Geographic Areas of China Circa 1970-90,” International Journal of Cancer, Vol. 102, No. Copyright © 2013 SciRes. AID  The Relationship between Human Papillomavirus and Oesophageal Squamous Cell Carcinoma in China—A Review of the Evidence 30 3, 2002, pp. 271-274. doi:10.1002/ijc.10706 [31] Y. Qin, L. Wang, Z. Fan, D. Kwong and X. Guan, “Com- parative Genomic Hybridization Analysis of Genetic Ab- errations Associated with Development of Esophageal Squamous Cell Carcinoma in Henan, China,” World Jour- nal of Gastroenterology, Vol. 14, No. 12, 2008, pp. 1828- 1835. doi:10.3748/wjg.14.1828 [32] C. Yang, “Research on Esophage al Cancer in China: A R e- view,” Cancer Research, Vol. 40, No. 8, 1980, pp. 2633- 2644. [33] X. Castellsagué, N. Muñoz, E. De Stefani, C. Victora, R. Castelletto, P. Rolón and M. Quintana, “Independent and Joint Effects of Tobacco Smoking and Alcohol Drinking on the Risk of Esophageal Cancer in Men and Women,” International Journal of Cancer, Vol. 82, No. 5, 1999, pp. 657-664. doi:10.1002/(SICI)1097-0215(19990827)82:5<657::AID- IJC7>3.0.CO;2-C [34] C. Han, G. Qiao, N. L. Hubbert, L. Li, C. Sun, Y. Wang, M. Yan, D. Xu, Y. Li, D. R. Lowy, et al., “Serologic As- sociation between Human Papillomavirus Type 16 Infec- tion and Esophageal Cancer in Shaanxi Province, China,” Journal of the National Cancer Institute, Vol. 88, No. 20, 1996, pp. 1467-1471. doi:10.1093/jnci/88.20.1467 [35] J. Lagergren, Z. Wang, R. Bergstrom, J. Dillner and O. Nyren, “Human Papillomavirus Infection and Esophageal Cancer: A Nationwide Seroepidemiologic Case-Control Study in Sweden,” Journal of the National Cancer Insti- tute, Vol. 91, No. 2, 1999, pp. 156-162. doi:10.1093/jnci/91.2.156 [36] C. Bosetti, C. La Vecchia, R. Talamini, L. Simonato, P. Zambon, E. Negri, D. Trichopoulos, P. Lagiou, R. Bar- dini and S. Franceschi, “Food Groups and Risk of Squa- mous Cell Esophageal Cancer in Northern Italy,” Journal of Cancer, Vol. 87, No. 2, 2000, pp. 289-294. [37] G. Launoy, C. Milan, N. E. Day, M. P. Pienkowski, M. Gignoux and J. Faivre, “Diet and Squamous-Cell Cancer of the Oesophagus: A French Multicentre Case-Control Study,” International Journal of Cancer, Vol. 76, No. 1, 1998, pp. 7-12. doi:10.1002/(SICI)1097-0215(19980330)76:1<7::AID-IJ C2>3.0.CO;2-4 [38] A. Ghavamzadeh, A. Moussavi, M. Jahani, M. Rastegar- panah and M. Iravani, “Esophageal Cancer in Iran,” Semi- nars in Oncology, Vol. 28, No. 2, 2001, pp. 153-157. doi:10.1016/S0093-7754(01)90086-7 [39] F. Islami, P. Boffetta, J.-S. Ren, L. Pedoeim, D. Khatib an d F . Kamang a r , “Hi g h- T e mp e r a tu re Beverages and Foods and Esophageal Cancer Risk—A Systematic Review,” In- ternational Journal of Cancer, Vol. 125, No. 3, 2009, pp. 491-524. doi:10.1002/ijc.24445 [40] F. Chang, S. Syrjänen, L. Wang and K. Syrjänen, “Infec- tious Agents in the Etiology of Esophageal Cancer,” Gas- troenterology, Vol. 103, No. 4, 1992, pp. 1335-1348. [41] V. Del, M. White, S. Enam, S. Oviedo, M. Bromer, R. Thomas, H. Parkman and K. Khalili, “Detection of JC Virus DNA Sequences and Expression of Viral T Antigen and Agnoprotein in Esophageal Carcinoma,” Cancer, Vol. 103, No. 3, 2005, pp. 516-527. doi:10.1002/cncr.20806 [42] M. Poljak, A. Cerar and K. Seme, “Human Papillomavi- rus Infection in Esophageal Carcinomas: A Study of 121 lesions Using Multiple Broad-Spectrum Polymerase C hain Reactions and Literature Review,” Human Pathology, Vol. 29, No. 3, 1998, pp. 266-271. doi:10.1016/S0046-8177(98)90046-6 [43] M. Selgrad, P. Malfertheiner, L. Fini, A. Goel, C. R. Boland and L. Ricciardiello, “The Role of Viral and Bac- terial Pathogens in Gastrointestinal Cancer,” Journal of Cellular Physiology, Vol. 216, No. 2, 2008, pp. 378-388. doi:10.1002/jcp.21427 [44] M. Sur and K. Cooper, “The Role of the Human Papillo- mavirus in Esophageal Cancer,” Pathology, Vol. 30, No. 4, 1998, pp. 358-354. doi:10.1080/00313029800169616 [45] M. Tandi Matsha, R. Erasmus, A. Kafuko, D. Mugwanya, A. Stepien and M. I. Parker, “Human Papillomavirus As- sociated with Cancer,” Journal of Clinical Pathology, Vol. 55, No. 8, 2002, pp. 587-590. doi:10.1136/jcp.55.8.587 [46] K. Syrjänen, “Histological Changes Identical to Those of Condylomatous Lesions Found in Esophageal Squamous Cell Carcinomas,” Archiv für Geschwulstforschung, Vol. 52, No. 4, 1982, pp. 283-292. [47] L. Li, “Morbidity and Mortality of Malignant Tumours in Some Chinese Cities and Counties,” First Edition, Peo- ple’s Medical Publishing House, Beijing, 2007. [48] C. Hennekens and J. Buring, “Case-Control Studies,” In: S. M. Boston, Ed., Epidemiology in Medicine, First Edi- tion, Little, Brown and Company, Toronto, 1987, pp. 132- 152. [49] R. Friis and T. Sellers, “Study Designs: Ecologic, Cross- Sectional, Case-Control,” In: Epidemiology for Public Health Practice, Fourth Edition, Jones and Bartlett Pub- lishers, LLC, Sudbury, 2009, pp. 262-275. [50] A. Dean, T. Arner, G. Sunki, R. Friedman, M. Lantinga, S. Sangam, J. Zubieta, K. Sullivan, K. Brendel, Z. Gao, et al., “Epi Info, a Database and Statistics Program for Pub- lic Health Professionals,” 3.5.3 Edition, Centres for Dis- eas Control and Prevention (CDC), Atlanta, 2007. [51] M. Mori, R. Shimono, T. Inoue, H. Kuwano, K. Sugima- chi and R. Zhang, “Papillomavirus and Esophageal Can- cer in the Japanese and Chinese,” The American Journal of Gastroenterology, Vol. 84, No. 9, 1989, pp. 1126- 1127. [52] G.-F. Gao, M. J. Roth, W.-Q. Wei, C. C. Abnet, F. Chen, N. Lu, F.-H. Zhao, X.-Q. Li, G.-Q. Wang, P. R. Taylor, et al., “No Association between HPV Infection and the Neo- plastic Progression of Esophageal Squamous Cell Carci- noma: Result from a Cross-Sectional Study in a High- Risk Region of China,” International Journal of Cancer, Vol. 119, No. 6, 2006, pp. 1354-1359. [53] S. Lu, F. Luo and H. Li, “Detection of Human Papillo- mavirus in Esophageal Squamous Cell Carcinoma and Adjacent Tissue Specimens in Linxian,” Chinese Journal of Oncology, 1995, Vol. 17, No. 5, pp. 321-324. [54] C.-S. Song, J.-H. Cui, Z.-P. Wu, G. Yang and J.-J. Tan, “Relationship between Detection pf Human Papilloma Copyright © 2013 SciRes. AID  The Relationship between Human Papillomavirus and Oesophageal Squamous Cell Carcinoma in China—A Review of the Evidence 31 Virus Genotypes and Esophageal Squamous Cell Carci- noma,” Chinese Journal of Cancer Prevention and Treat- ment, Vol. 16, No. 5, 2009, pp. 329-331. [55] B. Cao, X. Tian, Y. Li, P. Jiang, T. Ning, H. Xing, Y. Zhao, C. Zhang, X. Shi, D. Chen, et al., “LMP7/TAP2 Gene Polymorphisms and HPV Infection in Esophageal Carcinoma Patients from a High Incidence Area in China,” Carcinogenesis, Vol. 26, No. 7, 2005, pp. 1280- 1284. [56] B. Chen, “Polymerase Chain Reaction in Detection of Human Papillomavirus DNA in Esophageal Carcinoma,” Chinese Medical Journal, Vol. 73, No. 11, 1993, pp. 667- 669. [57] B. Chen, H. Yin and N. Dhurandhar, “Detection of Hu- man Papillomavirus DNA in Esophageal Squamous Cell Carcinomas by the Polymerase Chain Reaction Using General Consensus Primers,” Human Pathology, Vol. 25, No. 9, 1994, pp. 920-923. [58] M. Dai, W. Zhang, G. Clifford, T. Gheit, B. He, K. Mi- chael, T. Waterboer, P. Hainaut, M. Tommasino and S. Franceschi, “Human Papillomavirus Infection among 100 Oesophageal Cancer Cases in the People’s Republic of China,” International Journal of Cancer, Vol. 121, No. 6, 2007, pp. 1396-1398. [59] E. de Villiers, D. Lavergne, F. Chang, K. Syrjänen, P. Tosi, M. Cintorino, R. Santopietro and S. Syrjänen, “An Interlaboratory Study to Determine the Presence of Hu- man Papillomavirus DNA in Esophageal Carcinoma from China,” International Journal of Cancer, Vol. 81, No. 2, 1999, pp. 225-228. [60] D. He, S. Tsao and H. Bu, “Human Papillomavirus Infec- tion and Esophageal Squamous Cell Carcinoma,” Chinese Journal of Pathology, Vol. 25, No. 6, 1996, pp. 351-354. [61] J. Koshiol, W. Q. Wei, A. R. Kreimer, W. Chen, P. Gravitt, J. S. Ren, C. C. Abnet, J. B. Wang, F. Kamangar, D. M. Lin, et al., “No Role for Human Papillomavirus in Esophageal Squamous Cell Carcinoma in China,” Inter- national Journal of Cancer, Vol. 127, No. 1, 2010, pp. 93-100. [62] D. Lavergne and E. M. de Villiers, “Papillomavirus in Esophageal Papillomas and Carcinomas,” International Journal of Cancer, Vol. 80, No. 5, 1999, pp. 681-684. [63] S. Li, Y. Li, L. Wang, X. Wu, L. Zhou, X. Zhao, H. Liu and Y. Zeng, “Detection of Human Papillomavirus in Tissues of Esophageal Carcinomas by Polymerase Chain Reaction,” Chinese Journal of Experimental and Clinical Virology, Vol. 22, No. 4, 2008, pp. 251-253. [64] T. Li, Z. M. Lu, M. Guo, Q. J. Wu, K. N. Chen, H. P. Xing, Q. Mei and Y. Ke, “p53 Codon 72 Polymorphism (C/G) and the Risk of Human Papillomavirus-Associated Carcinomas in China,” Cancer, Vol. 95, No. 12, 2002, pp. 2571-2576. [65] T. Li, Z.-M. Lu, K.-N. Chen, M. Guo, H. -P. Xing, Q. Mei, H.-H. Yang, J. F. Lechner and Y. Ke, “Human Papillo- mavirus Type 16 is an Important Infectious Factor in the High Incidence of Esophageal Cancer in Anyang Area of China,” Carcinogenesis, Vol. 22, No. 6, 2001, pp. 929- 934. [66] P. Liao, J. Qin, T. Zeng, F. Li, J. Cai and L. He, “A 1:2 Matched Case-Control Study on the Interaction of HPV16E6 and HLA-DR9 Allele to Esophageal Cancer in Kazakh Ethnicity, Xinjiang,” Chinese Journal of Epide- miology, Vol. 30, No. 9, 2009, pp. 951-954. [67] M. Liu, H. Zeng, X. Zhang, J. Zhu, J. Huang, X. Zhang and M. Xia, “Study on Human Papillomavirus Infection and Loss of Heterozygosity of Microsatellite in Esophag- eal Cancer,” Chinese Journal of Epidemiology, Vol. 28, No. 12, 2007, pp. 1203-1206. [68] Y. Liu, X. Li, G. Jin, X. Yan, J. Yang, J. Wang, Y. Li, F. Wang and X. Zhang, “HPV Detection and FHIT Expres- sion in Esophageal Squamous Carcinoma from High In- cidence Area in Cixian County,” Chinese Journal of Can- cer, Vol. 22, No. 5, 2003, pp. 492-495. [69] X. Lu, Y. Zhang, R. Lin, X. Liang, Y. Zhang, X. Wang, Y. Zhang, Y. Wang and H. Wen, “p53 Polymorphism in Human Papillomavirus-Associated Kazakh’s Esophageal Cancer in Xinjiang, China,” World Journal of Gastroen- terology, Vol. 10, No. 19, 2004, pp. 2775-2778. [70] X. M. Lu, S. Monnier-Benoit, L. Z. Mo, S. Y. Xu, J. L. Prétet, Z. Liu, D. A. Vuitton and C. Mougin, “Human Papillomavirus in Esophageal Squamous Cell Carcinoma of the High-Risk Kazakh Ethnic Group in Xinjiang, China,” European Journal of Surgical Oncology, Vol. 34, No. 7, 2008, pp. 765-770. [71] Z. Lu, K. Chen and M. Guo, “Detection of HPV in Hu- man Oesophageal Cancer in High Incidence Area and Its Correlation with p53 Expression,” Chinese Journal of Oncology, Vol. 23, No. 3, 2001, pp. 220-223. [72] D. Peixoto Guimaraes, S. Hsin Lu, P. Snijders, R. Wil- motte, R. Herrero, G. Lenoir, R. Montesano, C. J. L. M. Meijer, J. Walboomers and P. Hainaut, “Absence of As- sociation between HPV DNA, TP53 Codon 72 Polymor- phism, and Risk of Oesophageal Cancer in a High-Risk Area of China,” Can c er Letters, Vol. 162, No. 2, 2001, pp. 231-235. [73] Z. Y. Shen, S. P. Hu, L. C. Lu, C. Z. Tang, Z. S. Kuang, S. P. Zhong and Y. Zeng, “Detection of Human Papillo- mavirus in Esophageal Carcinoma,” Journal of Medical Virology, Vol. 68, No. 3, 2002, pp. 412-416. [74] K. Shuyama, A. Castillo, F. Aguayo, Q. Sun, N. Khan, C. Koriyama and S. Akiba, “Human Papillomavirus in High- and Low-Risk Areas of Oesophageal Squamous Cell Car- cinoma in China,” British Journal of Cancer, Vol. 96, No. 10, 2007, pp. 1554-1559. [75] H. Si, S. Tsao, C. Poon and A. Cheung, “Human Papil- lomavirus Infection and Loss of Heterozygosity in Eso- phageal Squamous Cell Carcinoma,” Cancer Letter, Vol. 213, No. 2, 2004, pp. 231-239. [76] H. X. Si, S. W. Tsao, C. S. P. Poon, L. D. Wang, Y. C. Wong and A. L. Cheung, “Viral Load of HPV in Eso- phageal Squamous Cell Carcinoma,” International Jour- nal of Cancer, Vol. 103, No. 4, 2003, pp. 496-500. [77] L. Suzuk, A. Noffsinger, Y. Hui and C. Fenoglio-Preiser, “Detection of Human Papillomavirus in Esophageal Squa- mous Cell Carcinoma,” Cancer, Vol. 78, No. 4, 1996, pp. 704-710. Copyright © 2013 SciRes. AID  The Relationship between Human Papillomavirus and Oesophageal Squamous Cell Carcinoma in China—A Review of the Evidence 32 [78] W. Xu, L. Zhang, Z. Lu, J. Li, Y. Ke and G. Xu, “Detec- tion of Human Papillomavirus Type 16 E6 mRNA in Carcinomas of the Upper Digestive Tract,” Chinese Medi- cal Journal, Vol. 83, No. 21, 2003, pp. 1910-1914. [79] X. Zhao, S. Li, Y. Li, X. Wang, Y. Li, X. Wu, L. Zhou, H. Liu and Y. Zeng, “Detection of Human Papillomavirus in Esophageal Carcinoma Tissues from Baoding City of Hebei Province,” Chinese Journal of Experimental and Clinical Virology, Vol. 23, No. 2, 2009, pp. 91-93. [80] X. Zhou, M. Guo, L. Quan, W. Zhang, Z. Lu, Q. Wang, Y. Ke and N. Xu, “Detection of Human Papillomavirus in Chinese Esophageal Squamous Cell Carcinoma and Its Adjacent Normal Epithelium,” World Journal of Gastro- enterology, Vol. 9, No. 6, 2003, pp. 1170-1173. [81] Y. Zhou, Y. Pan, S. Zhang, X. Shi, T. Ning and Y. Ke, “Increased Phosphorylation of p70 S6 Kinase Is Associ- ated with HPV16 Infection in Cervical Cancer and Eso- phageal Cancer,” British Journal of Cancer, Vol. 97, No. 2, 2007, pp. 218-222. [82] F. Chang, S. Syrjänen, Q. Shen, M. Cintorino, R. San- topietro, P. Tosi and K. Syrjänen, “Detection of HPV DNA in Esophageal Squamous Cell Carcinoma from the High Incidence Area of Linxian, China,” Scandinavian Journal of Gastroenterology, Vol. 35, No. 2, 2000, pp. 123-130. [83] J. Chen, Y. Zhou, J. Li and P. Wu, “Effect of HPV16 Infection on the Esophageal Carcinoma and the Gene Ex- pressions of p53 and p16,” China Journal of Laboratory Diagnosis, Vol. 8, No. 2, 2004, pp. 182-185. [84] L. Chen, L. Yang, Z. Sun, H. Zhang, T. Ren, B. Chang, L. Pang, Y. Qi, H. Li, J. Jiang, et al., “Correlation between HPV16 and Esophageal Cancer of Kazakh People in Xin- jiang,” Journal of Nongken Medicine, Vol. 30, No. 1, 2008, pp. 15-22. [85] M. Duan, R. Tian and X. Cao, “The Relationship between HPV and Esophageal Carcinoma,” Journal of Practical Medical Techniques, Vol. 14, No. 1A, 2005, pp. 11-12. [86] L. Y. Gu, L. M. Sai, X. L. Hou, G. Chen, F. Li, C. X. Liu, Y. Z. Chen, B. Chang, L. Yang, W. H. Liang, et al., “Correlation between HPV16 Infection and Esophageal Carcinoma of Kazakh People in Xinjiang,” China On- cology, Vol. 21, No. 9, 2010, pp. 668-672. [87] B. He, G. Duan and L. Cai, “Study on the Relationship between High Risk HPV Infection and p53 Polymor- phisms in Esophageal Cancer,” Strait Journal of Preven- tive Medicine, Vol. 13, No. 3, 2007, pp. 8-10. [88] M. Liu, X. Zhang, W. Fu, J. Yang and X. Zhu, “Study of Association between HPV Infection and Squamous Cell Carinoma as well as Dysplastic Tissue of the Oesopha- gus,” China Journal of Modern Medicine, Vol. 18, No. 14, 2008, pp. 2084-2086. [89] W. Liu, X. Jiang, M. Zhang and Z. Zhang, “The Rela- tionship between HPV16 and Expression of Cyclooxy- genase-2, P53 and Their Prognostic Roles in Esophageal Squamous Cell Carcinoma,” European Journal of Gas- troenterology & Hepatology, Vol. 22, No. 1, 2010, pp. 67-74. doi:10.1097/MEG.0b013e32832c7e76 [90] X. Wang, X. Tian, F. Liu, Y. Zhao, M. Sun, D. Chen, C. Lu, Z. Wang, X. Shi, Q. Zhang, et al., “Detection of HPV DNA in Esophageal Cancer Specimens from Different Regions and Ethnic Groups: A Descriptive Study,” BMC Cancer, Vol. 10, No. 1, 2010, p. 19. doi:10.1186/1471-2407-10-19 [91] S. Zhou, I. Sheyhidin, T. Yang, L. Zhang, A. Hasim, X. Lu, M. Niyaz and T. Liu, “Relationship between Human Papillomavirus 16 and Esophageal Squamous Cell Car- cinoma in Uygur Population in Xinjiang Uygur Autono- mous Region,” World Chinese Journal of Digestology, 2009, Vol. 17, No. 31, pp. 3214-3217. [92] M.-C. Cui, Y. Li, X. He, X.-L. Wang, L.-D. Wang and H.-S. Liu, “Study of Human Papillomavirus in Biospy Tissue Specimens of Esophageal Carcinomas in Linzhou City,” Chinese Journal of Experimental and Clinical Vi- rology, Vol. 25, No. 1, 2011, pp. 39-41. [93] A. Goto, C.-P. Li, S. Ota, T. Niki, Y. Ohtsuki, S. Kitajima, S. Yonezawa, C. Koriyama, S. Akiba, H. Uchima, et al., “Human Papillomavirus Infection in Lung and Esophag- eal Cancers: Analysis of 485 Asian Cases,” Journal of Medical Virology, Vol. 83, No. 8, 2011, pp. 1383-1390. doi:10.1002/jmv.22150 [94] H. Kawaguchi, S. Ohno, K. Araki, M. Miyazaki, H. Saeki, M. Watanabe, S. Tanaka and K. Sugimachi, “p53 Poly- morphism in Human Papillomavirus-Associated Esopha- geal,” Cancer Cancer Researchearch, Vol. 60, 2000, pp. 2753-2755. [95] Q. Ma, H. Jiang, Y. Feng, X. Wang, Y. Zhou, K. Liu and Z. KJia, “Detection of Human Papillomavirus DNA in Squamus Cell Carcinoma of the Esophagus,” World Chi- nese Journal of Digestology, Vol. 8, No. 11, 2000, pp. 1218-1224. [96] L. Yang, L. Chen, Z.-Z. Sun, H.-Y. Zhang, X.-Y. Tian, Y. Qi, J. Zhu, L. Yang, J.-M. Qin and F. Li, “Analysis of Human Papillomavirus DNA Infection in Kazakh Eso- phageal Carcinomas in Xinjiang Province,” World Chi- nese Journal of Digestology, Vol. 15, No. 9, 2007, pp. 2114-2119. [97] G. Ding, L. Wang, S.-H. Li, C. Feng, Y.-R. Zhang, J.-L. Ren, Z. Fan, R. Wang, X. He and T. Guo, “Correlation between Papillomavirus Infection and Esophageal Squa- mous Cell Carcinoma and Gastric Adenocarcinoma in High-Incidence Area of Henan Province,” Chinese Jour- nal of Cancer Prevention and Treatment, Vol. 16, No. 4, 2009, pp. 252-255. [98] G.-C. Ding, J.-L. Ren, F.-B. Chang, J.-L. Li, L. Yaun, X. Song, S.-L. Zhou, T. Guo, Z.-M. Fa, Y. Zeng, et al., “Hu- man Papillomavirus DNA and p16 INK4A Expression in Concurrent Esophageal and Gastric Cardia Cancers,” World Journal of Gastroenterology, Vol. 16, No. 46, 2010, pp. 5901-5906. doi:10.3748/wjg.v16.i46.5901 [99] D. Zhang, Q. Zhang, L. Zhou, L. Huo, Y.-R. Zhang, Z. Shen and Y. Zhu, “Comparison of Prevalence, Viral Load, Physical Status and Expression of Human Papillimavi- rus-16, -18 and -58 in Esophageal and Cervical Cancer: A Case-Control Study,” BMC Cancer, Vol. 10, No. 650, 2010, p. 650. doi:10.1186/1471-2407-10-650 [100] C. Xu, X. Qian, X. Zhou, Q. Zhao and Y. Li, “Expression Copyright © 2013 SciRes. AID  The Relationship between Human Papillomavirus and Oesophageal Squamous Cell Carcinoma in China—A Review of the Evidence 33 HPV 16-E6 and E7 Oncoproteins in Squamous Cell Car- cinoma Tissues of Esophageal Cancer and Non-Cancer Tissues,” Chinese Journal of Oncology, Vol. 23, No. 2, 2004, pp. 165-168. [101] J. Liu, S. Qin and W. Zhang, “Re lationship between HPV - E6 p53 Protein and Esophageal Squamous Cell Carci- noma,” World Chinese Journal of Digestology, Vol. 8, No. 5, 2000, pp. 494-496. [102] F. Chang, S. Syrjänen, Q. Shen, H. Ji and K. Syrjänen, “Human Papillomavirus (HPV) DNA in Esophageal Pre- cancer Lesions and Squamous Cell Carcinomas from China,” International Journal of Cancer, Vol. 45, No. 1, 1990, pp. 21-25. doi:10.1002/ijc.2910450106 [103] Y. Li, G. Huang, H. Xiao, Y. Huang, T. Mao and W. Deng, “The Status of Human Papillomavirus 16 DNA in the Tissues of Human Esophagus Carcinoma,” Journal of West China University of Medical Sciences, Vol. 22, No. 2, 1991, pp. 157-160. [104] J. Li, Y. Zhang and D. Gao, “Study on the Interrelation- ship between Human Papilloma Virus Infection and Lan- gerhans Cells in Carcinogenesis of Esophagus,” Chinese Journal of Pathology, Vol. 25, No. 2, 1996, pp. 83-85. [105] F. Chang, S. Syrjänen, L. Wang, Q. Shen and K. Syrjänen, “p53 Overexpression and Human Papillomavirus (HPV) Infection in Oesophageal Squamous Cell Carcinomas De- rived from a High-Incidence Area in China,” AntiCancer Researchearch, Vol. 17, No. 1B, 1997, pp. 709-715. [106] L. Zhu, X. Su, K. Chen, R. Yang, H. Xing, J. Cui and Y. Ke, “Detection Rate of Human Papillomavirus-16 in Eso- phageal Squamous Cell Carcinoma from Different Chi- nese Populations,” Chinese Journal of Cancer, Vol. 24, No. 7, 2005, pp. 870-873. [107] P. Yao, G. Li, J. Li, H. Xia, X. Yang, H. Huang, Y. Fu, R. Wang, X. Wang and J. Sha, “Evidence of Human Papil- loma Virus Infection and Its Epidemiology in Esophageal Squamous Cell Carcinoma,” World Journal of Gastroen- terology, Vol. 12, No. 9, 2006, pp. 1352-1355. [108] F. Chang, S. Syrjänen, Q. Shen, L. Wang and K. Syrjänen, “Screening for Human Papillomavirus Infections in Eso- phageal Squamous Cell Carcinomas by in Situ Hybridiza- tion,” Cancer, Vol. 72, No. 9, 1993, pp. 2525-2530. doi:10.1002/1097-0142(19931101)72:9<2525::AID-CNC R2820720902>3.0.CO;2-L [109] P. Zhou, X.-Z. Wang, Z.-L. Zhu, J. Liu and X. Li, “Ex- pression of HPV16/18 in Esophageal Squamous Carci- noma in Taixing Area,” Journal of China Medical Uni- versity, Vol. 40, No. 2, 2011, pp. 172-174. [110] L. Jin and Q. Cao, “Expression and Effect of Human Pa- pillomavirus in Esophageal Carcinoma,” Chinese Journal of Gastroenterology, Vol. 12, No. 1, 2007, pp. 36-39. [111] Z. X. Qi, X. Xu, B. Zhang, M. Du, H. Yang, L. Zheng, J. Li and Z. Shen, “Relationship between HPV 16/18 and p53, p21WAF1, MDM2, Ki67 and Cyclin D1 Expression in Esophageal Squamous Cell Carcinoma: Comparative Study by Using Tissue Microarray Technology,” Experi- mental Oncology, Vol. 28, No. 3, 2006, pp. 235-240. [112] P. O. Fidalgo, M. L. Cravo, P. P. Chaves, C. N. Leitão and F. C. Mira, “High Prevalence of Human Papilloma- virus in Squamous Cell Carcinoma and Matched Normal Esophageal Mucosa. Assessment by Polymerase Chain Reaction,” Cancer, Vol. 76, No. 9, 1995, pp. 1522-1528. doi:10.1002/1097-0142(19951101)76:9<1522::AID-CNC R2820760904>3.0.CO;2-3 [113] T. Kahn, E. Schwarz and H. zur Hausen, “Molecular Cloning and Characterization of the DNA of a New Hu- man Papillomavirus (HPV 30) from a Laryngeal Carci- noma,” International Journal of Cancer, Vol. 37, No. 1, 1986, pp. 61-65. doi:10.1002/ijc.2910370111 [114] V. Furrer, M. Benitez, M. Furnes, H. Lanfranchi and N. Modesti, “Biopsy vs. Superficial Scraping: Detection of Human Papillomavirus 6, 11, 16, and 18 in Potentially Malignant and Malignant Oral Lesions,” Journal of Oral Pathology & Medicine, Vol. 35, No. 6, 2006, pp. 338- 344. doi:10.1111/j.1600-0714.2006.00423.x [115] HPV Vaccination, “Should it be Recommended or Re- quired?” Rapid Public Health Policy Response Project, Jacobs Institute of Women’s Health, School of Public Health and Health Services, The George Washington University, Washington DC, 2007. [116] J. Li, L. Li, J. Ma, L. Wei, M. Niyazi, C. Li, A. Xuh, J. Wanga, H. Lianga, J. Belinsoni, et al., “Knowledge and Attitudes about Human Papillomavirus (HPV) and HPV Vaccines among Women Living in Metropolitan and Ru- ral Regions of China,” Vaccine, Vol. 27, No. 8, 2009, pp. 1210-1215. doi:10.1016/j.vaccine.2008.12.020 [117] J. Shi, Y. Qiao, J. Smith, B. Dondog, Y. Bao, M. Dai, G. Clifford and S. Franceschi, “Epidemiology and Preven- tion of Human Papillomavirus and Cervical Cancer in China and Mongolia,” Vaccine, Vol. 26S, No. 12, 2008, pp. M53-M59. doi:10.1016/j.vaccine.2008.05.009 [118] http://www.merck.com/newsroom/press_releases/product/ 2006_0608.html [119] M. Chang, S. You, C. Chen, C. Liu, C. Lee, S. Lin, H. Chu, T. Wu, S. Yang, H. Kuo, et al., “Decreased Inciden- ce of Hepatocellular Carcinoma in Hepatitis B Vaccinees: A 20-Year Follow-Up Study,” Journal of the National Cancer Institute, Vol. 101, 2009, pp. 1348-1355. doi:10.1093/jnci/djp288 [120] H. Chen, M. Chang, Y. Ni, H. Hsu, P. Lee, C. Lee and D. Chen, “Seroepidemiology of Hepatitis B Virus Infection in Children: Ten Years of Mass Vaccination in Taiwan,” Journal of the American Medical Association, Vol. 276, No. 11, 1996, pp. 906-908. doi:10.1001/jama.1996.03540110060032 [121] M. Campo, “Papillomas and Cancer in Cattle,” Cancer Survivors, Vol. 6, No. 1, 1987, pp. 39-54. [122] D. Xing, W. Tan and D. Lin, “Genetic Polymorphisms and Susceptibility to Esophageal Cancer among Chinese Population,” Oncology Reports, Vol. 10, No. 5, 2003, pp. 1615-1623. [123] F. Chang, S. Syrjänen, Q. Shen, M. Cintorino, R. San- topietro, P. Tosi and K. Syrjänen, “Evaluation of HPV, CMV, HSV and EBV in Esophageal Squamous Cell Car- cinomas from a High-Incidence Area of China,” Anti- Cancer Research, Vol. 20, No. 5C, 2000, pp. 3935-3940. [124] X. Wang, X. Tian, F. Liu, Y. Zhao, M. Sun, D. Chen, C. Copyright © 2013 SciRes. AID  The Relationship between Human Papillomavirus and Oesophageal Squamous Cell Carcinoma in China—A Review of the Evidence Copyright © 2013 SciRes. AID 34 Lu, Z. Wang, X. Shi, Q. Zhang, et al., “Detection of HPV DNA in Esophageal Cancer Specimens from Different Regions and Ethnic Groups: A Descriptive Study,” BMC Cancer, Vol. 10, No. 19, 2010, p. 19. Abbreviations BPV: Bovine papillomavirus CICAMS: Cancer Institute & Hospital Chinese Academy of Medical Sciences CDC: Centre for Disease Control CNKI: Chinese National Knowledge Infrastructure CP: Consensus primer FISH: Filter in situ hybridisation GP: General primer HCC: Hepatocellular carcinoma HB: Histological biopsy HPV: Human papillomavirus IHC: Imm unoh i s t ochemistry ISH: In situ hybridisation IARC: International Agency on Research on Cancer OC: Oesophageal cancer OSCC: Oesophageal squamous cell carcinoma PCR: Polymerase chain reaction
|