Paper Menu >>
Journal Menu >>
 J. Biomedical Science and Engineering, 2010, 3, 1006-1012 JBiSE doi:10.4236/jbise.2010.310131 Published Online October 2010 (http://www.SciRP.org/journal/jbise/). Published Online October 20 10 in SciRes. http://www.scirp.org/journal/jbise Immunohistochemical characterization of the rabbit tracheal cartilages Richard D. Wemer1, Michael Detamore2, Robert A. Weatherly3 1Department of Otolaryngology-Head and Neck Surgery, Park Nicollet Clinics, St. Louis Park, USA 2Department of Chemical and Petroleum Engineering, University of Kansas, Kansas City, USA 3Department of Otolaryngology-Head and Neck Surgery, University of Kansas, Kansas City, USA Email: wemerr@parknicollet.com; detamore@ku.edu; rweatherly@kumc.edu Received 5 August 2010; revised 18 August 2010; accepted 27 August 2010. ABSTRACT The objective of this study was to immunohisto- chemically elucidate the major extracellular matrix constituents of rabbit tracheal cartilage. The impe- tus for this project is the need for crucial design and validation criteria for tissue engineering jux- taposed with the conspicuous lack of trachea ex- tracellular matrix data in the literature. Tracheal tissue specimens were harvested from New Zealand White rabbits, and were immunostained for colla- gen I, collagen II, aggrecan and decorin; and a Verhoeff-Van Gieson stain was performed to visu- alize elastin. The most striking result was the highly organized relationship between distinct fibrous (containing collagen I, decorin and elastin) and hyaline-like (containing collagen II and aggrecan) regions of the tracheal wall. The tracheal cartilage stained strongly with collagen II throughout, with periodic bands of aggrecan in the tracheal arches, meaning that there were areas void of aggrecan immunostaining alternating with areas with strong aggrecan immunostaining. In contrast, the periph- ery of the cartilage and the perichondrium itself exhibited strong collagen I staining and no collagen II staining. Elastin fibers and decorin were also detected along the periphery of the cartilage in the perichondrium and corresponded highly with the distribution of collagen I staining. The body of the rabbit trachea is therefore composed of a hya- line-cartilage structure primarily made of collagen II and bands of aggrecan, surrounded by a fibrous region composed of elastin and collagen I, indica- tive of a flexible tissue with distinct regions of compressive integrity. This information will be a valuable reference to future tissue engineering ef- forts in the creation of a biosynthetic substitute for laryngotracheal reconstruction. Keywords: Trachea; Collagen; Aggrecan; Decorin; Elastin. 1. INTRODUCTION The treatment of subglottic stenosis is a difficult clinical problem that may require invasive measures at both pri- mary and donor sites. After failure of more conservative measures, open airway surgery is often performed, which requires donor cartilage tissue to be harvested, usually a segment of autologous costal cartilage. Such procedures are complicated by long healing times, donor site complications including pain and pneumothorax, size and shape mismatches, and prolonged hospital stays. Tissue-engineered cartilage offers a viable alternative to these highly morbid procedures. Donor site complica- tions could be eliminated, and tissue could be shaped appropriately for specific defects in the tracheal wall. Tissue engineering attempts for the trachea are still pre- liminary, from a clinical perspective, at this time. Nev- ertheless, tracheal tissue engineering is a burgeoning field [1-13], and the level of urgency is high for provid- ing crucial characterization data for tissue engineers, both to develop new ideas for design strategies for reca- pitulating the native tracheal structure, and to elucidate validation criteria. We have used rabbits in previous tracheal defect re- construction studies [14-16], as rabbits are an ideal ani- mal model for early-stage in vivo investigations. A re- view of animal models for trachea research suggested that large animals such as goats and sheep should be used for tracheal tissue engineering based on size and cell number [17]. However, the rabbit has heretofore been a more commonly used animal model for tracheal tissue engineering and is a logical stepping stone en route to larger animal models. For this reason, we have selected the rabbit for our immunohistochemical charac-  R. D. Wemer et al. / J. Biomedical Science and Engineering 3 (2010) 1006-1012 Copyright © 2010 SciRes. JBiSE 1007 terization of the trachea. In contrast to the trachea, the extracellular matrix of the vocal folds of the larynx (which lie just superior to the trachea) continues to be a popular research topic, including studies of collagen types, elastin, and pro- teoglycans [18-36]. However, despite the wide variety of trachea characterization efforts, ranging from camel tra- chea dimensions [37] to dolphin trachea biomechanics [38], and even speculation of airflow in dinosaur tra- cheas [39], very little is known about the distribution of key extracellular matrix components of the trachea. Evans and colleagues presented a nice series of matrix studies on the basement membrane zone associated with the trachea in monkeys and rats [40-42]. However, only a handful of studies have begun to elucidate the collagen and/or elastin content of the trachea in guinea pigs, mice and rats [43-47], in pigs [48, 49], and in humans [50, 51]. It is known that collagen fibers in the trachea run obliquely and are intermingled [43,51,52], that collagen I appears predominately in the perichondrium and colla- gen II appears predominately in the cartilaginous tra- cheal body [45-47,50]. It is also known that elastic fibers may be abundant in the perichondrium of the trachea [36,43,44], forming an extensive network that extends into the posterior membranous tracheal wall [36]. With regard to elastin, it is noteworthy that a pair of studies found trappin-2 (also known as elafin), an elastase in- hibitor, in the trachea [48,49]. In addition, specific glycosaminoglycans (GAGs) were identified immunohistochemically in rat tracheas, although the heterogeneous distributions of chondro- itin-4-sulfate and chondroitin-6-sulfate were not in agreement [47]. Toluidine blue O staining in humans also revealed a heterogeneous GAG distribution [51], although the distributions of specific proteoglycans was not identified. It is also known that regions of the trachea are calcified, although there is a debate as to whether the tissue is commonly ossified [46,47,50,53]. What was not known prior to the current study was the distribution of key proteoglycans, which have im- portant functional roles, and whether their distribution coincided with the distribution of collagen types I and II. Moreover, it was unknown whether the distribution of collagens I and II observed in the trachea of rats [45-47] and humans [50] would be observed in the rabbit trachea as well. Therefore, the objective of the current study was to identify the distributions of elastin, collagen I, and collagen II; as well as two key proteoglycans (aggrecan and decorin) in the rabbit trachea. These matrix compo- nents were chosen based on their relevance to tissue en- gineering, implications for mechanical performance, and to establish relative distribution and abundance for vali- dation of engineered constructs. Given that collagen II and aggrecan are associated with hyaline cartilage, whereas elastin, collagen I and decorin are associated primarily with fibrous tissues [54], we hypothesized that aggrecan would coincide with collagen II distribution, and that decorin would coincide with collagen I and elastin distribution. The relative distributions of these key extracellular matrix components will be crucial to tissue engineering efforts that address surgical repair of laryngotracheal stenosis. 2. METHODS 2.1. Specimen Preparation Four New Zealand White rabbits were obtained and sac- rificed at adult age. All rabbits were male and weighed approximately 9 lbs. They were sacrificed after under- going a single cardiovascular physiology experiment not relevant to tracheal protein structure or synthesis. Imme- diately after sacrifice, the larynx-trachea complexes were collected and stored in PBS-saturated gauze at –20˚C prior to sectioning. Serial 10 µm frozen sections were taken from each of the samples. Sections were mounted on slides, fixed in chilled acetone for 20 min, and then stored at –20˚C until further use. 2.2. Verhoeff-Van Gieson Elastic T iss ue St ain Fixed rabbit tissue samples were stained for elastin. The samples were first placed in an iron hematoxylin solution for 10 min and then rinsed in distilled water and differentiated in 2% ferric chloride. After rinsing in distilled water and placing in 95% alcohol, the samples were counterstained with Van Gieson’s solu- tion (Sigma Aldrich; St. Louis, MO) for 4 min. The samples were then dehydrated in graded alcohol and then cleared in xylene and mounted. 2.3. Immunohistochemistry Slides were placed in a Biogenex i6000 (San Ramon, CA) autostainer and rehydrated for 5 min in PBS. En- dogenous peroxidase activity was quenched with 1% hydrogen peroxide in methanol for 30 min. Specimens were blocked with serum from the secondary antibody host for 20 min and followed by incubation with the primary antibody for 60 min (dilutions provided in Ta- ble 1). Mouse monoclonal IgG anti-collagen I and mouse monoclonal IgG anti-collagen II antibodies were obtained from Accurate Chemical and Scientific (West- bury, NY) and Chondrex, LLC (Redmond, WA), respec- tively. Mouse monoclonal IgG anti-aggrecan and sheep polyclonal IgG anti-decorin were obtained from Abcam (Cambridge, MA). Negative controls were assessed by omission of the primary antibody, and the absence of non-specific staining was verified. The specimens were then incubated with the appro- 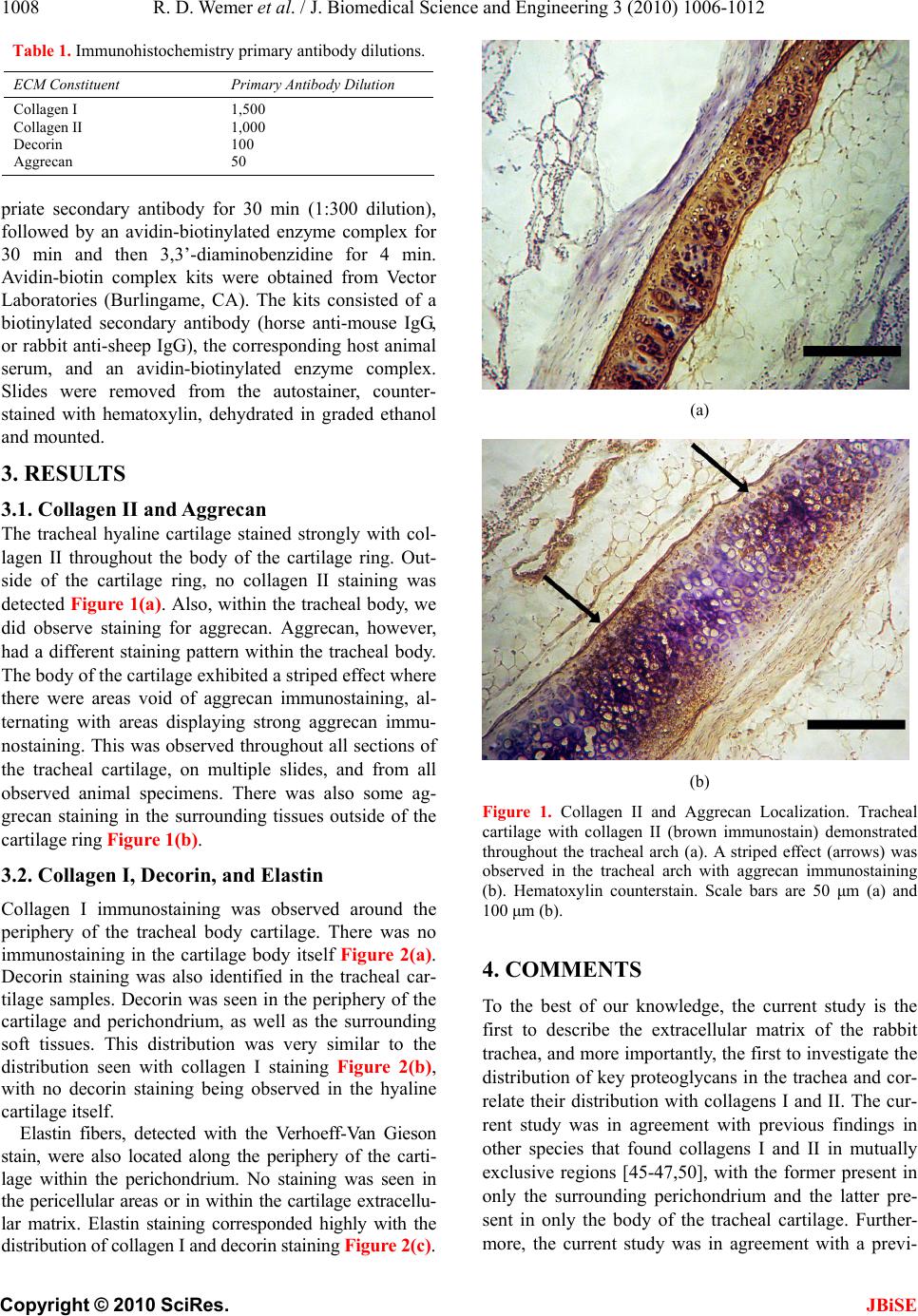 R. D. Wemer et al. / J. Biomedical Science and Engineering 3 (2010) 1006-1012 Copyright © 2010 SciRes. JBiSE 1008 Table 1. Immunohistochemistry primary antibody dilutions. ECM Constituent Primary Antibody Dilution Collagen I Collagen II Decorin Aggrecan 1,500 1,000 100 50 priate secondary antibody for 30 min (1:300 dilution), followed by an avidin-biotinylated enzyme complex for 30 min and then 3,3’-diaminobenzidine for 4 min. Avidin-biotin complex kits were obtained from Vector Laboratories (Burlingame, CA). The kits consisted of a biotinylated secondary antibody (horse anti-mouse IgG, or rabbit anti-sheep IgG), the corresponding host animal serum, and an avidin-biotinylated enzyme complex. Slides were removed from the autostainer, counter- stained with hematoxylin, dehydrated in graded ethanol and mounted. 3. RESULTS 3.1. Collagen II and Aggrecan The tracheal hyaline cartilage stained strongly with col- lagen II throughout the body of the cartilage ring. Out- side of the cartilage ring, no collagen II staining was detected Figure 1(a). Also, within the tracheal body, we did observe staining for aggrecan. Aggrecan, however, had a different staining pattern within the tracheal body. The body of the cartilage exhibited a striped effect where there were areas void of aggrecan immunostaining, al- ternating with areas displaying strong aggrecan immu- nostaining. This was observed throughout all sections of the tracheal cartilage, on multiple slides, and from all observed animal specimens. There was also some ag- grecan staining in the surrounding tissues outside of the cartilage ring Fig ure 1(b). 3.2. Collagen I, Decorin, and Elastin Collagen I immunostaining was observed around the periphery of the tracheal body cartilage. There was no immunostaining in the cartilage body itself Figure 2(a). Decorin staining was also identified in the tracheal car- tilage samples. Decorin was seen in the periphery of the cartilage and perichondrium, as well as the surrounding soft tissues. This distribution was very similar to the distribution seen with collagen I staining Figure 2(b), with no decorin staining being observed in the hyaline cartilage itself. Elastin fibers, detected with the Verhoeff-Van Gieson stain, were also located along the periphery of the carti- lage within the perichondrium. No staining was seen in the pericellular areas or in within the cartilage extracellu- lar matrix. Elastin staining corresponded highly with the distribution of collagen I and decorin staining Figur e 2(c). (a) (b) Figure 1. Collagen II and Aggrecan Localization. Tracheal cartilage with collagen II (brown immunostain) demonstrated throughout the tracheal arch (a). A striped effect (arrows) was observed in the tracheal arch with aggrecan immunostaining (b). Hematoxylin counterstain. Scale bars are 50 μm (a) and 100 μm (b). 4. COMMENTS To the best of our knowledge, the current study is the first to describe the extracellular matrix of the rabbit trachea, and more importantly, the first to investigate the distribution of key proteoglycans in the trachea and cor- relate their distribution with collagens I and II. The cur- rent study was in agreement with previous findings in other species that found collagens I and II in mutually exclusive regions [45-47,50], with the former present in only the surrounding perichondrium and the latter pre- sent in only the body of the tracheal cartilage. Further- more, the current study was in agreement with a previ- 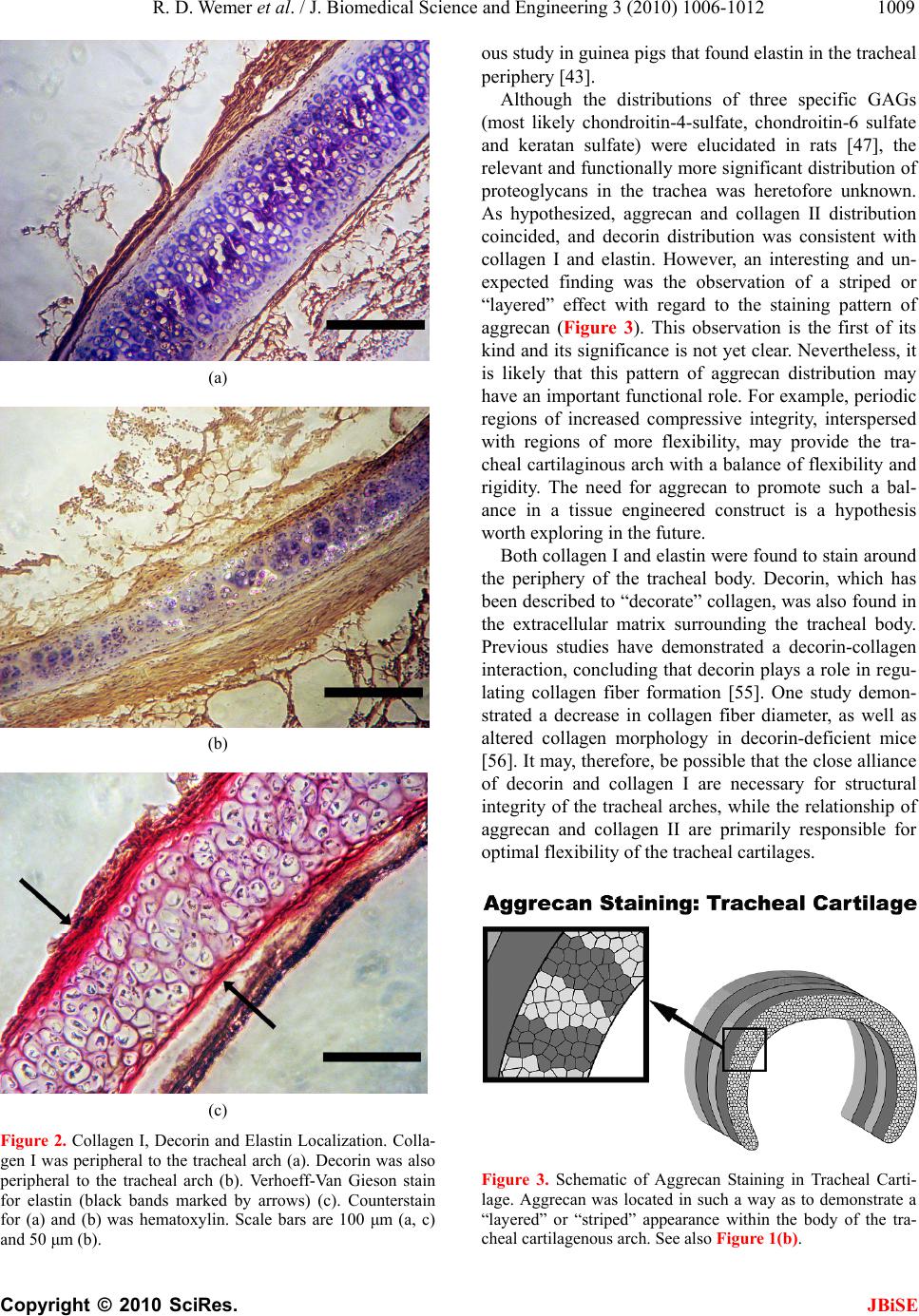 R. D. Wemer et al. / J. Biomedical Science and Engineering 3 (2010) 1006-1012 Copyright © 2010 SciRes. JBiSE 1009 (a) (b) (c) Figure 2. Collagen I, Decorin and Elastin Localization. Colla- gen I was peripheral to the tracheal arch (a). Decorin was also peripheral to the tracheal arch (b). Verhoeff-Van Gieson stain for elastin (black bands marked by arrows) (c). Counterstain for (a) and (b) was hematoxylin. Scale bars are 100 μm (a, c) and 50 μm (b). ous study in guinea pigs that found elastin in the tracheal periphery [43]. Although the distributions of three specific GAGs (most likely chondroitin-4-sulfate, chondroitin-6 sulfate and keratan sulfate) were elucidated in rats [47], the relevant and functionally more significant distribution of proteoglycans in the trachea was heretofore unknown. As hypothesized, aggrecan and collagen II distribution coincided, and decorin distribution was consistent with collagen I and elastin. However, an interesting and un- expected finding was the observation of a striped or “layered” effect with regard to the staining pattern of aggrecan (Figure 3). This observation is the first of its kind and its significance is not yet clear. Nevertheless, it is likely that this pattern of aggrecan distribution may have an important functional role. For example, periodic regions of increased compressive integrity, interspersed with regions of more flexibility, may provide the tra- cheal cartilaginous arch with a balance of flexibility and rigidity. The need for aggrecan to promote such a bal- ance in a tissue engineered construct is a hypothesis worth exploring in the future. Both collagen I and elastin were found to stain around the periphery of the tracheal body. Decorin, which has been described to “decorate” collagen, was also found in the extracellular matrix surrounding the tracheal body. Previous studies have demonstrated a decorin-collagen interaction, concluding that decorin plays a role in regu- lating collagen fiber formation [55]. One study demon- strated a decrease in collagen fiber diameter, as well as altered collagen morphology in decorin-deficient mice [56]. It may, therefore, be possible that the close alliance of decorin and collagen I are necessary for structural integrity of the tracheal arches, while the relationship of aggrecan and collagen II are primarily responsible for optimal flexibility of the tracheal cartilages. Figure 3. Schematic of Aggrecan Staining in Tracheal Carti- lage. Aggrecan was located in such a way as to demonstrate a “layered” or “striped” appearance within the body of the tra- cheal cartilagenous arch. See also Figure 1(b).  R. D. Wemer et al. / J. Biomedical Science and Engineering 3 (2010) 1006-1012 Copyright © 2010 SciRes. JBiSE 1010 The biochemical characterization of the rabbit trachea is an important step for a tissue engineering solution to the treatment of airway stenosis. This animal model has been employed for reasons presented earlier, as well as for the rabbit laryngotracheal segment’s size closely ap- proximating that of a young pediatric population, of high relevance to tissue engineering for use in treating human disease. In demonstrating the composition of a portion of the laryngotracheal complex, we can then attempt to engineer a tissue that possesses properties similar to that of the native tissue. Future studies will be required to elucidate whether any part of the tracheal body possesses an osseous character (e.g., alkaline phosphatase activity and collagen I in addition to calcium) [46,47,50,53]. Moreover, other trachea characterization efforts will be crucial for construct design and analysis, in particular, studies of the biomechanics of the trachea [51,57-59], the fluid dynamics of air flow through the trachea [60-65], and characterization of surrounding tissues such as the tracheal smooth muscle. The identification of native tracheal proteins and pro- teoglycans is critical to the actual construction of tis- sue-engineered implants. The identification of proteins such as collagen I and II, as well as proteoglycans like decorin and aggrecan, may ultimately lend information as to what substrates are required to produce a suitable cartilage graft. We must, however, remain cautious when considering the need to replicate native tracheal cartilage. The biochemical composition of cartilage is complex, and an exact reproduction of the structure may be neither feasible nor warranted to successfully reconstruct the trachea after injury to a tracheal segment. For example, a newly formed cartilage segment may merely need to possess rigidity, but not maximal flexibility, depending on the size and length of the diseased tracheal segment to be replaced. Additional aspects of tracheal reconstruc- tion such as mucosal resurfacing and vascular supply to large segments, as highlighted by the Leuven Tracheal Transplant Group from Belgium [66], will also need to be addressed as the process of engineered cartilage re- placement for tracheal defects becomes more clearly defined. In the current study, we have demonstrated that the body of the tracheal ring is composed of collagen II and bands of aggrecan (hyaline-like tissue), surrounded by a supporting matrix composed of collagen I, elastin, and decorin (fibrous-like tissue). Looking forward, the in- formation obtained in the current study can be applied to the construction of a bioengineered tracheal graft. In an endeavor to replicate the tracheal cartilage ultrastructure, an ideal graft would possess enough rigidity to withstand stresses, but remain flexible enough to allow for motion and other functional measures. Future studies as de- scribed above will be required to correlate the structure of the tracheal cartilage to its function (fluid mechanics of airflow and biomechanical integrity). 5. ACKNOWLEDGEMENTS All of the authors in this study had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. REFERENCES [1] Tan, Q., Steiner, R., Hoerstrup, S.P and Weder, W. (2006) Tissue-engineered trachea: History, problems and the fu- ture. European Journal of Cardio-Thoracic Surgery, 30 (5), 782-786. [2] Kojima, K. and Vacanti, C.A. (2004) Generation of a tissue-engineered tracheal equivalent. Biotechnology and Applied Biochemistry, 39(Pt 3), 257-262. [3] Bucheler, M. and Haisch, A. (2003) Tissue engineering in otorhinolaryngology. DNA Cell Biology, 22(9), 549-564. [4] Grillo, H.C. (2002) Tracheal replacement: A critical re- view. The Annals of Thoracic Surgery, 73(6), 1995-2004. [5] Grillo, H.C. (2003) Tracheal replacement. The Journal of Thoracic and Cardiovascular Surgery, 125(4), 975. [6] Grillo, H.C. (2005) Bioengineered airway tissue. The Journal of Thoracic and Cardiovascular Surgery, 129(5), 1208. [7] Birchall, M. and Macchiarini, P. (2008) Airway trans- plantation: A debate worth having? Transplantation, 85 (8), 1075-1080. [8] Doss, A.E., Dunn, S.S., Kucera, K.A., Clemson, L.A. and Zwischenberger, J.B. (2007) Tracheal replacements: Part 2. ASAIO Journal, 53(2), 631-639. [9] Kucera, K.A., Doss, A.E., Dunn, S.S., Clemson L.A. and Zwischenberger J.B. (2007) Tracheal replacements: Part 1. ASAIO Journal, 53(4), 497-505. [10] Macchiarini, P., Jungebluth, P., Go, T., Asnaghi, M.A., Rees, L.E., Cogan, T.A., Dodson, A., Martorell, J., Bel- lini, S., Parnigotto, P.P., Dickinson, S.C., Hollander, A.P., Mantero, S., Conconi, M.T. and Birchall, M.A. (2008) Clinical transplantation of a tissue-engineered airway. Lancet, 372(9655), 2023-2030. [11] Steger, V., Hampel, M., Trick, I., Muller, M. and Walles, T. (2008) Clinical tracheal replacement: Transplantation, bioprostheses and artificial grafts. Expert Review of Medical Devices, 5(5), 605-612. [12] Asnaghi, M.A., Jungebluth, P., Raimondi, M.T., Dickin- son, S.C., Rees, L.E., Go, T., Cogan, T.A., Dodson, A., Parnigotto, P.P., Hollander, A.P., Birchall, M.A., Conconi, M.T., Macchiarini, P. and Mantero, S. (2009) A dou- ble-chamber rotating bioreactor for the development of tissue-engineered hollow organs: From concept to clini- cal trial. Biomaterials, 30(29), 5260-5269. [13] Jungebluth, P., Go, T., Asnaghi, A., Bellini, S., Martorell, J., Calore, C., Urbani, L., Ostertag, H., Mantero, S., Conconi, M.T. and Macchiarini, P. (2009) Structural and morphologic evaluation of a novel detergent-enzymatic tissue-engineered tracheal tubular matrix. The Journal of Thoracic and Cardiovascular Surgery, 138(3), 586-593. [14] Grimmer, J.F., Gunnlaugsson, C.B., Alsberg, E., Murphy, H.S., Kong, H.J., Mooney, D.J. and Weatherly, R.A.  R. D. Wemer et al. / J. Biomedical Science and Engineering 3 (2010) 1006-1012 Copyright © 2010 SciRes. JBiSE 1011 (2004) Tracheal reconstruction using tissue-engineered car- tilage. Archives of Otolaryngology-Head & Neck Surgery, 130(10), 1191-1196. [15] Robey, T.C., Eiselt, P.M., Murphy, H.S., Mooney, D.J. and Weatherly, R.A. (2000) Biodegradable external tra- cheal stents and their use in a rabbit tracheal reconstruc- tion model. Laryngoscope, 110(11), 1936-1942. [16] Robey, T.C., Valimaa, T., Murphy, H.S., Tormala, P., Mooney, D.J. and Weatherly, R.A. (2000) Use of internal bioabsorbable PLGA “finger-type” stents in a rabbit tra- cheal reconstruction model. Archives of Otolaryngol- ogy-Head & Neck Surgery, 126(8), 985-991. [17] Hallers, E.J., Rakhorst, G., Marres, H.A., Jansen, J.A., Kooten, T.G., Schutte, H.K., Loon, J.P., Houwen, E.B. and Verkerke, G.J. (2004) Animal models for tracheal re- search. Biomaterials, 25(9), 1533-1543. [18] Buhler, R.B., Sennes, L.U., Mauad, T., Melo, E.C., Silva, L.F. and Saldiva, P.H. (2008) Collagen fiber and versican distribution within the lamina propria of fetal vocal folds. Laryngoscope, 118(2), 371-374. [19] Butler, J.E., Hammond, T.H. and Gray, S.D. (2001) Gender-related differences of hyaluronic acid distribution in the human vocal fold. Laryngoscope, 111 (5 ), 907-911. [20] Hammond, T.H., Gray, S.D. and Butler, J.E. (2000) Age- and gender-related collagen distribution in human vocal folds. The Annals of Otology, Rhinology, and Laryngol- ogy, 109(10 Pt 1), 913-920. [21] Gray, S.D., Titze, I.R., Alipour, F. and Hammond, T.H. (2000) Biomechanical and histologic observations of vocal fold fibrous proteins. The Annals of Otology, Rhi- nology, and Laryngology, 109(1), 77-85. [22] Gray, S.D., Titze, I.R., Chan, R. and Hammond, T.H, (1999) Vocal fold proteoglycans and their influence on biomechanics. Laryngoscope, 109(6), 845-854. [23] Hammond, T.H., Zhou, R., Hammond, E.H., Pawlak, A. and Gray, S.D. (1997) The intermediate layer: A mor- phologic study of the elastin and hyaluronic acid con- stituents of normal human vocal folds. Journal of Voice, 11 (1) , 59-66. [24] Pawlak, A.S., Hammond, T., Hammond, E. and Gray, S.D. (1996) Immunocytochemical study of proteoglycans in vocal folds. The Annals of Otology, Rhinology, and Laryngology, 105(1), 6-11. [25] Chan, R.W., Fu, M., Young, L. and Tirunagari, N. (2007) Relative contributions of collagen and elastin to elasticity of the vocal fold under tension. Annals of Biomedical Engineering, 35(8), 1471-1483. [26] Melo, E.C., Lemos, M., Aragao, X.F.J., Sennes, L.U., Nascimento, S.P.H. and Tsuji, D.H. (2003) Distribution of collagen in the lamina propria of the human vocal fold. Laryngoscope, 113(12), 2187-2191. [27] Sivasankar, M. and Ivanisevic, A. (2007) Atomic force microscopy investigation of vocal fold collagen. Laryn- goscope, 117(10), 1876-1881. [28] Tateya, T., Tateya, I. and Bless, D.M. (2006) Collagen subtypes in human vocal folds. The Annals of Otology, Rhinology, and Laryngology, 115(6), 469-476. [29] Tateya, T., Tateya, I. and Bless, D.M. (2007) Im- muno-scanning electron microscopy of collagen types I and III in human vocal fold lamina propria. The Annals of Otology, Rhinology, and Laryngology, 116(2), 156-159. [30] Sakae, F.A., Imamura, R., Sennes, L.U., Mauad, T., Sal- diva, P.H. and Tsuji, D.H. (2008) Disarrangement of col- lagen fibers in Reinke’s edema. Laryngoscope, 118(8), 1500-1503. [31] Sato, K., Hirano, M. and Nakashima, T. (2003) 3D struc- ture of the macula flava in the human vocal fold. Acta Otolaryngologica, 123(2), 269-273. [32] Sato, K., Hirano, M. and Nakashima, T. (2002) Age- related changes of collagenous fibers in the human vocal fold mucosa. The Annals of Otology, Rhinology, and Laryngology, 111(1), 15-20. [33] Sato, K. and Hirano, M. (1995) Histologic investigation of the macula flava of the human vocal fold. The Annals of Otology, Rhinology, and Laryngology, 104(2), 138-143. [34] Hirano, S., Minamiguchi, S., Yamashita, M., Ohno, T., Kanemaru, S.I. and Kitamura, M. (2008) Histologic characterization of human scarred vocal folds. Journal of Voice, 23(4), 399-407, Epub. [35] Ximenes, F.J.A, Tsuji, D.H., Nascimento, P.H. and Sen- nes, L.U. (2003) Histologic changes in human vocal folds correlated with aging: A histomorphometric study. The Annals of Otology, Rhinology, and Laryngology, 112 (10), 894-898. [36] Kamel, K.S., Beckert, L.E. and Stringer, M.D. (2009) Novel insights into the elastic and muscular components of the human trachea. C linic al Ana tomy, 22(6), 689-697. [37] Al-Zghoul, M.F., Ismail, Z.B., Al-Rukibat, R.K. and Al- Majali, A.M. (2006) A quantitative study on the trachea of young Arabian camels (Camelus dromedarius). Jour- nal of Camel Practice and Research, 13(2), 129-133. [38] Cozzi, B., Bagnoli, P., Acocella, F. and Costantino, M.L. (2005) Structure and biomechanical properties of the trachea of the striped dolphin Stenella coeruleoalba: Evi- dence for evolutionary adaptations to diving. The Ana- tomical Record. Part A, Discoveries in Molecular, Cellu- lar, and Evolutionary Biology, 284(1), 500-510. [39] Pierson, D.J. (2009) The physiology of dinosaurs: Circu- latory and respiratory function in the largest animals ever to walk the earth. Respiratory Care, 54(7), 887-911. [40] Evans, M.J., Fanucchi, M.V., Baker, G.L., Winkle, L.S., Pantle, L.M., Nishio, S.J., Schelegle, E.S., Gershwin, L.J., Miller, L.A., Hyde, D.M. and Plopper, C.G. (2004) The remodelled tracheal basement membrane zone of infant rhesus monkeys after 6 months of recovery. Clinical and Experimental Allergy: Journal of the British Society for Allergy and Clinical Immunology, 34(7), 1131-1136. [41] Evans, M.J., Winkle, L.S., Fanucchi, M.V., Toskala, E., Luck, E.C., Sannes, P.L. and Plopper, C.G. (2000) Three-dimensional organization of the lamina reticularis in the rat tracheal basement membrane zone. American Journal of Respiratory Cell and Molecular Biology, 22 (4), 393-397. [42] Evans, M.J., Fanucchi, M.V., Baker, G.L., Winkle, L.S., Pantle, L.M., Nishio, S.J., Schelegle, E.S., Gershwin, L.J., Miller, L.A., Hyde, D.M., Sannes, P.L. and Plopper, C.G. (2003) Atypical development of the tracheal basement membrane zone of infant rhesus monkeys exposed to ozone and allergen. American Journal of Physiology. Lung Cellular and Molecular Physiology, 285(4), L931-L939. [43] Amiri, M.H. and Gabella, G. (1988) Structure of the guinea-pig trachea at rest and in contraction. Anatomy and Embryology, 178(5), 389-397. [44] Maki, J.M., Sormunen, R., Lippo, S., Kaarteenaho, W.R,  R. D. Wemer et al. / J. Biomedical Science and Engineering 3 (2010) 1006-1012 Copyright © 2010 SciRes. JBiSE 1012 Soininen, R. and Myllyharju, J. (2005) Lysyl oxidase is essential for normal development and function of the respiratory system and for the integrity of elastic and collagen fibers in various tissues. The American Journal of Pathology, 167(4), 927-936. [45] Sasano, Y., Takahashi, I., Zhu, J.X., Ohtani, H., Mizogu- chi, I. and Kagayama, M. (2001) Gene and protein ex- pressions of type I collagen are regulated tissue-speci- fically in rat hyaline cartilages in vivo. European Journal of Morphology, 39(3), 149-154. [46] Sasano, Y., Takahashi, I., Mizoguchi, I., Kagayama, M., Takita, H. and Kuboki, Y. (1998) Type X collagen is not localized in hypertrophic or calcified cartilage in the de- veloping rat trachea. Anatomy and Embryology, 197(5), 399-403. [47] Sasano, Y., Mizoguchi, I., Furusawa, M., Aiba, N., Ohtani, E., Iwamatsu, Y. and Kagayama, M. (1993) The process of calcification during development of the rat tracheal cartilage characterized by distribution of alkaline phosphatase activity and immunolocalization of types I and II collagens and glycosaminoglycans of proteogly- cans. Anatomy and Embryology, 188(1), 31-39. [48] Tamechika, I., Itakura, M., Saruta, Y., Furukawa, M., Kato, A., Tachibana, S. and Hirose, S. (1996) Accelerated evolution in inhibitor domains of porcine elafin family members. The Journal of Biological Chemistry, 271(12), 7012-7018. [49] Suzuki, Y., Furukawa, M., Abe, J., Kashiwagi, M. and Hirose, S. (2000) Localization of porcine trappin-2 (SKALP/elafin) in trachea and large intestine by in situ hybridization and immunohistochemistry. Histochemistry and Cell Biology, 114(1), 15-20. [50] Kusafuka, K., Yamaguchi, A., Kayano, T. and Takemura, T. (2001) Ossification of tracheal cartilage in aged hu- mans: A histological and immunohistochemical analysis. Journal of Bone and Mineral Metabolism, 19(3), 168-174. [51] Roberts, C.R., Rains, J.K., Pare, P.D., Walker, D.C., Wiggs, B. and Bert, J.L. (1998) Ultrastructure and tensile properties of human tracheal cartilage. Journal of Bio- mechanics, 31(1), 81-86. [52] Rao, R.A., Mehta, M.R. and Toussaint, K.C. (2009) Fou- rier transform-second-harmonic generation imaging of biological tissues. Optics Express, 17(17), 14534-14542. [53] Maki, K., Hayashi, S., Nishioka, T., Kimura, M. and Noguch, T. (2000) A new type of matrix vesicles is found in fetal bovine tracheal cartilage. Connect Tissue Re- search, 41(2), 109-115. [54] Detamore, M.S. and Athanasiou, K.A. (2003) Structure and function of the temporomandibular joint disc: impli- cations for tissue engineering. Journal of Oral and Max- illofacial Surgery, 61(4), 494-506. [55] Poole, A.R., Webber, C., Pidoux, I., Choi, H. and Rosenberg, L.C. (1986) Localization of a dermatan sul- fate proteoglycan (DS-PGII) in cartilage and the presence of an immunologically related species in other tissues. The Journal of Histochemistry and Cytochemistry: Official Journal of the Histochemistry Society, 34(5), 619-625. [56] Danielson, K.G., Baribault, H., Holmes, D.F., Graham, H., Kadler, K.E. and Iozzo, R.V. (1997) Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. Journal of Cell Biology, 136, 729-743. [57] Teng, Z., Ochoa, I., Li, Z., Lin, Y., Rodriguez, J.F., Bea, J.A. and Doblare, M. (2008) Nonlinear mechanical prop- erty of tracheal cartilage: A theoretical and experimental study. Journal of Biomechanics, 41(9), 1995-2002. [58] Miller, T.L., Altman, A.R., Tsuda, T. and Shaffer, T.H. (2007) An ultrasound imaging method for in vivo tra- cheal bulk and Young’s moduli of elasticity. Journal of Biomechanics, 40(7), 1615-1621. [59] Rains, J.K., Bert, J.L., Roberts, C.R. and Pare, P.D. (1992) Mechanical properties of human tracheal cartilage. Journal of Applied Physiology, 72(1), 219-225. [60] Jayaraju, S.T., Paiva, M., Brouns, M., Lacor, C. and Ver- banck, S. (2008) Contribution of upper airway geometry to convective mixing. Journal of Applied Physiology, 105(6), 1733-1740. [61] Russo, J., Robinson, R. and Oldham, M.J. (2008) Effects of cartilage rings on airflow and particle deposition in the trachea and main bronchi. Medical Engineering & Phys- ics, 30(5), 581-589. [62] Rakesh, V., Rakesh, N.G,. Datta, A.K., Cheetham, J. and Pease, A.P. (2008) Development of equine upper airway fluid mechanics model for Thoroughbred racehorses. Equine Veterinary Journal , 40(3), 272-279. [63] Nazridoust, K. and Asgharian, B. (2008) Unsteady-state airflow and particle deposition in a three-generation hu- man lung geometry. Inhalation Toxicology, 20(6), 595- 610. [64] Isaacs, K.K., Schlesinger, R.B. and Martonen, T.B. (2006) Three-dimensional computational fluid dynamics simula- tions of particle deposition in the tracheobronchial tree. Journal of Aerosol Medicine, 19(3), 344-352. [65] Martonen, T.B., Zhang, Z., Yu, G. and Musante, C.J. (2001) Three-dimensional computer modeling of the human upper respiratory tract. Cell Biochemistry and Biophysics, 35(3), 255-261. [66] Delaere, P., Vranckx, J., Verleden, G., Leyn, P. and Raemdonck, D. (2010) Tracheal allotransplantation after withdrawal of immunosuppressive therapy. The New England Journal of Medicine, 362(2), 138-145. |

