 Food and Nutrition Sciences, 2013, 4, 305-314 http://dx.doi.org/10.4236/fns.2013.43041 Published Online March 2013 (http://www.scirp.org/journal/fns) Analysis of Volatile Compounds and Identification of Characteristic Aroma Components of Toona sinensis (A. Juss.) Roem. Using GC-MS and GC-O Changjin Liu1, Jie Zhang1, Zhongkai Zhou1*, Zetian Hua2, Hongying Wan1, Yanhui Xie1, Zhiwei Wang1, Li Deng3 1Key Laboratory of Food Nutrition and Safety, Ministry of Education, Tianjin University of Science and Technology, Tianjin, China; 2Tianjin Tianlong Agricultural S & T Co., Ltd., Tianjin, China; 3Tianjin Chunfa Biotechnology Group CO., Ltd., Tianjin, China. Email: *zhongkai_zhou@hotmail.com Received December 3rd, 2012; revised January 22nd, 2013; accepted February 30th, 2013 ABSTRACT In this study, volatile compounds present in Toona sinensis (A. Juss.) Roem (TS) were investigated and their character- istic aromatic components were identified using Headspace Solid-phase Microextraction (HS-SPME) followed by Gas Chromatography-Mass Spectrometry (GC-MS) and Gas Chromatography-Olfactometry (GC-O). The optimum condi- tions for extracting the volatiles from TS were achieved with the experimental parameters including the use of a 65 μm polydimethylsiloxane/divinyl benzene (PDMS/DVB) fibre, an extraction temperature of 40˚C and an extraction time of 30 min. Under these conditions, 56 volatile compounds were separated and 53 were identified by GC-MS. Among them, 21 sulfide compounds (42.146%) and 27 terpenes(55.984%) were found to be the major components. The sample was analyzed by GC-O and 26 elutes were sniffed and their sensory descriptions evaluated by an odor panelists. Analysis of the data indicated, two compounds cis and trans isomers of 2-Mercapto-3,4-dimethyl-2,3-dihydrothiophene were major contributors to the characteristic aroma of TS. Keywords: Toona sinensis (A. Juss.) Roem.; Volatile Compounds; Characteristic Aroma Components; HS-SPME; GC-MS; GC-O 1. Introduction Toona sinensis (A. Juss.) Roem. (TS), is a tree commonly named Chinese toon or Chinese Mahogany and belongs to the family of Meliaceae. It widely distributes in China and some other Asian countries. Its tender leaves and young sprouts have been commonly used as a spice in China since the Han Dynasty around two thousand years ago. Due to its very unique flavour, TS is a popular tradi- tional spice in the Chinese diet. The unique aroma can be sensed among almost all parts of TS, and provides a pleasant fragrance when its buds germinate. The edible buds are picked in the early spring annually, so TS is also recognized as a seasonal vegetable as well. As a fresh foliar vegetable, the delicious buds are usually cooked with other foods such as eggs and bean curds, in dishes known as Scrambled eggs with Chinese to on and Tofu with Chinese toon. Almost every part of TS has been widely used in Chi- nese traditional medicine for treatment of enteritis, dys- entery and itch during the period of ancient China [1]. Comprehensive investigations have demonstrated the pharmacological and health promoting properties of its high content flavonoid content [2,3]. Besides its medici- nal functions, the most conspicuous trait of TS is its ap- pealing flavours which are the results of its volatile com- pounds. However, limited studies are available on the volatile compounds in TS. Thus, experiments in this study have been designed to identify and characterize impor- tant volatile compounds in TS. As a solvent-free extraction method, HS-SPME has shown many advantages for volatile compound analysis compared to traditional methods such as simultaneous distillation-extraction, and steam distillation [4]. For in- stance, the volatile compounds in garlic tend to be de- graded during solvent extraction and steam distillation but not with HS-SPME [5]. In the analysis of volatiles from chili peppers, a 50/30 μm divinylbenzene/carboxen/ polydimethylsiloxane (DVB/CAR/PDMS) extraction me- dium was showed to give the highest extraction effi- ciency with ground samples [6]. These studies also con- cluded that HS-SPME was a practical method to deter- mine intermediate volatile compounds and even very *Corresponding author. Copyright © 2013 SciRes. FNS  Analysis of Volatile Compounds and Identification of Characteristic Aroma Components of Toona sinensis (A. Juss.) Roem. Using GC-MS and GC-O 306 volatile constituents produced enzymatically after the rup- ture of plant cells. Flavours present in TS is likely to be a mixture of very volatile and intermediate volatile com- pounds generated by physical cell disruption, thus, the HS-SPME technique may be a suitable technique for studying these compounds. GC-MS and GC-O have been widely used in flavour analysis and are very useful tools for identification of characteristic aroma compounds [7]. However, these combined techniques have not yet been applied to TS flavour analysis so far. Thus, this is the first attempt to use GC-MS and GC-O for TS flavour compound analysis after extraction using HS-SPME. 2. Materials and Methods 2.1. Chemicals Chromatographically pure standards used for identifica- tion were supplied by several companies: (Z)-1-(Methy- sulfany)-1-propene and (E)-1-(Methysulfany)-1-propene were supplied by Heowns (Atlana, USA). 2,5-Dimethyl- thiophene and 3,4-Dimethylthiophene were purchased from AccuStandard (New Haven CT, USA). An n-Al- kane (C7-C33) mix in Hexane (Supelco, Bellefonte, USA) was also used to calculate the retention index (RI) of each component. 2.2. Sample Collection and Preparation Fresh buds of TS were collected from Linfen city in Shanxi province, China, during spring, 2012. Before analysis, they were manually ground at room temperature using a mortar in order to release the fragrance ade- quately. A 10.0 g aliquot of sample was immediately transferred into a 40 ml gas-tight glass vessel and incu- bated at room temperature for 30 min to achieve partition equilibration for the volatile compounds. Four extraction fibres; 30/50 μm DVB/CAR/PDMS, 65 μm PDMS/DVB, 75 μm CAR/PDMS and 100 μm PDMS (Supelco, Bellefonte, USA) were evaluated. Be- fore extraction, the fibres were preconditioned for 30 min in the injection port of the GC as indicated by the manu- facturer. The fibre was then inserted into the vessel and exposed to the headspace. The procedure was repeated using different extraction times (10 min to 50 min) and temperatures (10˚C to 50˚C) to determine the optimal HS-SPME conditions. Finally the fibre was removed and the components were desorbed in the GC injection port for 5 min. 2.3. GC-MS Operation Conditions A Varian 4000 GC-MS (Walnut Creek, CA, USA) was used for separation and qualitative determination of the volatiles. Ultra-high purity helium was used as the carrier gas at a flow rate of 1.5 ml/min into the column. Injec- tion was at 250˚C in split mode (20:1) onto a 30 m × 0.25 m DB-5 capillary column with 0.25 μm film thickness (Varian, USA). The oven temperature was set at 40˚C for 3 min, then increased to 150˚C at 4˚C/min, and kept at this temperature for 1 min, ultimately increased to 260˚C at 8˚C/min, and kept at this temperature for 6 min. MS conditions were: ionization energy, 70 eV, full scan mode with a scan frequency of 1.2 scan/s and a scan range of 50 - 550 amu in all experiments and the transfer line was at 250˚C. The ion trap was operated at 220˚C in the electron impact mode. 2.4. GC-O Operation Conditions The GC-O analysis was conducted using an Agilent 5975 gas chromatograph equipped with a FID detector (Agilent Technologies, SantaClara, CA, USA) and a sniffing port (Sniffer 9000, Brechbühler, Switzerland). Injection and separation conditions were as indicated above. Gas chro- matography effluents of TS extracts were split between the sniffing port and the FID at a ratio of 1:1. The tem- perature of the sniffing port was 240˚C. The sniffing port was supplied with humidified air at 40˚C with a flow of 600 ml/min in order to avoid nasal dehydration. 2.5. Sniffing Test Sniffing tests were performed on eight chromatographic runs by four panelists (two males and two females in the age range of 20 to 30 years old). A panel consisting of 4 people was thought to be suitable for sniffing test based on previous studies [8,9]. An aroma evaluation approach outlined by Lv et al. [10] was used to describe aroma properties and intensities at the sniffing port under the conditions outlined above (see Section 2.4). According to the experience of previous TS flavour study, vocabulary pool for describing TS aroma was made. Prior to the ex- perience, the panelists were familiarized with the pool of aromatic compounds and instructed on how to use suit- able descriptors to describe the individual compounds in TS. They were asked describe the aroma properties and intensities of the compounds eluted from the GC column. An independent recorder was requested to write down the panelist’s evaluation instantly. A compound was deemed as aromatically active if detected in at least half of all sniffs (four of eight runs). The intensity of each com- pound was evaluated by using a five-point intensity in- terval scale (1-very mild; 2-mild; 3-moderate; 4-strong; 5-very strong). A score was not given if no aroma was perceived. Finally the recorded data were collected, the mean values of related compounds were calculated to the nearest whole number (showed as the numbers of “*”) and the most frequent descriptions of each compound Copyright © 2013 SciRes. FNS 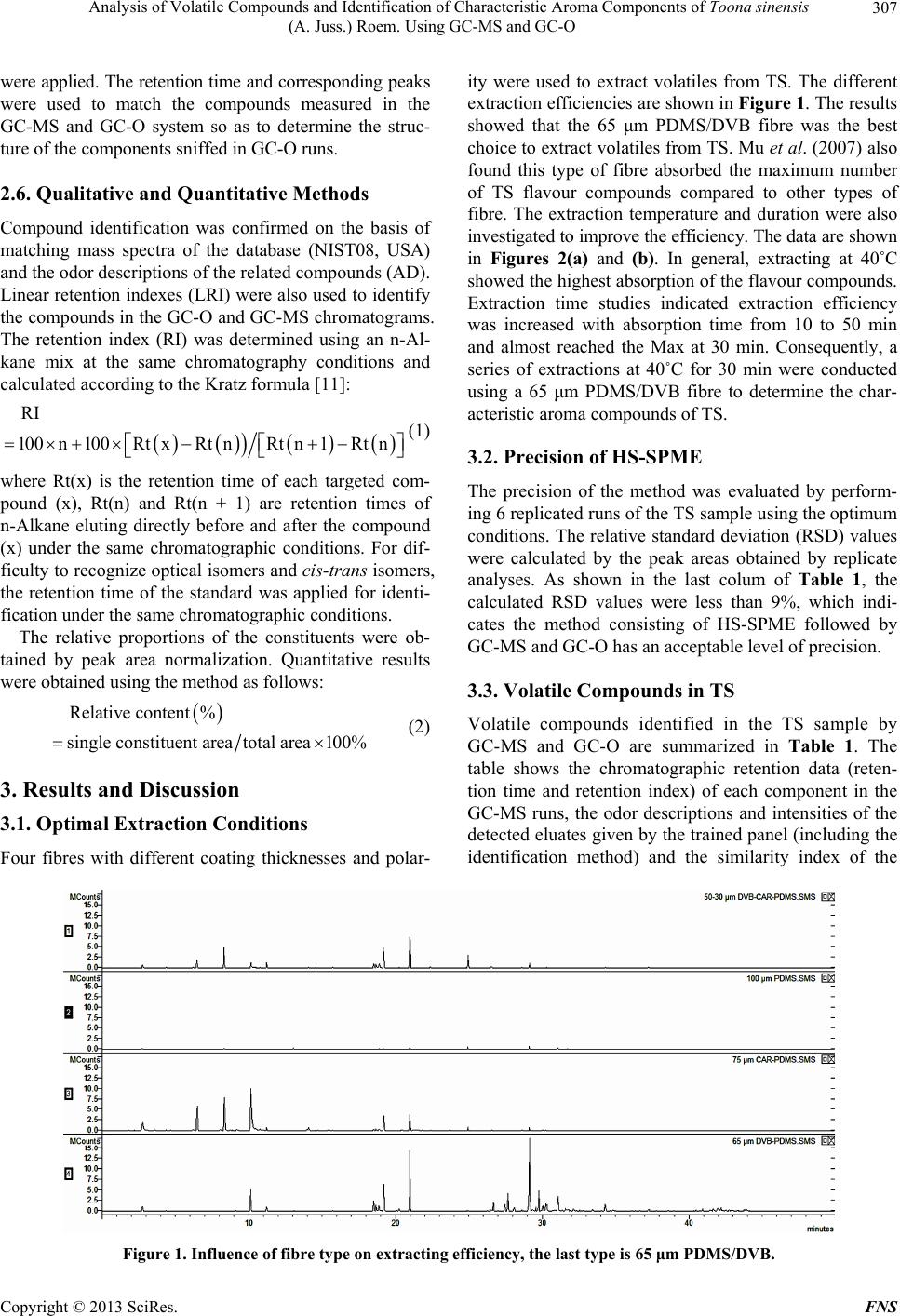 Analysis of Volatile Compounds and Identification of Characteristic Aroma Components of Toona sinensis (A. Juss.) Roem. Using GC-MS and GC-O Copyright © 2013 SciRes. FNS 307 were applied. The retention time and corresponding peaks were used to match the compounds measured in the GC-MS and GC-O system so as to determine the struc- ture of the components sniffed in GC-O runs. 2.6. Qualitative and Quantitative Methods Compound identification was confirmed on the basis of matching mass spectra of the database (NIST08, USA) and the odor descriptions of the related compounds (AD). Linear retention indexes (LRI) were also used to identify the compounds in the GC-O and GC-MS chromatograms. The retention index (RI) was determined using an n-Al- kane mix at the same chromatography conditions and calculated according to the Kratz formula [11]: RI 100n100Rt xRt nRt n1Rt n (1) ity were used to extract volatiles from TS. The different extraction efficiencies are shown in Figure 1. The results showed that the 65 μm PDMS/DVB fibre was the best choice to extract volatiles from TS. Mu et al. (2007) also found this type of fibre absorbed the maximum number of TS flavour compounds compared to other types of fibre. The extraction temperature and duration were also investigated to improve the efficiency. The data are shown in Figures 2(a) and (b). In general, extracting at 40˚C showed the highest absorption of the flavour compounds. Extraction time studies indicated extraction efficiency was increased with absorption time from 10 to 50 min and almost reached the Max at 30 min. Consequently, a series of extractions at 40˚C for 30 min were conducted using a 65 μm PDMS/DVB fibre to determine the char- acteristic aroma compounds of TS. 3.2. Precision of HS-SPME where Rt(x) is the retention time of each targeted com- pound (x), Rt(n) and Rt(n + 1) are retention times of n-Alkane eluting directly before and after the compound (x) under the same chromatographic conditions. For dif- ficulty to recognize optical isomers and cis-trans isomers, the retention time of the standard was applied for identi- fication under the same chromatographic conditions. The precision of the method was evaluated by perform- ing 6 replicated runs of the TS sample using the optimum conditions. The relative standard deviation (RSD) values were calculated by the peak areas obtained by replicate analyses. As shown in the last colum of Table 1, the calculated RSD values were less than 9%, which indi- cates the method consisting of HS-SPME followed by GC-MS and GC-O has an acceptable level of precision. The relative proportions of the constituents were ob- tained by peak area normalization. Quantitative results were obtained using the method as follows: 3.3. Volatile Compounds in TS Relative content% single constituent areatotal area100% (2) Volatile compounds identified in the TS sample by GC-MS and GC-O are summarized in Table 1. The table shows the chromatographic retention data (reten- tion time and retention index) of each component in the GC-MS runs, the odor descriptions and intensities of the detected eluates given by the trained panel (including the identification method) and the similarity index of the 3. Results and Discussion 3.1. Optimal Extraction Conditions Four fibres with different coating thicknesses and polar- Figure 1. Influence of fibre type on extracting efficiency, the last type is 65 μm PDMS/DVB.  Analysis of Volatile Compounds and Identification of Characteristic Aroma Components of Toona sinensis (A. Juss.) Roem. Using GC-MS and GC-O 308 (a) (b) Figure 2. (a) Effect of absorption temperatures on peak area of TS volatile compounds using a 65 μm PDMS-DVB fibre for 30 min; (b) Effect of absorption time on peak area of TS volatile compounds using a 65 μm PDMS-DVB fibre at 40˚C. unknown compared with the spectrum of the MS data- base. Mean- while, the relative amounts of these volatile compounds are also listed in Table 1. A total ion chro- matogram of volatile constituents in TS is given in Fig- ure 3. The analysis of TS volatile compounds revealed a total of 56 compounds with 53 of these identified. The major classes included 24 terpenes (55.984%), 7 thiophenes (32.660%) and 11 thioethers (8.764%). In addition, there were 2 aldehydes (0.049%), 2 thioesters (0.782%, 1 ester (0.296%), 1 thiapyrans (0.030%), and 1 ketone (0.010%). Among all the separated compounds, β-caryophyllene (21.450%) followed by cis-2-Mercapto-3,4-dimethyl-2,3- dihydrothiophene (16.960%), trans-2-Mercapto-3,4-dime- thyl-2,3-dithydrothiophene (9.484%), 3,4-Dimethylthio- phene (6.010%), Aristolene (5.413%), Germacrene D Copyright © 2013 SciRes. FNS  Analysis of Volatile Compounds and Identification of Characteristic Aroma Components of Toona sinensis (A. Juss.) Roem. Using GC-MS and GC-O 309 Table 1. Volatile compounds present in TS determined by GC-MS and GC-O runs. NO. Retention Time (min) RIa Compounds Aroma property Aroma intensity MIb Similc RCdRSDe (%) 1 2.762 610 Methylthiirane Fresh, garlic *** MS, RIL, AD 780 1.9026.47 2 4.357 696 (Z)-1-(Methysulfany)-1-propene Garlic, onion * MS, RS, AD 894 0.1504.95 3 4.658 712 (E)-1-(Methysulfany)-1-propene Garlic, onion * MS, RS, AD 909 0.0536.96 4 6.157 789 5-Methyl-2,3-dihydrothiophene MS, RIL 819 0.0256.56 5 6.500 802 Hexanal Grass, leafy ** MS, RIL, AD 790 0.0022.31 6 8.341 863 (E)-2-Hexenyl Grass, leafy ** MS, RIL, AD 872 0.0475.21 7 9.146 876 2,5-Dimethylthiophene Fried, onion, rubber ** MS, RS, AD 900 0.1302.34 8 10.133 887 3,4-Dimethylthiophene Fried, onion, rubber *** MS, RS, AD 905 6.0102.17 9 10.456 894 (Z,E)-Bis(1-propenyl)sulfide Onion, garlic ** MS, RIL, AD 761 0.0315.25 10 10.746 901 (E,E)-Bis(1-propenyl)sulfide Onion, garlic ** MS, RIL, AD 845 0.0264.12 11 11.218 916 α-Pinene Fruity, resin ** MS, RIL, AD 888 1.3655.67 12 12.819 967 α-Thujene MS, RIL 849 0.0343.32 13 13.472 987 β-Pinene MS, RIL 860 0.0854.67 14 14.079 1005 cis-2-Ethyl-3-methylthiophene MS, RIL 767 0.0203.46 15 15.055 1030 Limonene MS, RIL 874 0.0833.56 16 15.726 1047 cis-Ocimene MS, RIL 893 0.2562.59 17 16.602 1070 3-Ethyltetrahydro-2H-thiopyran MS, RIL 789 0.0304.69 18 17.981 1105 2-Ethenyl-1,3,3-trimethylcyclohexene MS, RIL 809 0.0413.45 19 18.500 1119 3-Ethyl-1,2-dithiacyclohex-4-ene Pungent, sulphur ** MS, RIL, AD 845 3.7217.56 20 18.677 1124 3-Ethyl-1,2-dithiacyclohex-5-ene Pungent, sulphur * MS, RIL, AD 789 0.9416.54 21 18.853 1128 2-Ethyl-1,3-dithiacyclohex-4-ene Pungent, sulphur ** MS, RIL, AD 756 1.7107.45 22 19.225 1138 cis-2-Mercapto-3,4-dimethyl-2,3- dihydrothiophene Cooked, TS, rubber**** MS, RIL 845 9.484 4.65 23 20.051 1160 Allyl dithiopropanoate Onion, garlic ** MS, AD 867 0.2015.56 24 20.266 1166 Prop-1-enyl dithiopropanonate MS, RIL 745 0.5817.24 25 20.408 1170 2-Ethyl-5-propyl thiophene 754 0.0316.14 26 20.990 1185 trans-2-Mercapto-3,4-dimethyl-2,3- dihydrothiophene Fresh, TS ***** MS, RIL 827 16.960 4.44 27 21.313 1194 2-Ethyl-1,3-dithiacyclohex-4-ene Pungent, sulphur * MS, RIL, AD 748 0.1014.78 28 22.793 1236 1,2-Dithiocane MS, RIL 856 0.0075.21 29 23.704 1262 5,5-Dimethyl-1,3-dithian-2-one MS, RIL 611 0.0328.45 30 24.958 1298 Pelargonic acid methyl ester MS, RIL 787 0.2963.43 31 26.267 1337 δ-elemene MS, RIL 874 0.1122.35 32 26.662 1349 α-Cubebene Herb * MS, RIL, AD 877 3.4262.79 33 27.456 1369 Ylangene Fruity, sweet * MS, RIL, AD 849 1.8155.63 34 27.668 1373 α-Copaene Cinnamon, floral * MS, RIL, AD 886 4.4147.45 35 28.093 1392 α-Elemene Flower, sweet * MS, RIL, AD 867 1.4865.43 36 28.605 1408 (Z)-Caryophyllene MS, RIL 875 0.5444.78 37 29.140 1425 β-Caryophyllene Fruity, sweet, flower*** MS, RIL, AD 885 21.4505.79 38 29.347 1431 δ-Elemene MS, RIL 865 0.3525.89 39 29.560 1438 α-Guaiene: MS, RIL 881 0.7277.46 40 29.770 1444 Aristolene Flower, sweet * MS, RIL, AD 834 5.4132.35 41 30.048 1453 δ-Gurjunene Flower, sweet * MS, RIL, AD 864 1.6884.45 42 30.251 1459 α-Caryophyllene Mild, mint * MS, RIL, AD 900 3.1622.67 43 30.849 1478 γ-muurolene MS, RIL 874 0.1225.21 44 31.095 1486 Germacrene D Oily, green * MS, RIL, AD 911 4.8568.08 45 31.588 1501 α-Selinene MS, RIL 874 0.7274.52 Copyright © 2013 SciRes. FNS 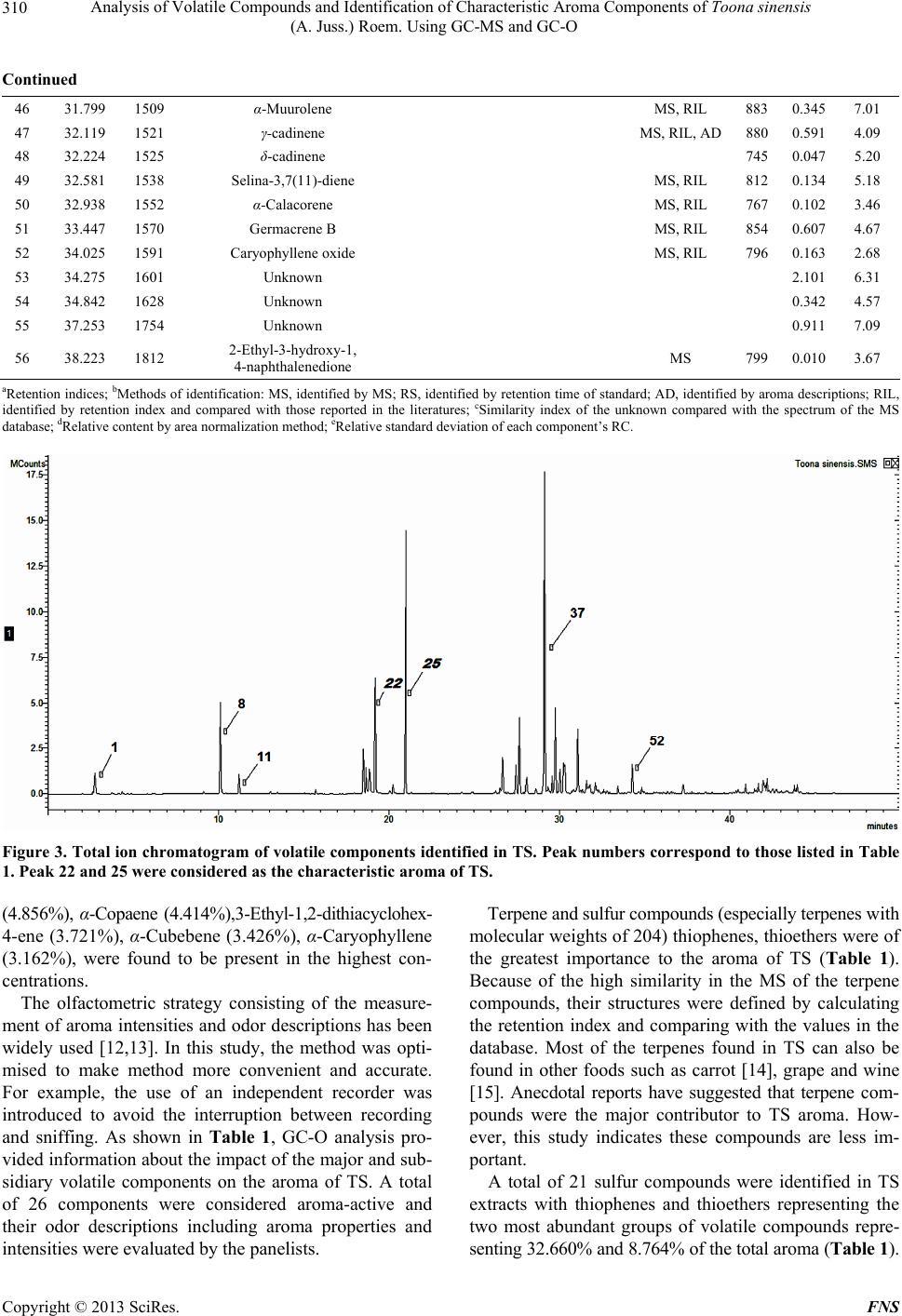 Analysis of Volatile Compounds and Identification of Characteristic Aroma Components of Toona sinensis (A. Juss.) Roem. Using GC-MS and GC-O 310 Continued 46 31.799 1509 α-Muurolene MS, RIL 883 0.3457.01 47 32.119 1521 γ-cadinene MS, RIL, AD 880 0.5914.09 48 32.224 1525 δ-cadinene 745 0.0475.20 49 32.581 1538 Selina-3,7(11)-diene MS, RIL 812 0.1345.18 50 32.938 1552 α-Calacorene MS, RIL 767 0.1023.46 51 33.447 1570 Germacrene B MS, RIL 854 0.6074.67 52 34.025 1591 Caryophyllene oxide MS, RIL 796 0.1632.68 53 34.275 1601 Unknown 2.1016.31 54 34.842 1628 Unknown 0.3424.57 55 37.253 1754 Unknown 0.9117.09 56 38.223 1812 2-Ethyl-3-hydroxy-1, 4-naphthalenedione MS 799 0.0103.67 aRetention indices; bMethods of identification: MS, identified by MS; RS, identified by retention time of standard; AD, identified by aroma descriptions; RIL, identified by retention index and compared with those reported in the literatures; cSimilarity index of the unknown compared with the spectrum of the MS database; dRelative content by area normalization method; eRelative standard deviation of each component’s RC. Figure 3. Total ion chromatogram of volatile components identified in TS. Peak numbers correspond to those listed in Table 1. Peak 22 and 25 were considered as the characteristic aroma of TS. (4.856%), α-Copaene (4.414%),3-Ethyl-1,2-dithiacyclohex- 4-ene (3.721%), α-Cubebene (3.426%), α-Caryophyllene (3.162%), were found to be present in the highest con- centrations. The olfactometric strategy consisting of the measure- ment of aroma intensities and odor descriptions has been widely used [12,13]. In this study, the method was opti- mised to make method more convenient and accurate. For example, the use of an independent recorder was introduced to avoid the interruption between recording and sniffing. As shown in Table 1, GC-O analysis pro- vided information about the impact of the major and sub- sidiary volatile components on the aroma of TS. A total of 26 components were considered aroma-active and their odor descriptions including aroma properties and intensities were evaluated by the panelists. Terpene and sulfur compounds (especially terpenes with molecular weights of 204) thiophenes, thioethers were of the greatest importance to the aroma of TS (Table 1). Because of the high similarity in the MS of the terpene compounds, their structures were defined by calculating the retention index and comparing with the values in the database. Most of the terpenes found in TS can also be found in other foods such as carrot [14], grape and wine [15]. Anecdotal reports have suggested that terpene com- pounds were the major contributor to TS aroma. How- ever, this study indicates these compounds are less im- portant. A total of 21 sulfur compounds were identified in TS extracts with thiophenes and thioethers representing the two most abundant groups of volatile compounds repre- senting 32.660% and 8.764% of the total aroma (Table 1). Copyright © 2013 SciRes. FNS  Analysis of Volatile Compounds and Identification of Characteristic Aroma Components of Toona sinensis (A. Juss.) Roem. Using GC-MS and GC-O 311 These sulfur compounds possess higher odor intensities and lower threshold values and have subsequently been confirmed to be the most important compounds of TS aromas. Large amounts of sulfur compounds can also be found in other foods such as cooked chicken [16], roast beef [17], heated leek [18], raw and processed garlic [19] and sliced onion [20]. Sniffing test of TS revealed 2,5-Dimethylthiophene and 3,4-Dimethylthiophene have an aroma of sulphur, fried onion and rubber (Table 1). Mu [21] also identified the two compounds in TS, but they did not describe their individual aromas. A small amount of Methylthiirane was found to be responsible for a “***” moderate pun- gent perception, and is also found to be one of the vola- tile components in garlic [22]. The thioethers, such as (Z)-1-(Methysulfany)-1-propene, (E)-1-(Methysulfany)-1- propene, (Z,E)-Bis(1-propenyl) sulfide and (E,E)-Bis(1- propenyl)sulfide are also found in onions and scallions [23]. Small amounts of aldehyde (0.049%) and ester (0.296%) compounds were also detected in TS (Table1). It is not surprising to see that TS contains Hexanal and (E)-2-Hexenal, which is common in many plant extracts and are perceived as green and grassy odors [24]. Abun- dant terpenes give an aroma of sweet, fruit and flower, but the threshold of these compounds appear to be slightly higher than that of other compounds, such as sulfur com- pounds. Consequently, only those constituents with higher concentrations can be perceived. For example, β-Cary- ophyllene was found to have the highest concentration in TS, which is also present in many other plants such as clove [25] and cassia [26] as a major volatile compound. To the best of our knowledge, this study is the first report to identify a number of volatile compounds in TS, includ- ing cis-, trans-2-Mercapto-3,4-dimethyl-2,3-dihydrothio- phene, Allyl dithiopropanoate, α-Calacorene, Selina-3,7 (11)-diene, 3-Ethyl-1,2-dithiacyclohex-4-ene, 3-Ethyl-1,2- dithiacyclohex-5-ene, cis -2-Ethyl-3-methylthiophene, (Z)- Caryophyllene, α-Thujene 2-Ethyl-1,3-dithiacyclohex-4- ene,2-Ethenyl-1,3,3-trimethylcyclohexene and 5,5-Dime- thyl-1,3-dithian-2-one (Table 1). 3.4. Identification of the Key Aroma Compounds The most important aroma compounds of TS, isomers of 2-Mercapto-3,4-dimethyl-2,3-dihydrothiophene (the iden- tification method is discussed in the following paragraph), as indicated by peak 22 and 25, were first identified in this study. GC-O analysis indicated that the two peaks were demonstrated to be the characteristic peaks of TS. The two compounds eluted from the GC column one after another within two minutes, but their mass spectro- grams seemed to be nearly identical (Figures 4(a) and (b)), which implied the hypothesis that they might be isomers. Furthermore, the two mass spectrograms were difficult to identify even though many parallel tests were conducted (data not shown). By meticulously comparing the two mass spectrograms of 2-Mercapto-3,4-dimethyl-2,3-di- hydrothiophene and 3,4-Dimethylthiophene with com- pounds in the NIST08 database (Figures 4(a) and (b)), the similarity of these compounds was noticed. This ob- servation resulted in the hypothesis that peaks 22 and 25 corresponded to cis and trans isomers of 2-Mercapto-3,4- dimethyl-2,3-dihydrothiophene (Figures 5(a) and (b)). The presences of these two compounds may be due to the loss of H2S from 2-Mercapto-3,4-dimethyl-2,3-dihydrothio- phene to form 3,4-Dimethylthiophene which would ex- plain the similarity of the MS for the two compounds. This hypothesis is supported by the study that demon- strated 3,4-Dimethylthiophene can be produced by from bis(1-Propenyl) disulfide in a series of reactions [27] (Figure 6). Nevertheless, because of the lack of studies in this area, the identity of the target compounds were unknown before the present study. According to Kuo & Ho [23] the two peaks were located on their correspond- ing positions, that is, the former is cis-2-Mercapto-3,4- dimethyl-2,3-dihydrothiophene while the latter is trans-2- Mercapto-3,4-dimethyl-2,3-dihydrothiophene. Referring to the flavour formation mechanism, 3,4-Dimethylthio- phene and 2-Mercapto-3,4-dimethyl-2,3-dihydrothio- phene tracely exist in slicing onions and garlics, but the latter compound does not emanate its TS aroma in the allium vegetables, which may contribute to the other po- tent compounds such as allicin and allitride [28] and mask their odors. Due to the lack of the other pungent compounds and high amounts of 2-Mercapto-3,4-dime- thyl-2,3-dihydrothiophene (both isomers, the former smelt like cooked TS while the latter showed its fresh TS aroma) it is likely that 3,4-Dimethylthiophene was available by heating 2-Mercapto-3,4-dimethyl-2,3-dihydrothiophene in garlic [29], which implied they might be genarated from the same precursor in TS. However, the aroma chemisrty in TS is very complicated, and requires further investiga- tion. Although there have not been any reports on the mechanisms of TS aroma compound formation, a num- ber of studies on compound formation in garlic, onions and leeks [30,31] suggest it may be possible to elucidate these mechanisms. For instance, some sulfur compounds act as aroma precursors, in that, as they are broken down via physiological metabolism, and aroma-active products are rapidly formed. Further investigation into the reaction mechanisms, related enzymes and substrates in TS is required. 4. Conclusions This study indicated that HS-SPME coupled with GS-MS Copyright © 2013 SciRes. FNS 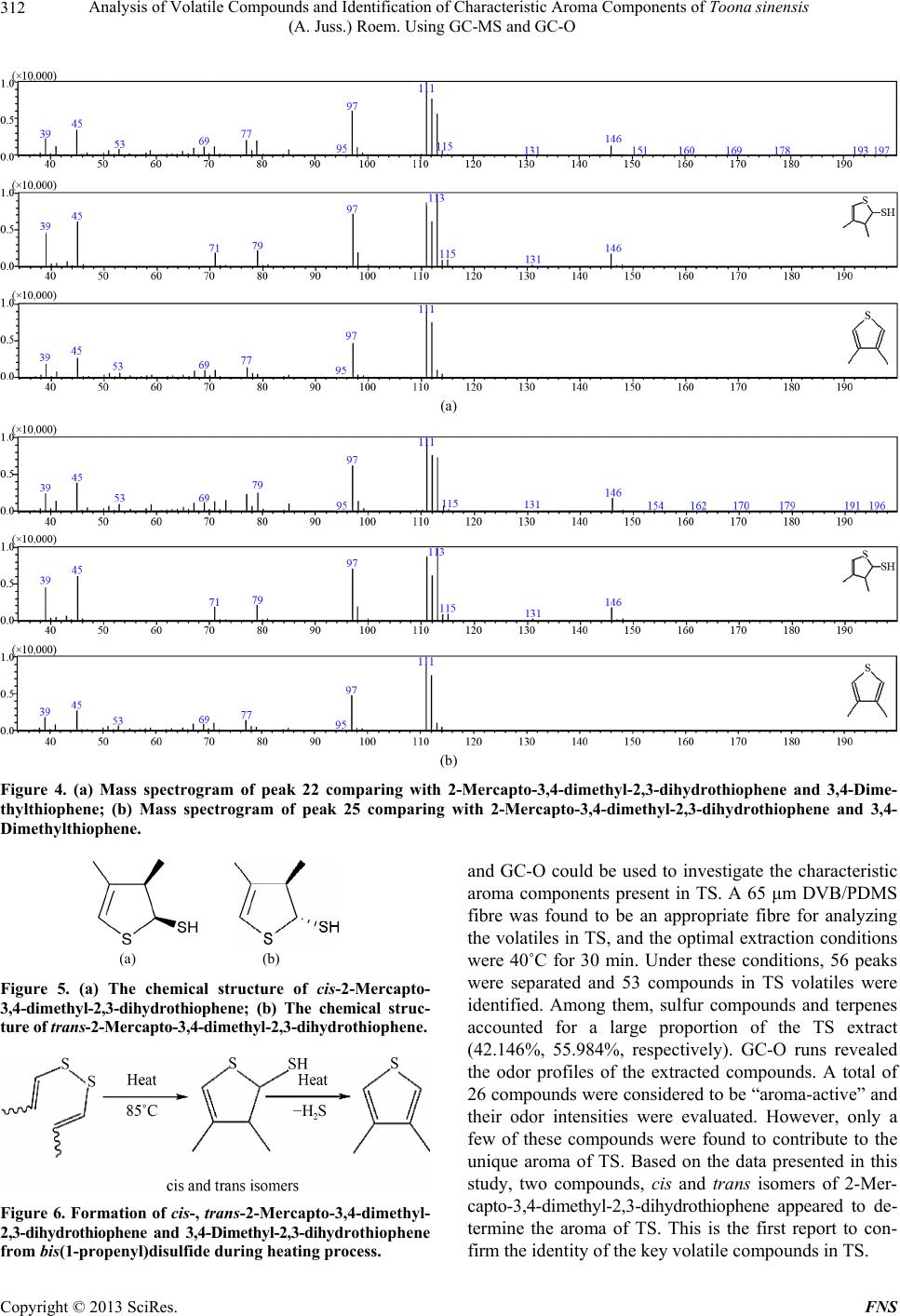 Analysis of Volatile Compounds and Identification of Characteristic Aroma Components of Toona sinensis (A. Juss.) Roem. Using GC-MS and GC-O Copyright © 2013 SciRes. FNS 312 (a) (b) Figure 4. (a) Mass spectrogram of peak 22 comparing with 2-Mercapto-3,4-dimethyl-2,3-dihydrothiophene and 3,4-Dime- thylthiophene; (b) Mass spectrogram of peak 25 comparing with 2-Mercapto-3,4-dimethyl-2,3-dihydrothiophene and 3,4- Dimethylthiophene. and GC-O could be used to investigate the characteristic aroma components present in TS. A 65 μm DVB/PDMS fibre was found to be an appropriate fibre for analyzing the volatiles in TS, and the optimal extraction conditions were 40˚C for 30 min. Under these conditions, 56 peaks were separated and 53 compounds in TS volatiles were identified. Among them, sulfur compounds and terpenes accounted for a large proportion of the TS extract (42.146%, 55.984%, respectively). GC-O runs revealed the odor profiles of the extracted compounds. A total of 26 compounds were considered to be “aroma-active” and their odor intensities were evaluated. However, only a few of these compounds were found to contribute to the unique aroma of TS. Based on the data presented in this study, two compounds, cis and trans isomers of 2-Mer- capto-3,4-dimethyl-2,3-dihydrothiophene appeared to de- termine the aroma of TS. This is the first report to con- firm the identity of the key volatile compounds in TS. (a) (b) Figure 5. (a) The chemical structure of cis-2-Mercapto- 3,4-dimethyl-2,3-dihydrothiophene; (b) The chemical struc- ture of trans-2-Mercapto-3,4-dimethyl-2,3-dihydrothiophene. Figure 6. Formation of cis-, trans-2-Mercapto-3,4-dimethyl- 2,3-dihydrothiophene and 3,4-Dimethyl-2,3-dihydrothiophene from bis(1-propenyl)disulfide during heating process.  Analysis of Volatile Compounds and Identification of Characteristic Aroma Components of Toona sinensis (A. Juss.) Roem. Using GC-MS and GC-O 313 5. Acknowledgements We would like to thank all the panelists participating in the sensory tests. Special thanks to Dr. Chris Blanchard (School of Biomedical Science, Charles Sturt University, Wagga, NSW 2650, Australia) for reviewing the manu- script. REFERENCES [1] J. M. Edmonds and M. Staniforth, “Plate 348. Toona sinensis,” Curtis’s Botanical Magazine, Vol. 15, No. 3, 1998, pp. 186-193. doi:10.1111/1467-8748.00169 [2] T. J. Hsieh, J. C. Wang, C. Y. Hu, C. T. Li, C. M. Kuo and S. L. Hsieh, “Effects of Rutin from Toona sinensis on the Immune and Physiological Responses of White Shrimp (Litopenaeus vannamei) under Vibrio alginolyticus Chal- lenge,” Fish & Shellfish Immunology, Vol. 25, No. 5, 2008, pp. 581-588. doi:10.1016/j.fsi.2008.07.014 [3] Y. C. Hseu, S. C. Chen, W. H. Lin, D. Z. Hung, M. K. Lin, Y. H. Kuo, M. T. Wang, H. J. Cho, L. Wang and H. L. Yang, “Toona sinensis (Leaf Extracts) Inhibit Vascular Endothelial Growth Factor (VEGF)-Induced Angiogene- sis in Vascular Endothelial Cells,” Journal of Ethno- pharmacology, Vol. 134, No. 1, 2011, pp. 111-121. doi:10.1016/j.jep.2010.11.058 [4] H. Kataoka, H. L. Lord and J. Pawliszyn, “Applications of Solid-Phase Microextraction in Food Analysis,” Jour- nal of Chromatography A, Vol. 880, No. 1-2, 2000, pp. 35-62. doi:10.1016/S0021-9673(00)00309-5 [5] S. N. Lee, N. S. Kim and D. S. Lee, “Comparative Study of Extraction Techniques for Determination of Garlic Fla- vor Components by Gas Chromatography-Mass Spec- trometry,” Analytical and Bioanalytical Chemistry, Vol. 377, No. 4, 2003, pp. 749-756. doi:10.1007/s00216-003-2163-z [6] S. B. Junior, A. M. T. De Melo, C. A. Zini and H. T. Godoy, “Optimization of the Extraction Conditions of the Volatile Compounds from Chili Peppers by Headspace Solid Phase Micro-Extraction,” Journal of Chromatog- raphy A, Vol. 1218, No. 21, 2011, pp. 3345-3350. doi:10.1016/j.chroma.2010.12.060 [7] B. D Acampora Zellner, P. Dugo, G. Dugo and L. Mon- dello, “Gas Chromatography-Olfactometry in Food Fla- vour Analysis,” Journal of Chromatography A, Vol. 1186, No. 1, 2008, pp. 123-143. doi:10.1016/j.chroma.2007.09.006 [8] Á. Högnadóttir and R. L. Rouseff, “Identification of Aroma Active Compounds in Orange Essence Oil Using Gas Chromatography-Olfactometry and Gas Chromatog- raphy-Mass Spectrometry,” Journal of Chromatography A, Vol. 998, No. 1-2, 2003, pp. 201-211. doi:10.1016/S0021-9673(03)00524-7 [9] S. Guillot, L. Peytavi, S. Bureau, R. Boulanger, J. P. Le- poutre, J. Crouzet and S. Schorr-Galindo, “Aroma Char- acterization of Various Apricot Varieties Using Head- space-Solid Phase Microextraction Combined with Gas Chromatography-Mass Spectrometry and Gas Chroma- tography-Olfactometry,” Food Chemistry, Vol. 96, No. 1, 2006, pp. 147-155. doi:10.1016/j.foodchem.2005.04.016 [10] H.-P. Lv, Q.-S. Zhong, Z. Lin, L. Wang, J.-F. Tan and L. Guo, “Aroma Characterisation of Pu-erh Tea Using Head- space-Solid Phase Microextraction Combined with GC/ MS and GC-Olfactometry,” Food Chemistry, Vol. 130, No. 4, 2012, pp. 1074-1081. doi:10.1016/j.foodchem.2011.07.135 [11] H. Van den Dool and P. Dec Kratz, “A Generalization of the Retention Index System Including Linear Tempera- ture Programmed Gas—Liquid Partition Chromatogra- phy,” Journal of Chromatography A, Vol. 11, No. 2, 1963, pp. 463-471. [12] G. Botelho, A. Mendes-Faia and M. C. Clímaco, “Char- acterisation of Free and Glycosidically Bound Odourant Compounds of Aragonez Clonal Musts by GC-O,” Ana- lytica Chimica Acta, Vol. 657, No. 2, 2010, pp. 198-203. doi:10.1016/j.aca.2009.10.030 [13] L. Culleré, F. San-Juan and J. Cacho, “Characterisation of Aroma Active Compounds of Spanish Saffron by Gas Chromatography-Olfactometry: Quantitative Evaluation of the Most Relevant Aromatic Compounds,” Food Chemis- try, Vol. 127, No. 4, 2011, pp. 1866-1871. doi:10.1016/j.foodchem.2011.02.015 [14] S. Kreutzmann, A. K. Thybo and W. L. P. Bredie, “Train- ing of a Sensory Panel and Profiling of Winter Hardy and Coloured Carrot Genotypes,” Food Quality and Prefer- ence, Vol. 18, No. 3, 2007, pp. 482-489. doi:10.1016/j.foodqual.2006.05.009 [15] J. Marais, “Terpenes in the Aroma of Grapes and Wines: A Review,” South African Journal for Enology and Viti- culture, Vol. 4, No. 2, 1983, pp. 49-60. [16] J. H. Kwon, K. Akram, K. C. Nam, E. J. Lee and D. U. Ahn, “Evaluation of Radiation-Induced Compounds in Irradiated Raw or Cooked Chicken Meat during Storage,” Poultry Science, Vol. 90, No. 11, 2011, pp. 2578-2583. doi:10.3382/ps.2010-01237 [17] S. Rochat, J. Y. S. Laumer and A. Chaintreau, “Analysis of Sulfur Compounds from the In-Oven Roast Beef Aroma by Comprehensive Two-Dimensional Gas Chromatogra- phy,” Journal of Chromatography A, Vol. 1147, No. 1, 2007, pp. 85-94. doi:10.1016/j.chroma.2007.02.039 [18] S. Wang, S. Yang, L. Ren, C. Qian, F. Liu and S. Jiang, “Determination of Organophosphorus Pesticides in Leeks (Allium porrum L.) by GC-FPD,” Chromatographia, Vol. 69, No. 1, 2009, pp. 79-84. doi:10.1365/s10337-008-0816-y [19] I. S. Chung, K. Y. Chae and K. H. Kyung, “Thermal Gen- eration and Antimicrobial Activity of Unusual Heterocyc- lic Sulfur Compounds in Garlic,” Food Science and Bio- technology, Vol. 17, No. 5, 2008, pp. 1032-1037. [20] C. C. Eady, T. Kamoi, M. Kato, N. G. Porter, S. Davis, M. Shaw, A. Kamoi and S. Imai, “Silencing Onion Lachry- matory Factor Synthase Causes a Significant Change in the Sulfur Secondary Metabolite Profile,” Plant Physiol- ogy, Vol. 147, No. 4, 2008, pp. 2096-2106. doi:10.1104/pp.108.123273 [21] R. Mu, X. Wang, S. Liu, X. Yuan, S. Wang and Z. Fan, Copyright © 2013 SciRes. FNS  Analysis of Volatile Compounds and Identification of Characteristic Aroma Components of Toona sinensis (A. Juss.) Roem. Using GC-MS and GC-O Copyright © 2013 SciRes. FNS 314 “Rapid Determination of Volatile Compounds in Toona sinensis (A. Juss.) Roem. by MAE-HS-SPME Followed by GC-MS,” Chromatographia, Vol. 65, No. 7-8, 2007, pp. 463-467. doi:10.1365/s10337-007-0183-0 [22] A. Bianchi, A. Zambonelli, A. Z. D’Aulerio and F. Belle- sia, “Ultrastructural Studies of the Effects of Allium sati- vum on Phytopathogenic Fungi in Vitro,” Plant Disease, Vol. 81, No. 11, 1997, pp. 1241-1246. doi:10.1094/PDIS.1997.81.11.1241 [23] M. C. Kuo and C. T. Ho, “Volatile Constituents of the Solvent Extracts of Welsh Onions (Allium fistulosum L. variety Maichuon) and Scallions (A. Fistulosum L. Vari- ety Caespitosum),” Journal of Agricultural and Food Chemistry, Vol. 40, No. 10, 1992, pp. 1906-1910. doi:10.1021/jf00022a036 [24] M. R. Corbo, R. Lanciotti, F. Gardini, M. Sinigaglia and M. E. Guerzoni, “Effects of Hexanal, trans-2-Hexenal, and Storage Temperature on Shelf Life of Fresh Sliced Apples,” Journal of Agricultural and Food Chemistry, Vol. 48, No. 6, 2000, pp. 2401-2408. doi:10.1021/jf991223f [25] R. Marín, M. A. Apel, R. P. Limberger, M. C. B. Raseira, J. F. M. Pereira, J. A. S. Zuanazzi and A. T. Henriques, “Volatile Components and Antioxidant Activity from Some Myrtaceous Fruits Cultivated in Southern Brazil,” Latin American Journal of Pharmacy, Vol. 27, No. 2, 2008, pp. 172-177. [26] I. A. Ogunwande, G. Flamini, P. L. Cioni, O. Omikorede, R. A. Azeez, A. A. Ayodele and Y. O. Kamil, “Aromatic Plants Growing in Nigeria: Essential Oil Constituents of Cassia alata (Linn.) Roxb. and Helianthus annuus L.,” Records of Natural Products, Vol. 4, No. 4, 2010, pp. 211-217. [27] E. Block, D. Putman and S. H. Zhao, “Allium Chemistry: GC-MS Analysis of Thiosulfinates and Related Com- pounds from Onion, Leek, Scallion, Shallot, Chive, and Chinese Chive,” Journal of Agricultural and Food Chem- istry, Vol. 40, No. 12, 1992, pp. 2431-2438. doi:10.1021/jf00024a018 [28] E. Block, “The Chemistry of Garlic and Onions,” Scien- tific American, Vol. 252, No. 3, 1985, pp. 114-119. doi:10.1038/scientificamerican0385-114 [29] E. Block and S. H. Zhao, “Onion Essential Oil Chemistry. cis-and trans-2-mercapto-3,4-dimethyl 2,3-dihydrothio- phene from Pyrolysis of Bis(1-propenyl) Disulfide,” Tet- rahedron Letters, Vol. 31, No. 35, 1990, pp. 4999-5002. doi:10.1016/S0040-4039(00)97788-8 [30] E. Block, T. Bayer, S. Naganathan and S. H. Zhao, “Al- lium Chemistry: Synthesis and Sigmatropic Rearrange- ments of Alk (en) yl 1-Propenyl Disulfide S-Oxides from Cut Onion and Garlic1,” Journal of the American Chemical Society, Vol. 118, No. 12, 1996, pp. 2799-2810. doi:10.1021/ja953444h [31] G. S. Nielsen and L. Poll, “Determination of Odor Active Aroma Compounds in Freshly Cut Leek (Allium ampelo- prasum Var. Bulga) and in Long-Term Stored Frozen Unblanched and Blanched Leek Slices by Gas Chroma- tography Olfactometry Analysis,” Journal of Agricultural and Food Chemistry, Vol. 52, No. 6, 2004, pp. 1642- 1646. doi:10.1021/jf030682k
|