Paper Menu >>
Journal Menu >>
 Journal of Cosmetics, Dermatological Sciences and Applications, 2013, 3, 99-106 http://dx.doi.org/10.4236/jcdsa.2013.31014 Published Online March 2013 (http://www.scirp.org/journal/jcdsa) 99 Suppression of Sebum Production and Accumulation by β-Cryptoxanthin Due to the Inhibition of the Expression of Diacylglycerol Acyltransferase-1 and Perilipin in Hamster Sebocytes* Takashi Sato1#, Yoshiyuki Shirakura2, Katsuyuki Mukai2, Akira Ito1 1Department of Biochemistry and Molecular Biology, School of Pharmacy, Tokyo University of Pharmacy and Life Sciences, Ha- chioji, Tokyo, Japan; 2R & D Center, Unitika Ltd., Kyoto, Japan. Email: #satotak@toyaku.ac.jp Received December 13th, 2012; revised January 15th, 2013; accepted January 24th, 2013 ABSTRACT Background: Acne vulgaris is characterized by the enhancement of sebaceous lipogenesis and sebum secretion, and apart from retinoids and some natural products there are few effective anti-acne agents that directly suppress sebum production and accumulation in sebaceous glands. Objective: We examined the effects of β-cryptoxanthin (β-CRX), which is a carotenoid pigment most abundant in Citrus unshiu Marcovich (Satsuma mandarin orange) and plays a role as a Vitamin A precursor on sebum production and accumulation in hamster sebaceous gland cells (sebocytes). Materi- als and methods: The regulation of sebum production was examined by the measurement of triacylglycerols (TGs), the major sebum component, and oil red O staining in insulin-differentiated hamster sebocytes. The expression of diacyl- glycerol acyltransferase-1 (DGAT-1), a rate-limiting enzyme of TG biosynthesis, and perilipin 1 (PLIN1), a lipid stor- age droplet protein, was analyzed using real-time PCR and Western blotting. Results: Hamster sebocytes constitutively produced TGs during cultivation and the production of TGs was enhanced by insulin treatment. Both constitutive and insulin-enhanced TG productions were dose- and time-dependently inhibited by β-CRX as well as 13-cis retinoic acid. In addition, the gene expression of DGAT-1 was suppressed by β-CRX in the sebocytes. Furthermore, the insulin-en- hanced sebum accumulation as lipid droplets was reduced in the β-CRX-treated cells. Moreover, β-CRX was found to suppress the gene expression and production of PLIN1 in insulin-differentiated hamster sebocytes. Conclusions: These results provide novel evidence that β-CRX is an effective candidate for acne therapy by its ability to exert dual inhibi- tory actions against DGAT-1-dependent TG production and PLIN1-mediated lipid-droplet formation in hamster sebo- cytes. Keywords: β-Cryptoxanthin; Sebocytes; Triacylglycerol Biosynthesis; Diacyglycerol Acyltransferase; Perilipin; Lipid-Droplet Formation; Sebum 1. Introduction The pathogenesis of acne, a common inflammatory skin disease [1,2], is characterized by: 1) excess sebum pro- duction in sebaceous glands; 2) the formation of micro- comedones, which is closely associated with the hyperk- eratinization of the follicular wall and infundibulum; 3) the hyperproliferation of Propionibacterium acnes (P. acnes); and 4) the induction of inflammatory reactions such as the acceleration of cytokine production and the biosynthesis of arachidonic acid metabolites in keratino- cytes, sebocytes, and invaded inflammatory cells [3,4]. The aggravation and duration of the inflammation are likely to result in acne scar that causes a psychological and social impact in the patient’s quality of life [4]. Sebum production in acne lesions has been reported to be increased by 5α-dihydrotestosterone, insulin, insu- lin-like growth factor 1, and prostaglandins [5-9]. In ad- dition, the biosynthesis of sebum components such as triacylglycerols (TGs) and sapienic acids is regulated by diacylglycerol acyltransferase (DGAT), a rate-limiting enzyme of TG synthesis [6,10], and ∆6 desaturase (FADS2) [11], respectively, in human and hamster se- bocytes. Furthermore, we have previously reported that the production of perilipin (PLIN), a lipid storage droplet protein, is augmented in differentiated hamster sebocytes and localized on the surface of intracellular lipid droplets, *Conflict of interest: The authors have no conflict of interest to declare. #Corresponding author. Copyright © 2013 SciRes. JCDSA  Suppression of Sebum Production and Accumulation by β-Cryptoxanthin Due to the Inhibition of the Expression of Diacylglycerol Acyltransferase-1 and Perilipin in Hamster Sebocytes 100 indicating that perilipin is a differentiation marker in ham- ster sebocytes [12]. On the other hand, retinoic acids such as tretinoin (all-trans retinoic acid; atRA) and isot- retinoin (13-cis retinoic acid; 13-cisRA) have been topi- cally and/or systemically used for acne therapy [13,14]. They have been reported to exhibit comedolytic, anti- inflammatory, and anti-lipogenetic actions in sebaceous glands and pilosebaceous units in humans, rats, and ham- sters in vivo and in vitro [14-19]. However, the use of retinoids in acne therapy has been limited in acceptance because of adverse effects such as skin irritation, scaling, and teratogenicity [13]. β-Cryptoxanthin (β-CRX), a carotenoid pigment most abundant in Citrus unshiu Marcovich (Satsuma mandarin orange), has been reported to exhibit multiple disease pre- ventive actions; e.g. anti-tumorigenic, anti-obesity, anti- atherogenic, and immunopotentiative ones [20-23]. In ad- dition, carotenoids such as β-CRX and β-carotene have been reported to be present in the blood of people from different countries including Japan, which is associated with some health benefits [22,24]. Indeed, epidemiologic studies have shown that higher intakes or blood levels of β-CRX result in a reduced risk of lung cancer and rheu- matoid arthritis development [25-27]. On the other hand, it has also been reported that low plasma level of Vita- min A is associated with the development and aggrava- tion of acne [28]. In addition, β-CRX is a vitamin A pre- cursor, which is oxidatively cleaved to vitamin A by β-carotene 15,15’-dioxygenase [29], and can be stably stored in some tissue for several months [30]. Taken to- gether with a recent report by Shirakura et al. [31] where β-CRX inhibits the intracellular lipid-droplet formation in mouse 3T3-L1 adipocytes, we hypothesize that, in- stead of retinoids such as atRA and 13-cisRA, β-CRX is an effective candidate for acne therapy by modulating sebaceous lipogenesis. However, it is not fully under- stood whether β-CRX directly suppresses sebum pro- duction and accumulation in sebaceous gland cells (se- bocytes) or not. In the present study, we demonstrated that β-CRX dose- and time-dependently inhibited the production and intra- cellular accumulation of sebum in insulin-differentiated hamster sebocytes. Furthermore, the β-CRX-mediated in- hibition of sebum production and accumulation is closely associated with the transcriptional suppression of DGAT- 1 and PLIN1, respectively, in differentiated hamster se- bocytes. 2. Materials and Methods 2.1. Cell Culture and Treatment Hamster sebocytes (2.4 × 104 cells/cm2) [32] were plated onto 96-well multiplates, 35-mm or 100-mm diameter culture dishes (Becton Dickinson, Tokyo, Japan) and then cultured for 24 h in DMEM/F12 (Invitrogen, Carls- bad, CA) supplemented with 6% heat-denatured fetal bovine serum (Nichirei Biosciences Inc., Tokyo, Japan), 2% human serum (C-C Biotech Co., Valley Center, CA), 0.68 mM L-glutamine (Invitrogen), and recombinant human epidermal growth factor (10 nM) (Progen Bio- technik GmbH, Heidelberg, Germany) to achieve com- plete cell adhesion as previously described [6,18]. The hamster sebocytes were treated every two days for up to 8 days with or without β-CRX (purity ≥ 95%; Shikoku Yashima Pure Chemicals, Tokushima, Japan) (Figure 1) or 13-cisRA (Sigma Chemical, St. Louis, MO) in the presence or absence of a sebocyte-differentiation inducer, insulin (10 nM) (Sigma Chemical) [12] in DMEM/F12 supplemented with heat-denatured fetal bovine serum, human serum, and L-glutamine. In this series of experi- ments, hamster sebocytes were used as far as the 3rd pas- sage level. 2.2. Analyses of Sebum Production and Accumulation After treating sebocytes with β-CRX, 13-cisRA, and/or insulin, the cells were subjected to the quantification of TGs, the major sebum component, using Liquitech TG-II (Roche Diagnostics, Tokyo, Japan) as previously de- scribed [12]. The amounts of intracellular TGs were cal- culated using an authentic trioleinate-standard solution (0.6 mg/ml). Intracellular DNA content was measured using salmon sperm DNA (6.25 - 100 mg/ml) and 3,5- diaminobenzoic acid dihydrochloride (Sigma Chemical). For the analysis of intracellular sebum accumulation, oil red O staining was performed. Briefly, the cells were washed once with Ca2+- and Mg2+-free phosphate-buff- ered saline [PBS(-)] and fixed with 4% paraformalde- hyde (Wako Pure Chemicals, Osaka, Japan) diluted with PBS(-) for 1 h at room temperature. The cells were washed with distilled H2O and then stained with 0.3% oil red O (Sigma Chemical) in isopropanol:distilled H2O (3:2, vol:vol) at 37˚C for 15 min. The stained cells were washed with distilled H2O, and then viewed with a light microscope furnished with a digital camera (Olympus Op- tical Co., Tokyo, Japan). 2.3. Real-Time PCR For the quantification of DGAT-1 and PLIN1 mRNA, total RNA was isolated from cells using ISOGEN (Nip- pon Gene, Toyama, Japan) and then the aliquot of RNA (500 ng) was subjected to reverse transcriptase reaction Figure 1. Chemical structure of β-cryptoxanthin (β-CRX). Copyright © 2013 SciRes. JCDSA  Suppression of Sebum Production and Accumulation by β-Cryptoxanthin Due to the Inhibition of the Expression of Diacylglycerol Acyltransferase-1 and Perilipin in Hamster Sebocytes 101 for the synthesis of cDNA using a PrimeScript RT re- agent Kit (Takara Bio, Shiga, Japan) according to the manufacturer’s instructions. Aliquots (an equivalent of 2.5 ng of total RNA) of the transcript were subjected to real-time PCR using SYBR Premix Ex Taq II (Takara Bio) and the following specific primers: human DGAT-1 (NM_012079); 5’-TCTACAAGCCCATGCTTCGAC-3’ (sense) and 5’-GGACGCTCACCAGGTACT-3’ (an- tisense), hamster PLIN1 (AB091681); 5’-ACCTTGCT GGATGGAGACC-3’ (sense) and 5’-CCAGGACCTTG TCTGAAGT-3’ (antisense), and hamster glyceralde- hyde-3-phosphate dehydrogenase (GAPDH) (X52123); 5’-CAGAACATCATCCCTGCAT-3’ (sense) and 5’-TA GGAACACGGAAGGCCAT-3’ (antisense) as previ- ously described [33]. The amplification cycle was per- formed at 94˚C for 5 s and 60˚C for 30 s using a Thermal Cycler Dice Real Time System TP-800 (Takara Bio). The obtained threshold cycle (CT) value for DGAT-1 and PLIN1 was normalized by that for GAPDH, and the relative expression level was expressed as the mean value of the control as 1. 2.4. Western Blot Analysis The harvested cell lysate (50 μg protein) was subjected to Western blot analysis using 12.5% acrylamide gel as previously described [33]. The membrane was reacted with rabbit anti-(human PLIN1) IgG, which was custom- ized by Operon Biotechnologies (Tokyo, Japan). To evaluate the level of β-actin as an internal control, the harvested cell lysates (50 μg protein) were similarly sub- jected to Western blot analysis using rabbit anti-(human β-actin) IgG (Medical & Biological Laboratories, Nagoya, Japan). Immunoreactive PLIN1 and β-actin were visual- ized with Amersham enhanced chemiluminescence-Wes- tern blotting detection reagents (GE Healthcare Bio- Sciences, Tokyo, Japan) according to the manufacturer’s instructions. Relative amounts of PLIN1 protein against β-actin were quantified by densitometric scanning using an Image Analyzer LAS-1000 Plus (GE Healthcare), and the relative expression level was expressed as the mean value of the control as 1. 2.5. Statistical Analysis A one-way ANOVA was used for the statistical analysis, and then the Fisher test was applied when multiple com- parisons were performed. 3. Results 3.1. Inhibition of TG Production and DGAT-1 Gene Expression by β-CRX in Hamster Sebocytes Since hamster sebocytes constitutively produce TGs dur- ing cultivation in vitro [32], we first examined the effect of β-CRX on constitutive TG production in cultured hamster sebocytes. As shown in Figure 2(A), β-CRX was found to decrease the level of TGs in a dose-de- pendent manner (70% inhibition at 10 μM). In addition, the sebocyte differentiation inducer, insulin (10 nM), was found to enhance the production of TGs in hamster se- bocytes, and the enhanced level of TGs was dose-de- pendently decreased by β-CRX (72% inhibition at 10 μM). Furthermore, a similar decrease in TG level was time-dependently observed in hamster sebocytes treated with β-CRX as well as 13-cis RA (Figure 3), as we pre- viously reported [18,34]. On the other hand, the gene expression of DGAT-1 was constitutively detectable, and was found to be increased in response to insulin treat- ment in hamster sebocytes (7.7 ± 3.9 fold, p < 0.05) (Figure 2(B)). In addition, β-CRX was found to suppress the gene expression of DGAT-1 in both the insulin-un- treated and treated hamster sebocytes (52% and 88% inhibition, respectively) (Figure 2(B)). Therefore, these results suggest that β-CRX inhibits the production of TGs due to the suppression of DGAT-1 expression in hamster sebocytes. 3.2. Suppression of Sebum Accumulation by Decreasing Gene Expression and Production of Perilipin in Hamster Sebocytes As the intracellular accumulation of sebum as lipid-dro- plets has been reported to be due to the increase of TG production in differentiated hamster sebocytes [32], oil red O staining revealed that the lipid-droplet formation was augmented in the insulin-differentiated hamster se- bocytes (Figures 4(B) vs. (A)). In addition, the enhanced sebum accumulation was found to be abolished by add- ing β-CRX (10 μM) (Figures 4(C) vs . (B)). On the other hand, perilipin, a lipid-droplet surface protein, has been reported to play an important role in the formation of intracellular lipid droplets in differentiated adipocytes, steroidogenic cells, and sebocytes [12,35]. As β-CRX inhibited sebum accumulation as lipid droplets (Figure 4), we examined whether β-CRX influenced the produc- tion of PLIN1 in hamster sebocytes. As shown in Figure 5, the production of PLIN1 was barely detectable in insu- lin-untreated sebocytes, but was augmented by insulin treatment. In addition, β-CRX was found to dose-depen- dently suppress the insulin-enhanced production of PLIN1 in hamster sebocytes. Furthermore, both basal and insulin-augmented levels of PLIN1 mRNA were found to be decreased in the β-CRX-treated cells (Figure 6). Thus, these results suggest that the suppression of PLIN1 pro- duction by β-CRX is associated with the inhibition of sebum accumulation in differentiated hamster sebocytes. Copyright © 2013 SciRes. JCDSA 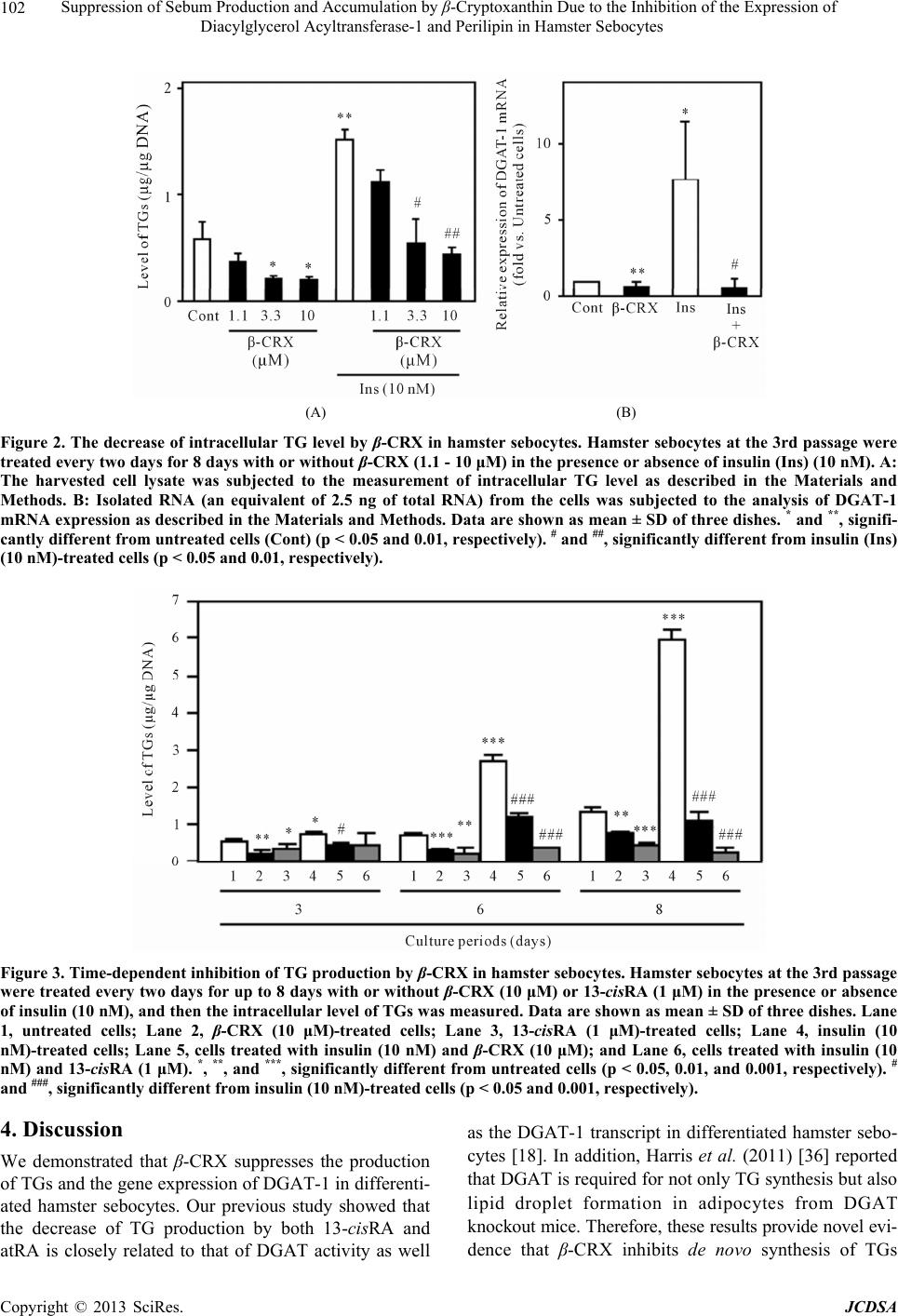 Suppression of Sebum Production and Accumulation by β-Cryptoxanthin Due to the Inhibition of the Expression of Diacylglycerol Acyltransferase-1 and Perilipin in Hamster Sebocytes Copyright © 2013 SciRes. JCDSA 102 (A) (B) Figure 2. The decrease of intracellular TG level by β-CRX in hamster sebocytes. Hamster sebocytes at the 3rd passage were treated every two days for 8 days with or without β-CRX (1.1 - 10 μM) in the presence or absence of insulin (Ins) (10 nM). A: The harvested cell lysate was subjected to the measurement of intracellular TG level as described in the Materials and Methods. B: Isolated RNA (an equivalent of 2.5 ng of total RNA) from the cells was subjected to the analysis of DGAT-1 mRNA expression as described in the Materials and Methods. Data are shown as mean ± SD of three dishes. * and **, signifi- cantly different from untreated cells (Cont) (p < 0.05 and 0.01, respectively). # and ##, significantly different from insulin (Ins) (10 nM)-treated cells (p < 0.05 and 0.01, respectively). Figure 3. Time-dependent inhibition of TG production by β-CRX in hamster sebocytes. Hamster sebocytes at the 3rd passage were treated every two days for up to 8 days with or without β-CRX (10 μM) or 13-cisRA (1 μM) in the presence or absence of insulin (10 nM), and then the intracellular level of TGs was measured. Data are shown as mean ± SD of three dishes. Lane 1, untreated cells; Lane 2, β-CRX (10 μM)-treated cells; Lane 3, 13-cisRA (1 μM)-treated cells; Lane 4, insulin (10 nM)-treated cells; Lane 5, cells treated with insulin (10 nM) and β-CRX (10 μM); and Lane 6, cells treated with insulin (10 nM) and 13-cisRA (1 μM). *, **, and ***, significantly different from untreated cells (p < 0.05, 0.01, and 0.001, respectively). # and ###, significantly different from insulin (10 nM)-treated cells (p < 0.05 and 0.001, respectively). 4. Discussion as the DGAT-1 transcript in differentiated hamster sebo- cytes [18]. In addition, Harris et al. (2011) [36] reported that DGAT is required for not only TG synthesis but also lipid droplet formation in adipocytes from DGAT knockout mice. Therefore, these results provide novel evi- dence that β-CRX inhibits de novo synthesis of TGs We demonstrated that β-CRX suppresses the production of TGs and the gene expression of DGAT-1 in differenti- ated hamster sebocytes. Our previous study showed that the decrease of TG production by both 13-cisRA and atRA is closely related to that of DGAT activity as well  Suppression of Sebum Production and Accumulation by β-Cryptoxanthin Due to the Inhibition of the Expression of Diacylglycerol Acyltransferase-1 and Perilipin in Hamster Sebocytes 103 Figure 4. β-CRX decreases sebum accumulation in insulin- differentiated hamster sebocytes. Hamster sebocytes treated with insulin (10 nM) and/or β-CRX (10 μM) as shown in Figure 2 were subjected to oil red O staining for the analy- sis of intracellular sebum accumulation as described in the Materials and Methods. A: untreated cells; B: insulin (10 nM)-treated cells; and C: cells treated with insulin (10 nM) and β-CRX (10 μM). Bars: 50 μm. Figure 5. β-CRX and 13-cisRA suppress the production of PLIN1 in insulin-differentiated hamster sebocytes. Cell ly- sate (50 μg protein) prepared from hamster sebocytes treat- ed every two days for 8 days with insulin (10 nM), β-CRX (1.1 - 10 μM), and/or 13-cisRA (1 μM) was subjected to Western blot analysis for PLIN1 and β-actin as described in the Materials and Methods. Data are shown as mean ± SD of four independent experiments. ***, significantly different from untreated cells (p < 0.001). # and ###, significantly dif- ferent from insulin (10 nM)-treated cells (p < 0.05 and 0.001, respectively). by suppressing DGAT-1 expression, which may in turn participate in the inhibition of lipid droplet formation in differentiated sebocytes. Furthermore, taken together with previous reports that TGs from sebaceous glands are a source of nutrition of P. acnes [1,3], β-CRX-decreased TG production is very likely to indirectly result in the preven- tion of P. acnes proliferation in acne lesions. The PAT-family of intracellular lipid storage droplet proteins such as PLIN1-5 has been reported to play im- portant roles in the regulation of TG storage and lipolysis in adipocytes and steroidogenic cells [35]. Our previous study showed that PLIN1 localizes to the surface of in- tracellular sebum-droplets in differentiated hamster se- bocytes [12]. In the present study, we found that not only intracellular lipid-droplet formation but also the gene expression and production of PLIN1 was suppressed by β-CRX in differentiated hamster sebocytes. A similar Figure 6. Suppression of PLIN1 mRNA expression by b- CRX in insulin-differentiated hamster sebocytes. Isolated RNA (an equivalent of 2.5 ng of total RNA) from the cells treated with insulin (10 nM), β-CRX (1.1 - 10 mM), and/or 13-cisRA (1 mM) as shown in Figure 5 was subjected to the analysis of PLIN1 mRNA expression as described in the Materials and Methods. Data are shown as mean ± SD of four different experiments. *, significantly different from untreated cells (p < 0.05). # and ##, significantly different from insulin (10 nM)-treated cells (p < 0.05 and 0.01, re- spectively). inhibition of lipid-droplet formation has been reported to be observed in β-CRX-treated mouse 3T3-L1 adipocytes [31]. Therefore, the β-CRX-inhibition of lipid-droplet for- mation is likely to include the transcriptional suppression of PLIN1 production in hamster sebocytes. Moreover, intracellular lipid-droplet formation has been reported to be associated with the protein kinase A (PKA)-dependent phosphorylation of PLIN, which facilitates the hormone- sensitive lipase-mediated lipolysis of neutral lipid drop- lets in adipocytes [37,38]. We previously reported that neither the level of cyclic AMP (cAMP) nor PKA activ- ity was augmented by 13-cisRA [18], of which its sup- pressive action against sebum accumulation is similar to that of β-CRX in differentiated hamster sebocytes. How- ever, β-carotene, which is structurally and functionally similar to β-CRX [39], has been reported to increase the level of cAMP and PKA activity in human pulmonary adenocarcinoma cells and epithelial cells from small air- ways and pancreatic ducts [40,41]. Thus, β-CRX may also influence the phosphorylation of PLIN1, which is coordinately associated with the abolishment of lipid- droplets in differentiated hamster sebocytes. Further ex- periments are needed to clarify these hypotheses. The development of comedones has been related to the enhancement of sebum production in sebaceous glands, under which various lipogenetic signaling through an- drogen and/or insulin-like growth factor 1/insulin path- ways is activated [42,43]. In the inflamed acne lesions resulting from comedogenesis, the expression and activa- tion of PPARγ have been reported to be augmented and closely associated with the aggravation of acne pathology [44,45]. Shirakura et al. [31] reported that β-CRX does not affect peroxisome proliferators activating receptor γ Copyright © 2013 SciRes. JCDSA  Suppression of Sebum Production and Accumulation by β-Cryptoxanthin Due to the Inhibition of the Expression of Diacylglycerol Acyltransferase-1 and Perilipin in Hamster Sebocytes 104 (PPARγ) activation. We have preliminarily demonstrated that β-CRX did not alter the PPARγ ligand, troglita- zone-augmented production of TGs and PLIN1 produc- tion in hamster sebocytes (Sato T and Ito A, unpublished data). Therefore, the inhibitory actions of β-CRX against sebum production and storage in sebocytes may at least partially account for the beneficial efficacy in the pre- vention of comedogenesis in acne lesions under non- inflammatory conditions. Carotenoids split into two groups; xanthophylls and carotenes [21]. Xanthophylls such as β-CRX exist as an ester form with fatty acids in fruits or vegetables [46,47]. In addition, xanthophyll esters have been reported to be present in human skin, where the xanthophylls are re- esterified following absorption [48]. Since the blood concentration of β-CRX has been reported to be higher than that of β-carotene or lycopene [47], it is suggested that β-CRX is easily absorbed and accumulated in the skin, where it may behave as Vitamin A for the control of cutaneous functions. Taken together with a report of El-Akawi et al. [28] that low plasma levels of Vitamin A are associated with acne development and aggravation, therefore, the supplementation of β-CRX is likely to be effective for the prevention of acne or acne maintenance. In conclusion, our findings provide novel evidence that β-CRX is an effective candidate for acne therapy by inhibiting not only sebaceous lipogenesis but also sebum accumulation against insulin-differentiated hamster se- bocytes, in which acne pathology is at least partly mim- icked in vitro. Furthermore, these findings may contrib- ute to a novel understanding of the molecular mecha- nisms of dietary carotenoids in the maintenance of skin barrier functions. 5. Acknowledgements This work was supported by a Grant-in-Aid for Scientific Research (C) (#22590506). We wish to thank Dr. K. Ki- tamura and Miss. M. Kuwata for their technical assis- tance. REFERENCES [1] H. Pawin, C. Beylot, M. Chivot, M. Faure, F. Poli, J. Revuz and B. Dréno, “Physiopathology of Acne Vulgaris: Recent Data, New Understanding of the Treatments,” European Journal of Dermatology, Vol. 14, No. 1, 2004, pp. 4-12. [2] C. C. Zouboulis, A. Eady, M. Philpott, L. A. Goldsmith, C. Orfanos, W. C. Cunliffe and R. Rosenfield, “What Is the Pathogenesis of Acne?” Experimental Dermatology, Vo. 14, No. 2, 2005, pp. 143-152. doi:10.1111/j.0906-6705.2005.0285a.x [3] H. C. Williams, R. P. Dellavalle and S. Garner, “Acne Vulgaris,” Lancet, Vol. 379, No. 9813, 2012, pp. 361- 372. doi:10.1016/S0140-6736(11)60321-8 [4] I. Kurokawa, F. W. Danby, Q. Ju, X. Wang, L. F. Xiang, L. Xia, W. Chen, I. Nagy, M. Picardo, D. H. Suh, R. Ganceviciene, S. Schagen, F. Tsatsou and C. C. Zoub- oulis, “New Developments in Our Understanding of Acne Pathogenesis and Treatment,” Experimental Dermatology, Vol. 18, No. 10, 2009, pp. 821-832. doi:10.1111/j.1600-0625.2009.00890.x [5] R. L. Rosenfield, A. Kentsis, D. Deplewski and N. Ciletti, “Rat Preputial Sebocyte Differentiation Involves Perox- isome Proliferator-Activated Receptors,” Journal of In- vestigative Dermatology, Vol. 112, No. 2, 1999, pp. 226- 232. doi:10.1046/j.1523-1747.1999.00487.x [6] C. Iwata, N. Akimoto, T. Sato, Y. Morokuma and A. Ito, “Augmentation of Lipogenesis by 15-Deoxy-δ12,14-Pros- taglandin J2 in Hamster Sebaceous Glands: Identification of Cytochrome P-450-Mediated 15-deoxy-δ12,14-Prostag- landin J2 Production,” Journal of Investigative Derma- tology, Vol. 125, No. 5, 2005, pp. 865-872. [7] T. Alestas, R. Ganceviciene, S. Fimmel, K. Müller-Decker and C. C. Zouboulis, “Enzymes Involved in the Biosyn- thesis of Leukotriene B4 and Prostaglandin E2 Are Active in Sebaceous Glands,” Journal of Molecular Medicine, Vol. 84, No. 1, 2006, pp. 75-87. doi:10.1007/s00109-005-0715-8 [8] T. M. Smith, Z. Cong, K. L. Gilliland, G. A. Clawson and D. M. Thiboutot, “Insulin-Like Growth Factor-1 Induces Lipid Production in Human SEB-1 Sebocytes via Sterol Response Element-Binding Protein-1,” Journal of Inves- tigative Dermatology, Vol. 126, No. 6, 2006, pp. 1226- 1232. doi:10.1038/sj.jid.5700278 [9] N. R. Trivedi, K. L. Gilliland, W. Zhao, W. Liu and D. M. Thiboutot, “Gene Array Expression Profiling in Acne Le- sions Reveals Marked Upregulation of Genes Involved in Inflammation and Matrix Remodeling,” Journal of Inves- tigative Dermatology, Vol. 126, No. 5, 2006, pp. 1071- 1079. doi:10.1038/sj.jid.5700213 [10] A. Turkish and S. L. Sturley, “Regulation of Triglyceride Metabolism. I. Eukaryotic Neutral Lipid Synthesis: ‘Many Ways to Skin ACAT or a DGAT’,” American Journal of Physiology. Gastrointestinal and Liver Physiology, Vol. 292, No. 4, 2007, pp. G953-957. [11] L. Ge, J. S. Gordon, C. Hsuan, K. Stenn and S. M. Prouty, “Identification of the Δ-6 Desaturase of Human Seba- ceous Glands: Expression and Enzyme Activity,” Journal of Investigative Dermatology, Vol. 120, No. 5, 2003, pp. 707-714. doi:10.1046/j.1523-1747.2003.12123.x [12] N. Akimoto, T. Sato, C. Iwata, M. Koshizuka, F. Shibata, A. Nagai, M. Sumida and A. Ito, “Expression of Perilipin A on the Surface of Lipid Droplets Increases along with the Differentiation of Hamster Sebocytes in Vivo and in Vitro,” Journal of Investigative Dermatology, Vol. 124, No. 6, 2005, pp. 1127-1133. doi:10.1111/j.0022-202X.2005.23718.x [13] A. Krautheim and H. P. Gollnick, “Acne: Topical Treat- ment,” Clinics in Dermatology, Vol. 22, No. 5, 2004, pp. 398-407. doi:10.1016/j.clindermatol.2004.03.009 [14] J. B. Bikowski, “Mechanisms of the Comedolytic and Copyright © 2013 SciRes. JCDSA  Suppression of Sebum Production and Accumulation by β-Cryptoxanthin Due to the Inhibition of the Expression of Diacylglycerol Acyltransferase-1 and Perilipin in Hamster Sebocytes 105 Anti-Inflammatory Properties of Topical Retinoids,” Jour- nal of Drugs in Dermatology, Vol. 4, No. 1, 2005, pp. 41- 47. [15] I. Tenaud, A. Khammari and B. Dreno, “In Vitro Modula- tion of TLR-2, CD1d and IL-10 by Adapalene on Normal Human Skin and Acne Inflammatory Lesions,” Experi- mental Dermatology, Vol. 16, No. 6, 2007, pp. 500-506. doi:10.1111/j.1600-0625.2007.00552.x [16] C. C. Zouboulis, B. Korge, H. Akamatsu, L. Q. Xia, S. Schiller, H. Gollnick and C. E. Orfanos, “Effects of 13-cis- Retinoic Acid, All-trans-Retinoic Acid, and Acitretin on the Proliferation, Lipid Synthesis and Keratin Expression of Cultured Human Sebocytes in Vitro,” Journal of Inves- tigative Dermatology, Vol. 96, No. 5, 1991, pp. 792-797. doi:10.1111/1523-1747.ep12471782 [17] M. J. Kim, N. Ciletti, S. Michel, U. Reichert and R. L. Rosenfield, “The Role of Specific Retinoid Receptors in Sebocyte Growth and Differentiation in Culture,” Journal of Investigative Dermatology, Vol. 114, No. 2, 2000, pp. 349-353. doi:10.1046/j.1523-1747.2000.00868.x [18] T. Sato, A. Takahashi, M. Kojima, N. Akimoto, M. Yano and A. Ito, “A Citrus Polymethoxy Flavonoid, Nobiletin In- hibits Sebum Production and Sebocyte Proliferation, and Augments Sebum Excretion in Hamsters,” Journal of Inves- tigative Dermatology, Vol. 127, No. 12, 2007, pp. 2740- 2748. [19] A. M. Nelson, K. L. Gilliland, Z. Cong and D. M. Thi- boutot, “13-cis Retinoic Acid Induces Apoptosis and Cell Cycle Arrest in Human SEB-1 Sebocytes,” Journal of In- vestigative Dermatology, Vol. 126, No. 10, 2006, pp. 2178- 2189. doi:10.1038/sj.jid.5700289 [20] A. J. Young and G. M. Lowe, “Antioxidant and Prooxi- dant Properties of Carotenoids,” Archives of Biochemistry and Biophysics, Vol. 385, No. 1, 2001, pp. 20-27. doi:10.1006/abbi.2000.2149 [21] A. Matsumoto, H. Mizukami, S. Mizuno, K. Umegaki, J. Nishikawa, K. Shudo, H. Kagechika and M. Inoue, “β- Cryptoxanthin, a Novel Natural RAR Ligand, Induces ATP-Binding Cassette Transporters in Macrophages,” Bio- chemical Pharmacology, Vol. 74, No. 2, 2007, pp. 256- 264. doi:10.1016/j.bcp.2007.04.014 [22] G. Maiani, M. J. Castón, G. Catasta, E. Toti, I. G. Cam- brodón, A. Bysted, F. Granado-Lorencio, B. Olmedilla- Alonso, P. Knuthsen, M. Valoti, V. Böhm, E. Mayer-Mie- bach, D. Behsnilian and U. Schlemmer, “Carotenoids: Actual Knowledge on Food Sources, Intakes, Stability and Bioavailability and Their Protective Role in Hu- mans,” Molecular Nutrition and Food Research, Vol. 53 Suppl. 2, 2009, pp. S194-218. doi:10.1002/mnfr.200800053 [23] K. Takayanagi, S. Morimoto, Y. Shirakura, K. Mukai, T. Sugiyama, Y. Tokuji and M. Ohnishi, “Mechanism of Visceral Fat Reduction in Tsumura Suzuki Obese, Diabe- tes (TSOD) Mice Orally Administered β-Cryptoxanthin from Satsuma Mandarin Oranges (Citrus Unshiu Marc),” Journal of Agricultural and Food Chemistry, Vol. 59, No. 23, 2011, pp. 12342-12351. doi:10.1021/jf202821u [24] M. Sugiura, H. Matsumoto, M. Kato, Y. Ikoma, M. Yano and A. Nagao, “Multiple Linear Regression Analysis of the Seasonal Changes in the Serum Concentration of β- Cryptoxanthin,” Journal of Nutritional Science and Vita- minology, Vol. 50, No. 3, 2004, pp. 196-202. doi:10.3177/jnsv.50.196 [25] S. Männistö, S. A. Smith-Warner, D. Spiegelman, D. Al- banes, K. Anderson, P. A. van den Brandt, J. R. Cerhan, G. Colditz, D. Feskanich, J. L. Freudenheim, E. Giovan- nucci, R. A. Goldbohm, S. Graham, A. B. Miller, T. E. Rohan, J. Virtamo, W. C. Willett and D. J. Hunter, “Die- tary Carotenoids and Risk of Lung Cancer in a Pooled Analysis of Seven Cohort Studies,” Cancer Epidemiology, Biomarkers and Prevention, Vol. 13, No. 1, 2004, pp. 40- 48. doi:10.1158/1055-9965.EPI-038-3 [26] J. M. Yuan, D. O. Stram, K. Arakawa, H. P. Lee and M. C. Yu, “Dietary Cryptoxanthin and Reduced Risk of Lung Cancer: The Singapore Chinese Health Study,” Cancer Epi- demiology, Biomarkers and Prevention, Vol. 12, No. 9, 2003, pp. 890-898. [27] D. J. Pattison, D. P. Symmons, M. Lunt, A. Welch, S. A. Bingham, N. E. Day and A. J. Silman, “Dietary β-Cryp- toxanthin and Inflammatory Polyarthritis: Results from a Population-Based Prospective Study,” The American Jour- nal of Clinical Nutrition, Vol. 82, No. 2, 2005, pp. 451- 455. [28] Z. El-Akawi, N. Abdel-Latif and K. Abdul-Razzak, “Does the Plasma Level of Vitamins A and E Affect Acne Con- dition,” Clinical and Experimental Dermatology, Vol. 31, No. 3, 2006, pp. 430-434. doi:10.1111/j.1365-2230.2006.02106.x [29] T. M. Redmond, S. Gentleman, T. Duncan, S. Yu, B. Wiggert, E. Gantt and F. X. Cunningham Jr., “Identifica- tion, Expression, and Substrate Specificity of a Mammal- ian β-Carotene 15,15'-Dioxygenase,” The Journal of Bio- logical Chemistry, Vol. 276, No. 9, pp. 6560-6565. doi:10.1074/jbc.M009030200 [30] M. Sugiura, M. Kato, H. Matsumoto, A. Nagao and M. Yano, “Serum Concentration of β-Cryptoxanthin in Japan Reflects the Frequency of Satsuma Mandarin (Citrus un- shiu Marc.) Consumption,” Journal of Health Sciences, Vol. 48, No. 4, 2002, pp. 350-353. doi:10.1248/jhs.48.350 [31] Y. Shirakura, K. Takayanagi, K. Mukai, H. Tanabe and M. Inoue, “β-Cryptoxanthin Suppresses the Adipogenesis of 3T3-L1 Cells via RAR Activation,” Journal of Nutri- tional Science and Vitaminology, Vol. 57, No. 6, 2011, pp. 426-431. doi:10.3177/jnsv.57.426 [32] T. Sato, N. Imai, N. Akimoto, T. Sakiguchi, K. Kitamura and A. Ito, “Epidermal Growth Factor and 1α,25-Dihy- droxyvitamin D3 Suppress Lipogenesis in Hamster Seba- ceous Gland Cells in Vitro,” Journal of Investigative Der- matology, Vol. 117, No. 4, 2001, pp. 965-970. doi:10.1046/j.0022-202x.2001.01516.x [33] T. Sato, T. Shirane, N. Noguchi, M. Sasatsu and A. Ito, “Novel Anti-Acne Actions of Nadifloxacin and Clinda- mycin That Inhibit the Production of Sebum, Prostag- landin E2 and Promatrix Metalloproteinase-2 in Hamster Sebocytes,” Journal of Dermatology, Vol. 39, No. 9, 2012, pp. 774-780. Copyright © 2013 SciRes. JCDSA  Suppression of Sebum Production and Accumulation by β-Cryptoxanthin Due to the Inhibition of the Expression of Diacylglycerol Acyltransferase-1 and Perilipin in Hamster Sebocytes Copyright © 2013 SciRes. JCDSA 106 doi:10.1111/j.1346-8138.2012.01525.x [34] K. Iinuma, T. Sato, N. Akimoto, N. Noguchi, M. Sasatsu, S. Nishijima, I. Kurokawa and A. Ito, “Involvement of Propionibacterium acnes in the Augmentation of Lipo- genesis in Hamster Sebaceous Glands in Vivo and in Vi- tro,” Journal of Investigative Dermatology, Vol. 129, No. 9, 2009, pp. 2113-2119. doi:10.1038/jid.2009.46 [35] A. R. Kimmel, D. L. Brasaemle, M. McAndrews-Hill, C. Sztalryd and C. Londos, “Adoption of PERILIPIN as a Unifying Nomenclature for the Mammalian PAT-Family of Intracellular Lipid Storage Droplet Proteins,” Journal of Lipid Research, Vol. 51, No. 3, 2010, pp. 468-471. doi:10.1194/jlr.R000034 [36] C. A. Harris, J. T. Haas, R. S. Streeper, S. J. Stone, M. Kumari, K. Yang, X. Han, N. Brownell, R. W. Gross, R. Zechner and R. V. Farese Jr., “DGAT Enzymes Are Re- quired for Triacylglycerol Synthesis and Lipid Droplets in Adipocytes,” Journal of Lipid Research, Vol. 52, No. 4, 2011, pp. 657-667. doi:10.1194/jlr.M013003 [37] D. L. Brasaemle, B. Rubin, I. A. Harten, J. Gruia-Gray, A. R. Kimmel and C. Londos, “Perilipin A Increases Tria- cylglycerol Storage by Decreasing the Rate of Triacyl- glycerol Hydrolysis,” The Journal of Biological Chemis- tery, Vol. 275, No. 49, 2000, pp. 38486-38493. doi:10.1074/jbc.M007322200 [38] H. Wang, L. Hu, K. Dalen, H. Dorward, A. Marcinkie- wicz, D. Russell, D. Gong, C. Londos, T. Yamaguchi, C. Holm, M. A. Rizzo, D. Brasaemle and C. Sztalryd, “Ac- tivation of Hormone-Sensitive Lipase Requires Two Steps, Protein Phosphorylation and Binding to the PAT-1 Domain of Lipid Droplet Coat Proteins,” The Journal of Biological Chemistery, Vol. 284, No. 46, 2009, pp. 32116- 32125. doi:10.1074/jbc.M109.006726 [39] M. Kato, Y. Ikoma, H. Matsumoto, M. Sugiura, H. Hyo- do and M. Yano, “Accumulation of Carotenoids and Ex- pression of Carotenoid Biosynthetic Genes during Matu- ration in Citrus Fruit,” Plant Physiology, Vol. 134, No. 2, 2004, pp. 824-837. doi:10.1104/pp.103.031104 [40] H. A. Al-Wadei, T. Takahashi and H. M. Schuller, “Growth Stimulation of Human Pulmonary Adenocarcinoma Cells and Small Airway Epithelial Cells by β-Carotene via Ac- tivation of cAMP, PKA, CREB and ERK1/2,” Interna- tional Journal of Cancer, Vol. 118, No. 6, 2006, pp. 1370- 1380. doi:10.1002/ijc.21537 [41] H. A. Al-Wadei, M. Majidi, M. S. Tsao and H. M. Schul- ler, “Low Concentrations of β-Carotene Stimulate the Proliferation of Human Pancreatic Duct Epithelial Cells in a PKA-Dependent Manner,” Cancer Genomics and Pro- teomics, Vol. 4, No. 1, 2007, pp. 35-42. [42] W. J. Cunliffe, D. B. Holland, S. M. Clark and G. I. Sta- bles, “Comedogenesis: Some New Aetiological, Clinical and Therapeutic Strategies,” The British Journal of Der- matology, Vol. 142, No. 6, 2000, pp. 1084-1091. doi:10.1046/j.1365-2133.2000.03531.x [43] B. C. Melnik and G. Schmitz, “Role of Insulin, Insu- lin-Like Growth Factor-1, Hyperglycaemic Food and Milk Consumption in the Pathogenesis of Acne Vul- garis,” Experimental Dermatology, Vol. 18, No. 10, 2009, pp. 833-841. doi:10.1111/j.1600-0625.2009.00924.x [44] C. C. Zouboulis, “Sebaceous Gland Receptors,” Dermato- Endocrinology, Vol. 1, No. 2, 2009, pp. 77-80. [45] N. N. Elmongy and O. Shaker, “Expression of Perox- isome Proliferator Activator Receptor β/δ (PPAR β/δ) in Acne Vulgaris,” European Journal of Dermatology, Vol. 22, No. 1, 2012, pp. 42-45. [46] D. E. Breithaupt and A. Bamedi, “Carotenoid Esters in Vegetables and Fruits: A Screening with Emphasis on β- Cryptoxanthin Esters,” Journal of Agricultural and Food Chemistry, Vol. 49, No. 4, 2001, pp. 2064-2070. doi:10.1021/jf001276t [47] T. Wingerath, W. Stahl and H. Sies, “β-Cryptoxanthin Selectively Increases in Human Chylomicrons upon In- gestion of Tangerine Concentrate Rich in β-Cryptox- anthin Esters,” Archives of Biochemistry and Biophysics, Vol. 324, No. 2, 1995, pp. 385-390. doi:10.1006/abbi.1995.0052 [48] T. Wingerath, H. Sies and W. Stahl, “Xanthophyll Esters in Human Skin,” Archives of Biochemistry and Biophys- ics, Vol. 355, No. 2, 1998, pp. 271-274. doi:10.1006/abbi.1998.0734 |

