 Open Journal of Gastroenterology, 2013, 3, 49-54 OJGas http://dx.doi.org/10.4236/ojgas.2013.31008 Published Online February 2013 (http://www.scirp.org/journal/ojgas/) Alpha-fetoprotein testing for hepatocellular carcinoma may not be helpful in nonalcoholic steatohepatitis Linda L. Wong1,2*, Christopher J. Kim2, Sandi A. Kwee3,4, Brenda Y. Hernandez3 1University of Hawaii Cancer Center, Honolulu, USA 3Department of Surgery, University of Hawaii, John A. Burns School of Medicine, Honolulu, USA 2Department of Medicine, University of Hawaii, John A. Burns School of Medicine, Honolulu, USA 4PET Imaging Research, University of Hawaii, Honolulu, USA Email: *hepatoma@aol.com Received 21 October 2012; revised 22 November 2012; accepted 30 November 2012 ABSTRACT Background & Objectives: Diagnosing hepatocellular carcinoma (HCC) often utilizes serum tumor markers. Although the most commonly used tumor marker in clinical practice, alpha-fetoprotein (AFP) is not in- cluded in recent guidelines for diagnosing HCC. The overall performance characteristics of AFP as a tu- mor marker is viewed as insufficiently sensitive or specific. The diagnostic value of AFP specifically in nonalcoholic steatohepatitis (NASH) related HCC is unknown. We aimed to determine the utility of AFP testing in NASH-related HCC. Methods: Retrospec- tive review of 737 HCC patients referred from 1993- 2011 to a single facility treating the majority of chronic liver disease in Hawaii. HCC was diagnosed histologi- cally by percutaneous biopsy, liver biopsy at the time of surgery, or examination of the resected liver. Pa- tients were classified according to HCC risk factors including NASH, hepatitis B and C infection, and alcohol-related. Other data collected included: demo- graphics, ethnicity, presence of cirrhosis, tumor char- acteristics (size, number, vascular invasion), diabetes, hyperlipidemia, body mass index (BMI) and blood testing to calculate Model for End-Stage Liver Dis- ease (MELD) score. Elevated AFP was defined as >20 ng/mL. Sensitivity of AFP was determined and com- pared between various subgroups. Results: Elevated AFP levels were detected in 64.3% of patients. AFP sensitivity was 47% for NASH-related HCC (n = 100), and 67.2% for HCC with viral or alcoholic risk fac- tors (n = 637) (OR 0.43, 95% CI 0.28 - 0.66, p = 0.0001). Elevated AFP had higher sensitivity in fe- males (71.9% vs. 61.8%, OR 1.58, 95% CI 1.1 - 2.27, p = 0.013), non-diabetics (67.4% vs. 57.2%, OR 0.65, 95% CI 0.47 - 0.89, p = 0.0093), and cirrhotics (67.1% vs. 56.8%, OR 1.55, 95% CI 1.10 - 2.19, p = 0.0012). AFP did not vary significantly with regard to hyper- lipidemia or BMI. AFP was more sensitive in advanced disease including tumors > 5 cm, multiple tumors, or vascular invasion (all with p < 0.05). AFP did not vary with MELD score. Conclusions: Normal AFP is common in NASH-related HCC. Better tumor mark- ers may be needed to optimally screen and diagnose NASH-related HCC. Without more effective tumor markers, HCC detection relies heavily upon imaging and liver biopsy. Keywords: Hepatocellular Cancer; Nonalcoholic Steatohepatitis; Alpha-Fetoprotein 1. INTRODUCTION Primary liver cancer is the fifth most common cancer worldwide, and the third leading cause of cancer death. HCC is the major histologic subtype among primary liver cancers. Endemic areas of HCC include Southeast Asia and sub-Saharan Africa [1]. In areas of low preva- lence including North America and Europe, HCC inci- dence is rising [2,3]. In the United States, HCC had the highest increase in mortality, and second highest increase in incidence, of all cancers between 1995 and 2004 [4]. Chronic liver disease and cirrhosis are primary risk factors for HCC. Chronic viral infection with hepatitis B (HBV), hepatitis C (HCV), and alcoholic injury, are the predominant causes of liver disease [5]. In the United States, HBV rates are lower, whereas HCV is associated with 70% of HCC [6,7]. However, 15% - 50% of HCC cases are non-viral and non-alcohol related and classified as cryptogenic [7-9]. Metabolic disease including obesity, diabetes mellitus, and hyperlipidemia, are associated with non-viral and non-alcohol related HCC [7]. Perhaps a hepatic manifes- tation of metabolic disease, non-alcoholic fatty liver dis- ease (NAFLD) is the most common liver disease in the *Corresponding author. OPEN ACCESS  L. L. Wong et al. / Open Journal of Gastroenterology 3 (2013) 49-54 50 United States [10]. Characterizing a disease spectrum including simple steatosis, NASH, cirrhosis, and end- stage liver disease (ESLD), NAFLD may progress to HCC, and account for a substantial portion of crypto- genic HCC [11,12]. Diagnosing HCC utilizes liver imaging, histopathol- ogic evaluation, and tumor markers. Screening for HCC commonly uses liver ultrasound (US) and measuring AFP. Abnormal screening findings prompt diagnostic imaging with contrast-enhanced computed tomography (CT) and/or magnetic resonance imaging (MRI). Liver biopsy is used in scenarios with diagnostic ambiguity. AFP is a glycoprotein which is normally produced by the fetal liver, yolk sac, and the gastrointestinal tract. Although it is most commonly elevated in HCC, eleva- tions in serum AFP can be seen in various malignancies including testicular, bile duct, pancreatic, stomach, and colon cancer. Elevated AFP can also seen with non-ma- lignant conditions including hepatitis and cirrhosis [13]. AFP is the most commonly used tumor marker for HCC in clinical practice. It is easily obtainable and rela- tively inexpensive. However, recent guidelines do not include AFP in the diagnostic algorithm because of its overall sensitivity or specificity for HCC [14]. Defining an elevated AFP level > 20 ng/mL, confers a sensitivity of 60% and specificity of 80%. At a cut-off AFP level > 200 ng/mL, specificity approaches 100%, but sensitivity falls to 20% [15]. Yet, these performance characteristics for AFP are based largely upon studies of patients with chronic viral hepatitis developing HCC. Adding to the uncertain diagnostic utility of AFP has been the observa- tion that a significant number of small HCCs do not se- crete AFP. Although AFP has long been used as tumor marker for HCC, it is increasingly viewed to be unnecessary in a contemporary role for diagnosis. Part of the reason for this change is likely due to improvements in diagnostic imaging. Another possibility may be the changing preva- lence of chronic viral disease to fatty liver disease as the cause of chronic liver disease and HCC development. However, it is unclear whether there is a significant cor- relation between AFP and the development of HCC spe- cifically in the setting of NASH. The purpose of this study is to compare the frequency of elevated AFP with various risk factors, and estimate the relative diagnostic utility of AFP in NASH-related HCC. 2. METHODS This is a retrospective analysis of 737 HCC cases re- ferred to a Liver Center affiliated surgical group (LW) at Hawaii Medical Center-East (formerly St. Francis Medi- cal Center) from 1993-2011. This medical center was a tertiary center and sole clinic dedicated to liver diseases in Hawaii, the only liver transplant center in the State, and the primary referral center for hepatobiliary surgery for American territories of the Pacific Basin (including American Samoa, Guam, Saipan, and the Marshall Is- lands). Additionally, a number of patients were foreign nationals from Asian countries, including China, Japan, Korea, and the Philippines, who sought medical care in the United States. This center managed approximately 60% - 70% of HCC cases in the State of Hawaii. HCC was diagnosed histologically by percutaneous biopsy, liver biopsy at the time of surgery, or examina- tion of the resected liver. Consistent with the United Network for Organ Sharing (UNOS) policy regarding transplant for HCC, patients without histologic confir- mation were diagnosed with HCC if they had chronic liver disease and a liver lesion ≥ 2 cm in size on two im- aging studies (US, CT, or MRI) and one of the following: 1) vascular blush on CT or MRI 2) AFP > 200 ng/ml, or 3) arteriogram confirming the tumor [16]. The clinical presentation (i.e. reasons for referral and clinical workup leading to the diagnosis of HCC) for each patient were categorized as 1) symptomatic (i.e. abdominal pain or mass, weight loss, liver decompensa- tion, jaundice), 2) asymptomatic (e.g. workup prompted by incidental finding on a prior imaging test), and 3) asymptomatic—abnormal finding on screening. Although the Liver Center at our institution recom- mended that patients with viral hepatitis and chronic liver disease undergo HCC screening with AFP testing and US every six months, there was no uniform practice within our community that lead to this cohort. However, patients referred based on screening results, were identi- fied by AFP testing and/or imaging (either US, CT, or MRI) at various intervals, ranging from three to twelve months, consistent with National Comprehensive Cancer Network guidelines [17]. Demographic information, medical history, laboratory results, tumor characteristics, treatment, and survival data were collected via clinical interview by a single physi- cian without structured questionnaire and entered into a prospective database. Information on diabetes mellitus, hyperlipidemia, smoking, family history of HCC, and risk factors for HCC including viral hepatitis, alcohol abuse (defined as greater than two alcoholic beverages daily for at least ten years), and other chronic liver dis- eases, were included. Patients who had elevated lipid levels or took a lipid-lowering agent were classified as having hyperlipidemia. Measured height and weight were used to determine body mass index (BMI), with obesity defined as BMI ≥ 30. NASH was diagnosed based on liver biopsy, absence of significant alcohol use, negative hepatitis serologies, and no established etiology for liver disease after clinical work-up by a gastroenterologist and/or hepatologist. Laboratory data collected included bilirubin level, al- Copyright © 2013 SciRes. OPEN ACCESS 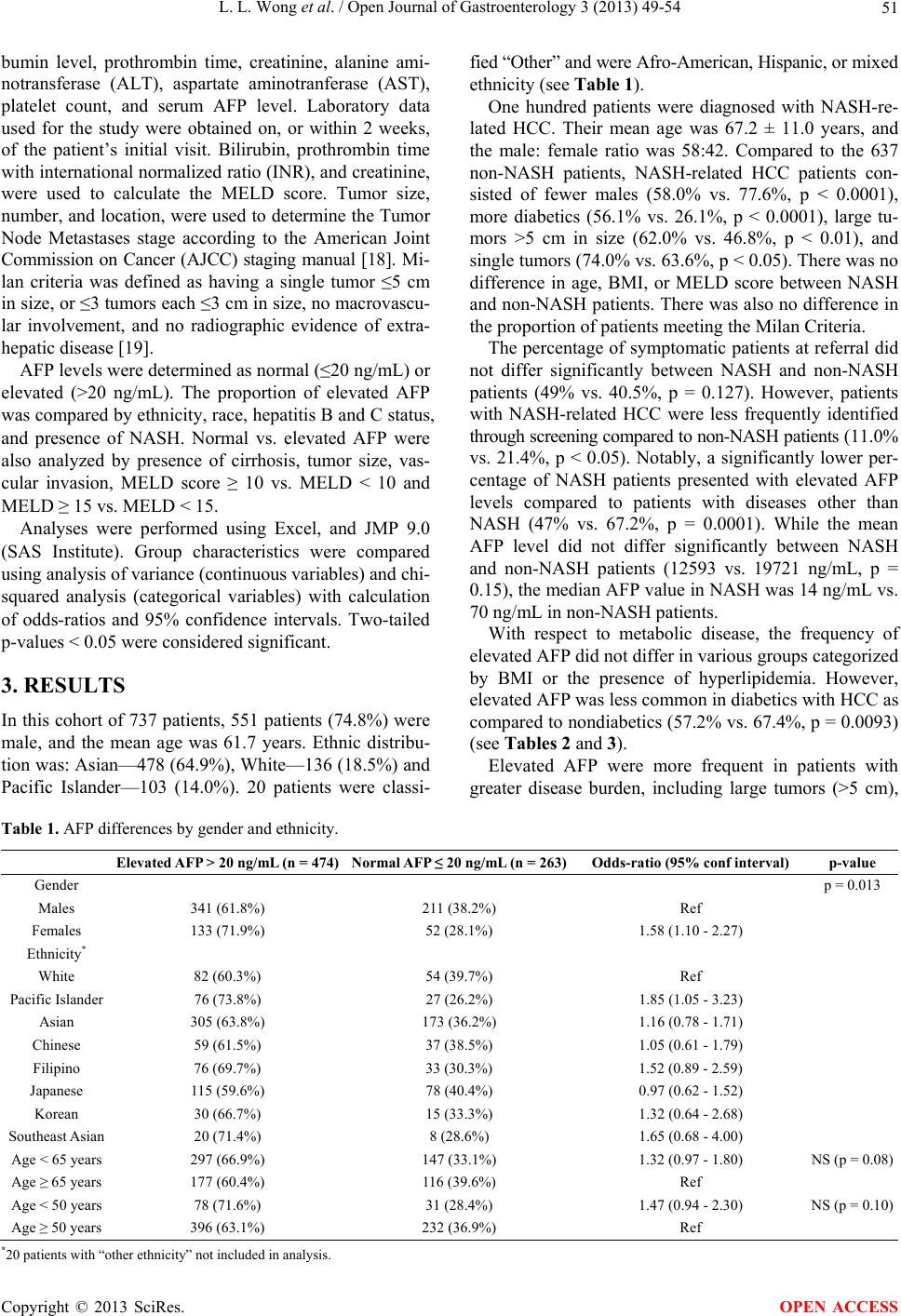 L. L. Wong et al. / Open Journal of Gastroenterology 3 (2013) 49-54 Copyright © 2013 SciRes. 51 OPEN ACCESS bumin level, prothrombin time, creatinine, alanine ami- notransferase (ALT), aspartate aminotranferase (AST), platelet count, and serum AFP level. Laboratory data used for the study were obtained on, or within 2 weeks, of the patient’s initial visit. Bilirubin, prothrombin time with international normalized ratio (INR), and creatinine, were used to calculate the MELD score. Tumor size, number, and location, were used to determine the Tumor Node Metastases stage according to the American Joint Commission on Cancer (AJCC) staging manual [18]. Mi- lan criteria was defined as having a single tumor ≤5 cm in size, or ≤3 tumors each ≤3 cm in size, no macrovascu- lar involvement, and no radiographic evidence of extra- hepatic disease [19]. AFP levels were determined as normal (≤20 ng/mL) or elevated (>20 ng/mL). The proportion of elevated AFP was compared by ethnicity, race, hepatitis B and C status, and presence of NASH. Normal vs. elevated AFP were also analyzed by presence of cirrhosis, tumor size, vas- cular invasion, MELD score ≥ 10 vs. MELD < 10 and MELD ≥ 15 vs. MELD < 15. Analyses were performed using Excel, and JMP 9.0 (SAS Institute). Group characteristics were compared using analysis of variance (continuous variables) and chi- squared analysis (categorical variables) with calculation of odds-ratios and 95% confidence intervals. Two-tailed p-values < 0.05 were considered significant. 3. RESULTS In this cohort of 737 patients, 551 patients (74.8%) were male, and the mean age was 61.7 years. Ethnic distribu- tion was: Asian—478 (64.9%), White—136 (18.5%) and Pacific Islander—103 (14.0%). 20 patients were classi- fied “Other” and were Afro-American, Hispanic, or mixed ethnicity (see Table 1). One hundred patients were diagnosed with NASH-re- lated HCC. Their mean age was 67.2 ± 11.0 years, and the male: female ratio was 58:42. Compared to the 637 non-NASH patients, NASH-related HCC patients con- sisted of fewer males (58.0% vs. 77.6%, p < 0.0001), more diabetics (56.1% vs. 26.1%, p < 0.0001), large tu- mors >5 cm in size (62.0% vs. 46.8%, p < 0.01), and single tumors (74.0% vs. 63.6%, p < 0.05). There was no difference in age, BMI, or MELD score between NASH and non-NASH patients. There was also no difference in the proportion of patients meeting the Milan Criteria. The percentage of symptomatic patients at referral did not differ significantly between NASH and non-NASH patients (49% vs. 40.5%, p = 0.127). However, patients with NASH-related HCC were less frequently identified through screening compared to non-NASH patients (11.0% vs. 21.4%, p < 0.05). Notably, a significantly lower per- centage of NASH patients presented with elevated AFP levels compared to patients with diseases other than NASH (47% vs. 67.2%, p = 0.0001). While the mean AFP level did not differ significantly between NASH and non-NASH patients (12593 vs. 19721 ng/mL, p = 0.15), the median AFP value in NASH was 14 ng/mL vs. 70 ng/mL in non-NASH patients. With respect to metabolic disease, the frequency of elevated AFP did not differ in various groups categorized by BMI or the presence of hyperlipidemia. However, elevated AFP was less common in diabetics with HCC as compared to nondiabetics (57.2% vs. 67.4%, p = 0.0093) (see Tables 2 and 3). Elevated AFP were more frequent in patients with greater disease burden, including large tumors (>5 cm), Table 1. AFP differences by gender and ethnicity. Elevated AFP > 20 ng/mL (n = 474) Normal AFP ≤ 20 ng/mL (n = 263)Odds-ratio (95% conf interval) p-value Gender p = 0.013 Males 341 (61.8%) 211 (38.2%) Ref Females 133 (71.9%) 52 (28.1%) 1.58 (1.10 - 2.27) Ethnicity* White 82 (60.3%) 54 (39.7%) Ref Pacific Islander 76 (73.8%) 27 (26.2%) 1.85 (1.05 - 3.23) Asian 305 (63.8%) 173 (36.2%) 1.16 (0.78 - 1.71) Chinese 59 (61.5%) 37 (38.5%) 1.05 (0.61 - 1.79) Filipino 76 (69.7%) 33 (30.3%) 1.52 (0.89 - 2.59) Japanese 115 (59.6%) 78 (40.4%) 0.97 (0.62 - 1.52) Korean 30 (66.7%) 15 (33.3%) 1.32 (0.64 - 2.68) Southeast Asian 20 (71.4%) 8 (28.6%) 1.65 (0.68 - 4.00) Age < 65 years 297 (66.9%) 147 (33.1%) 1.32 (0.97 - 1.80) NS (p = 0.08) Age ≥ 65 years 177 (60.4%) 116 (39.6%) Ref Age < 50 years 78 (71.6%) 31 (28.4%) 1.47 (0.94 - 2.30) NS (p = 0.10) Age ≥ 50 years 396 (63.1%) 232 (36.9%) Ref *20 patients with “other ethnicity” not included in analysis. 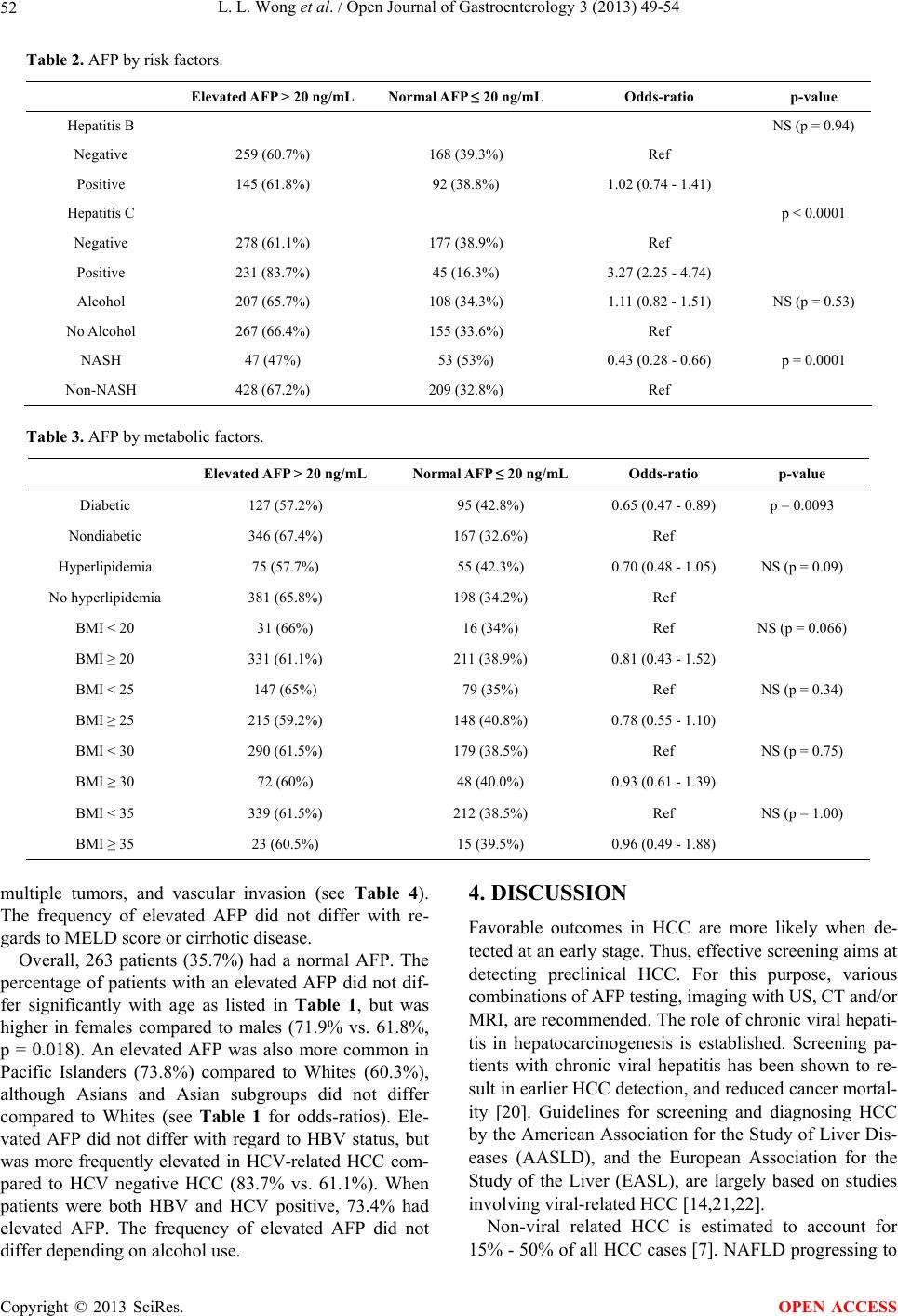 L. L. Wong et al. / Open Journal of Gastroenterology 3 (2013) 49-54 52 Table 2. AFP by risk factors. Elevated AFP > 20 ng/mL Normal AFP ≤ 20 ng/mL Odds-ratio p-value Hepatitis B NS (p = 0.94) Negative 259 (60.7%) 168 (39.3%) Ref Positive 145 (61.8%) 92 (38.8%) 1.02 (0.74 - 1.41) Hepatitis C p < 0.0001 Negative 278 (61.1%) 177 (38.9%) Ref Positive 231 (83.7%) 45 (16.3%) 3.27 (2.25 - 4.74) Alcohol 207 (65.7%) 108 (34.3%) 1.11 (0.82 - 1.51) NS (p = 0.53) No Alcohol 267 (66.4%) 155 (33.6%) Ref NASH 47 (47%) 53 (53%) 0.43 (0.28 - 0.66) p = 0.0001 Non-NASH 428 (67.2%) 209 (32.8%) Ref Table 3. AFP by metabolic factors. Elevated AFP > 20 ng/mL Normal AFP ≤ 20 ng/mL Odds-ratio p-value Diabetic 127 (57.2%) 95 (42.8%) 0.65 (0.47 - 0.89) p = 0.0093 Nondiabetic 346 (67.4%) 167 (32.6%) Ref Hyperlipidemia 75 (57.7%) 55 (42.3%) 0.70 (0.48 - 1.05) NS (p = 0.09) No hyperlipidemia 381 (65.8%) 198 (34.2%) Ref BMI < 20 31 (66%) 16 (34%) Ref NS (p = 0.066) BMI ≥ 20 331 (61.1%) 211 (38.9%) 0.81 (0.43 - 1.52) BMI < 25 147 (65%) 79 (35%) Ref NS (p = 0.34) BMI ≥ 25 215 (59.2%) 148 (40.8%) 0.78 (0.55 - 1.10) BMI < 30 290 (61.5%) 179 (38.5%) Ref NS (p = 0.75) BMI ≥ 30 72 (60%) 48 (40.0%) 0.93 (0.61 - 1.39) BMI < 35 339 (61.5%) 212 (38.5%) Ref NS (p = 1.00) BMI ≥ 35 23 (60.5%) 15 (39.5%) 0.96 (0.49 - 1.88) multiple tumors, and vascular invasion (see Table 4). The frequency of elevated AFP did not differ with re- gards to MELD score or cirrhotic disease. Overall, 263 patients (35.7%) had a normal AFP. The percentage of patients with an elevated AFP did not dif- fer significantly with age as listed in Table 1, but was higher in females compared to males (71.9% vs. 61.8%, p = 0.018). An elevated AFP was also more common in Pacific Islanders (73.8%) compared to Whites (60.3%), although Asians and Asian subgroups did not differ compared to Whites (see Table 1 for odds-ratios). Ele- vated AFP did not differ with regard to HBV status, but was more frequently elevated in HCV-related HCC com- pared to HCV negative HCC (83.7% vs. 61.1%). When patients were both HBV and HCV positive, 73.4% had elevated AFP. The frequency of elevated AFP did not differ depending on alcohol use. 4. DISCUSSION Favorable outcomes in HCC are more likely when de- tected at an early stage. Thus, effective screening aims at detecting preclinical HCC. For this purpose, various combinations of AFP testing, imaging with US, CT and/or MRI, are recommended. The role of chronic viral hepati- tis in hepatocarcinogenesis is established. Screening pa- tients with chronic viral hepatitis has been shown to re- sult in earlier HCC detection, and reduced cancer mortal- ity [20]. Guidelines for screening and diagnosing HCC by the American Association for the Study of Liver Dis- eases (AASLD), and the European Association for the Study of the Liver (EASL), are largely based on studies involving viral-related HCC [14,21,22]. Non-viral related HCC is estimated to account for 15% - 50% of all HCC cases [7]. NAFLD progressing to Copyright © 2013 SciRes. OPEN ACCESS 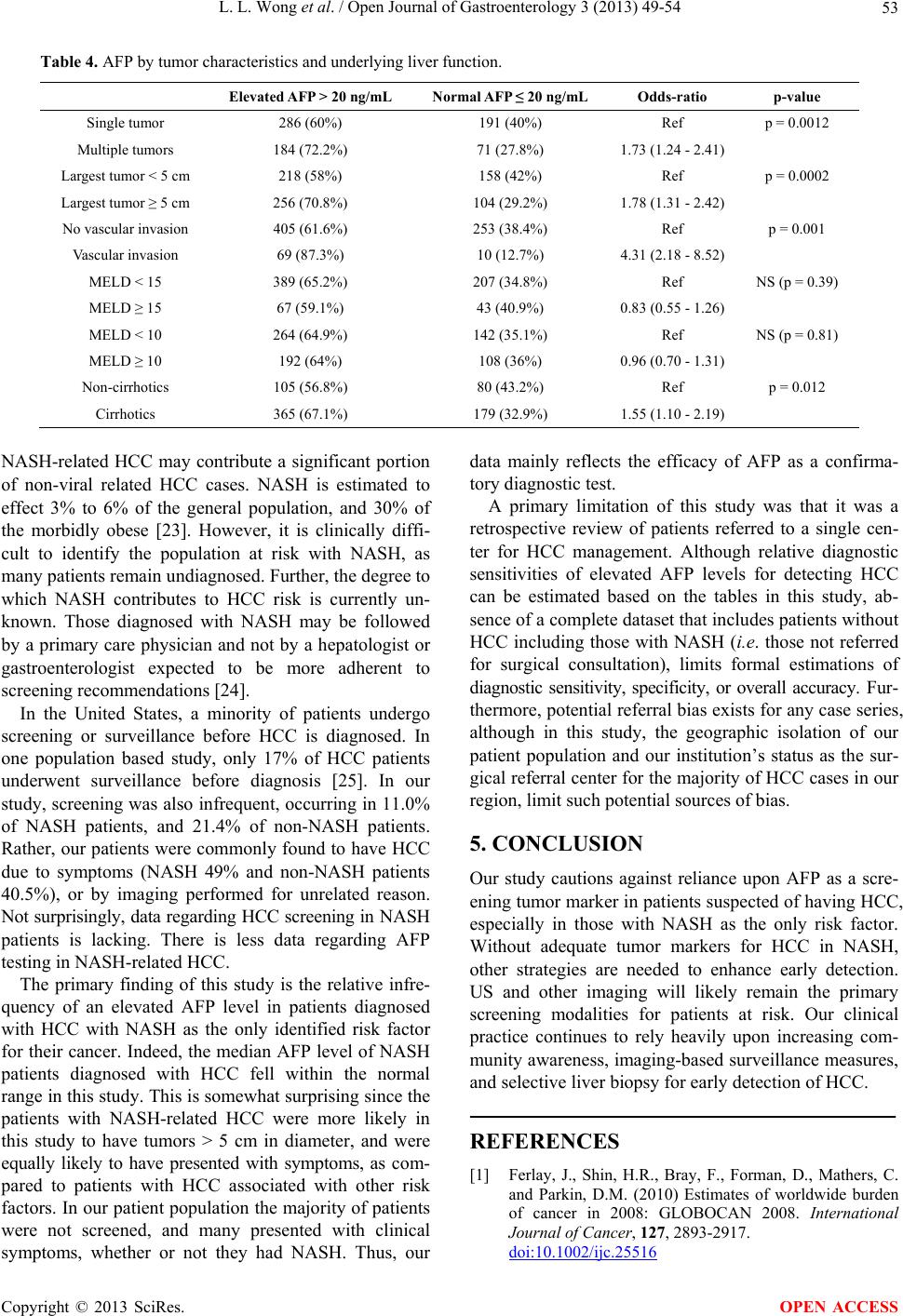 L. L. Wong et al. / Open Journal of Gastroenterology 3 (2013) 49-54 53 Table 4. AFP by tumor characteristics and underlying liver function. Elevated AFP > 20 ng/mL Normal AFP ≤ 20 ng/mL Odds-ratio p-value Single tumor 286 (60%) 191 (40%) Ref p = 0.0012 Multiple tumors 184 (72.2%) 71 (27.8%) 1.73 (1.24 - 2.41) Largest tumor < 5 cm 218 (58%) 158 (42%) Ref p = 0.0002 Largest tumor ≥ 5 cm 256 (70.8%) 104 (29.2%) 1.78 (1.31 - 2.42) No vascular invasion 405 (61.6%) 253 (38.4%) Ref p = 0.001 Vascular invasion 69 (87.3%) 10 (12.7%) 4.31 (2.18 - 8.52) MELD < 15 389 (65.2%) 207 (34.8%) Ref NS (p = 0.39) MELD ≥ 15 67 (59.1%) 43 (40.9%) 0.83 (0.55 - 1.26) MELD < 10 264 (64.9%) 142 (35.1%) Ref NS (p = 0.81) MELD ≥ 10 192 (64%) 108 (36%) 0.96 (0.70 - 1.31) Non-cirrhotics 105 (56.8%) 80 (43.2%) Ref p = 0.012 Cirrhotics 365 (67.1%) 179 (32.9%) 1.55 (1.10 - 2.19) NASH-related HCC may contribute a significant portion of non-viral related HCC cases. NASH is estimated to effect 3% to 6% of the general population, and 30% of the morbidly obese [23]. However, it is clinically diffi- cult to identify the population at risk with NASH, as many patients remain undiagnosed. Further, the degree to which NASH contributes to HCC risk is currently un- known. Those diagnosed with NASH may be followed by a primary care physician and not by a hepatologist or gastroenterologist expected to be more adherent to screening recommendations [24]. In the United States, a minority of patients undergo screening or surveillance before HCC is diagnosed. In one population based study, only 17% of HCC patients underwent surveillance before diagnosis [25]. In our study, screening was also infrequent, occurring in 11.0% of NASH patients, and 21.4% of non-NASH patients. Rather, our patients were commonly found to have HCC due to symptoms (NASH 49% and non-NASH patients 40.5%), or by imaging performed for unrelated reason. Not surprisingly, data regarding HCC screening in NASH patients is lacking. There is less data regarding AFP testing in NASH-related HCC. The primary finding of this study is the relative infre- quency of an elevated AFP level in patients diagnosed with HCC with NASH as the only identified risk factor for their cancer. Indeed, the median AFP level of NASH patients diagnosed with HCC fell within the normal range in this study. This is somewhat surprising since the patients with NASH-related HCC were more likely in this study to have tumors > 5 cm in diameter, and were equally likely to have presented with symptoms, as com- pared to patients with HCC associated with other risk factors. In our patient population the majority of patients were not screened, and many presented with clinical symptoms, whether or not they had NASH. Thus, our data mainly reflects the efficacy of AFP as a confirma- tory diagnostic test. A primary limitation of this study was that it was a retrospective review of patients referred to a single cen- ter for HCC management. Although relative diagnostic sensitivities of elevated AFP levels for detecting HCC can be estimated based on the tables in this study, ab- sence of a complete dataset that includes patients without HCC including those with NASH (i.e. those not referred for surgical consultation), limits formal estimations of diagnostic sensitivity, specificity, or overall accuracy. Fur- thermore, potential referral bias exists for any case series, although in this study, the geographic isolation of our patient population and our institution’s status as the sur- gical referral center for the majority of HCC cases in our region, limit such potential sources of bias. 5. CONCLUSION Our study cautions against reliance upon AFP as a scre- ening tumor marker in patients suspected of having HCC, especially in those with NASH as the only risk factor. Without adequate tumor markers for HCC in NASH, other strategies are needed to enhance early detection. US and other imaging will likely remain the primary screening modalities for patients at risk. Our clinical practice continues to rely heavily upon increasing com- munity awareness, imaging-based surveillance measures, and selective liver biopsy for early detection of HCC. REFERENCES [1] Ferlay, J., Shin, H.R., Bray, F., Forman, D., Mathers, C. and Parkin, D.M. (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International Journal of Cancer, 127, 2893-2917. doi:10.1002/ijc.25516 Copyright © 2013 SciRes. OPEN ACCESS  L. L. Wong et al. / Open Journal of Gastroenterology 3 (2013) 49-54 54 [2] El-Serag, H.B. and Mason, A.C. (1999) Rising incidence of hepatocellular carcinoma in the United States. New England Journal of Medicine, 340, 745-750. doi:10.1056/NEJM199903113401001 [3] Gomaa, A.I., Khan, S.A., Toledano, M.B., Waked, I. and Taylor-Robinson, S.D. (2008) Hepatocellular carcinoma: Epidemiology, risk factors and pathogenesis. World Jour- nal of Gastroenterology, 14, 4300-4308. doi:10.3748/wjg.14.4300 [4] US National Institute of Health (2010) SEER Cancer Statistics Review 1975-2004. http://www.SEER.cancer.gov [5] Perz, J.F., Armstrong, G.L., Farrington, L.A., Hutin, Y.J. and Bell, B.P. (2006) The contributions of hepatitis B vi- rus and hepatitis C virus infections to cirrhosis and pri- mary liver cancer worldwide. Journal of Hepatology, 45, 529-538. doi:10.1016/j.jhep.2006.05.013 [6] Hassan, M.M., Frome, A., Patt, Y.Z. and El-Serag H.B. (2002) Rising prevalence of hepatitis C virus infection among patients recently diagnosed with hepatocellular carcinoma in the United States. Journal of Clinical Gas- troenterology, 35, 266-269. doi:10.1097/00004836-200209000-00013 [7] El-Serag, H.B. (2004) Hepatocellular carcinoma: Recent trends in the United States. Gastroenterology, 127, S27- S34. doi:10.1053/j.gastro.2004.09.013 [8] Marrero, J.A., Fontana, R.J., Su, G.L., Conjeevaram, H.S., Emick, D.M. and Lok A.S. (2002) NAFLD may be a common underlying liver disease in patients with hepa- tocellular carcinoma in the United States. Hepatology, 36, 1349-1354. doi:10.1002/hep.1840360609 [9] Starley, B.Q., Calcagno, C.J. and Harrison, S.A. (2010) Nonalcoholic fatty liver disease and hepatocellular carci- noma: A weighty connection. Hepatology, 51, 1820-1832. doi:10.1002/hep.23594 [10] Ong, J.P. and Younossi, Z.M. (2007) Epidemiology and natural history of NAFLD and NASH. Clinics in Liver Disease, 11, 1-16. doi:10.1016/j.cld.2007.02.009 [11] Page, J.M. and Harrison, S.A. (2009) NASH and HCC. Clinics in Liver Disease, 13, 631-634. doi:10.1016/j.cld.2009.07.007 [12] Baffy, G., Brunt, E.M. and Caldwell, S.H. (2012) Hepa- tocellular carcinoma in non-alcoholic fatty liver disease: An emerging menace. Journal of Hepatology, 56, 1384- 1391. doi:10.1016/j.jhep.2011.10.027 [13] Di Bisceglie, A.M., Sterling, R.K., Chung, R.T., Everhart, J.E., Dienstag, J.L., Bonkovsky, H.L., Wright, E.C., Ever- son, G.T., Lindsay, K.L., Lok, A.S., Lee, W.M., Morgan, T.R., Ghany, M.G. and Gretch, D.R. (2005) Serum alpha- fetoprotein levels in patients with advanced hepatitis C: Results from the HALT-C Trial. Journal of Hepatology, 43, 434-441. doi:10.1016/j.jhep.2005.03.019 [14] Bruix, J. and Sherman, M. (2005) Management of hepa- tocellular carcinoma: An update. Hepatology, 42, 1208- 1236. doi:10.1002/hep.20933 [15] Gonzalez, S.A. and Keeffe, E.B. (2011) Diagnosis of heaptocellular carcinoma: role of tumor markers and liver biopsy. Clinics in Liver Disease, 15, 297-306. doi:10.1016/j.cld.2011.03.012 [16] United Network for Organ Sharing (2011) Policy 3.6.4.4. www.unos.org [17] National Comprehensive Cancer Network (2010) NCCN Clinical Practice Guidelines in Oncology: Hepatobiliary Cancers. V.2.2010. [18] Edge, S.B. (2010) American Joint Committee on Cancer staging manual. 7th Edition, Springer, New York. [19] Mazzaferro, V., Regalia, E., Doci, R., Andreola, S., Pul- virenti, A., Bozzetti, F., Montalto, F., Ammaturna, M., Morabito, A. and Gennan, L. (1996) Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. New England Journal of Medicine, 334, 693-699. doi:10.1056/NEJM199603143341104 [20] Zhang, B.H., Yang, B.H. and Tang, Z.Y. (2004) Ran- domized controlled trial of screening for hepatocellular carcinoma. Journal of Cancer Research in Clinical On- cology, 130, 417-422. doi:10.1007/s00432-004-0552-0 [21] Bruix, J., Sherman, M., Llovet, J.M., Beaugrand, M., Lencioni, R., Burroughs, A.K., Christensen, E., Pagliaro, L., Colombo, M. and Rodés J. (2001) Clinical manage- ment of hepatocellular carcinoma. Conclusions of the Barcelona 2000 EASL conference. European Association for the Study of the Liver. Journal of Hepatology, 35, 421-430. doi:10.1016/S0168-8278(01)00130-1 [22] Bruix, J. and Sherman, M. (2011) Management of hepa- tocellular carcinoma: An update. Hepatology, 53, 1020- 1022. doi:10.1002/hep.24199 [23] Torres, D.M. and Harrison, S.A. (2008) Diagnosis and therapy of nonalcoholic steatohepatitis. Gastroenterology, 134, 1682-1698. doi:10.1053/j.gastro.2008.02.077 [24] Patwardhan, V., Paul, S., Corey, K.E., Mazhar, S.M., Richter, J.M., Thiim, M. and Chung R.T. (2011) Hepato- cellular carcinoma screening rates vary by etiology of cir- rhosis and involvement of gastrointestinal sub-specialists. Digestive Disease Sciences, 56, 3316-3322. doi:10.1007/s10620-011-1836-2 [25] Davila, J.A., Morgan, R.O., Richardson, P.A., Du, X.L., McGlynn, K.A. and El-Serag, H.B. (2010) Use of sur- veillance for hepatocellular carcinoma among patients with cirrhosis in the United States. Hepatology, 52, 132- 141. doi:10.1002/hep.23615 Copyright © 2013 SciRes. OPEN ACCESS
|