Paper Menu >>
Journal Menu >>
 Vol.2, No.10, 1232-1237 (2010) Health doi:10.4236/health.2010.210183 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ Detection of the movement direction by the cells with directional receptive fields in the primary visual cortex of the cat Ausra Daugirdiene1*, Algimantas Svegzda2, Romualdas Satinskas2, Henrikas Vaitkevicius2 1Department Psychology Didactics, Vilnius Pedagogical University, Vilnius, Lithuania; *Corresponding Author: ausra.daugirdiene@gmail.com 2Department of General Psychology Vilnius University, Vilnius, Lithuania Received 24 July 2010; revised 2 August 2010; accepted 11 August 2010. ABSTRACT The study was performed on neurons with dire- ction selective (DS) receptive fields (RFs) in the primary visual cortex of the cat. Preferred dire- ctions (PDs) of these cells to a single light spot and a system of two identical light spots moving across the RF with a given angle between them were compared. Directional interactions appear- ed when the angles between the directions of the two moving spots were 30º or 60º. PD for 56% of the cells coincided with bisectors of these ang- les. These cells responded to a combination of the two moving stimuli as if only one stimulus moved in the RF in an intermediate direction. This direction coincided with PD of the DS neu- ron to a single spot. Also, the investigation rev- ealed that DS neurons responded to stimuli moving at such angles as 180º (to preferred and opposite directions simultaneously). In the fur- ther experiment we investigated responses of the DS cells in the primary visual cortex of RF. The angle between the directions of the two mo- ving spots was 60º. These cells responded to a combination of the two moving stimuli as if only one stimulus moved in RF in an intermediate direction. The more relative luminance of one of spots in pair was, the closer the intermediate dir- ection approached to the direction of this spot). Keywords: Cat; Primary Visual Cortex; Directionally Selective cells; Receptive Field (RF) 1. INTRODUCTION The perception of the motion of an object deals with the detection of the movement direction. A retina is the firs stage where DC cells are discovered in the cat’s visual system [1,2]. The DS cells respond preferentially to a stimulus moving in a particular direction (preferably to these cells). DS cells of the primary visual cortex of the cat do not have any direct projection from retina via the dorsal lateral geniculate nucleus (LGNd) [3-5]. This suggests that DS is mainly generated within the primary visual cortex. It is known that area 17 and 18 have parallel input fr- om LGNd [6], have same types of cells receptive fields (RFs) [7,8] and may be considered as a primary visual cortex [9]. The cortical mechanism of direction selectiv- ity is under constant interest [8,10,11]. The employment of various types of stimuli reveals the specific features of selectivity in RFs of DS cortical neurons [12-14], which can be useful in clarifying the basis of DS. The first aim of the study was to investigate how the PDs of DS cells depend on the interaction, which two stimuli evoke moving in different directions across the RF. It is known that there are neurons selective to direc- tion of the stimulus motion in the visual system that, as is believed, is related with the mechanisms of the mov- ement analysis [15,16]. The properties of this directional interaction in RF of DS neurons were the aim of the fur- ther study. We suggested elucidating them by exploring two moving light spots with different relative luminance contrast. We investigated how the PD of DS cells de- pends on the relative luminance of two light spots mov- ing in different directions across the RF. 2. METHODS Influence of the angular separation between stimuli. The investigation was performed on twenty adult cats (weight 2.5–3.5 kg). These animals were used for other studies as well. Surgery was carried out under deep an- esthesia with Nembutal (50 mg/kg), followed by atro-  A. Daugirdiene et al. / Health 2 (2010) 1232-1237 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 123 1233 pine sulphate (0.5 ml 0.1% solution), intramuscularly, using sterile technique. The femoral vein was cannulated and bilateral sympaticotomy performed. A headset was implanted in the frontal bone. A small craniotomy (5 mm) and duratomy was performed over the central pro- jection zone of area 17 near the border with area 18. A precise histological reconstruction of the individual ele- ctrode tracks was not done. We were confident that we recorded from neurons in cortical areas 17 and 18 on the basis of localization of the trepanation site, the depth of microelectrode position below the cortical surface and the characteristics of the RFs. During the experiment, the cat was fixed in an expe- rimental instrument by the headset and lightly anaesthe- tized with a mixture of nitrous oxide and oxygen (70:30). The animal was paralyzed with a permanent intravenous infusion of muscle–relaxant tubocurarine chloride 1.2 mg/kg per hour throughout the experiment. All the wo- unds were infiltrated with long lasting local anesthetics. Corneas were protected by neutral soft contact lenses and refractive errors corrected by spectacle lenses. The cat looked at a stimulus presented on the screen at a distance of 144 cm. All the stimulation was monocular. After the investigation was completed, the animal was sacrified by overdose of Nembutal. Protocols and methods corre- sponded to the requirements of the Lithuanian Commis- sion on Ethics for Studies involving Laboratory Ani- mals. Optical stimulation was carried out on the mechani- cally and optically supplemented two–canal automatic projector equipped with the electrical shutters, rotating optical prisms on step drivers and movable mirrors dri- ven by personal computer. A light spot (0.4 deg in di- ameter) or a system of two such spots moving over the semi–translucent tangent screen was used for stimulation. Background luminance was 0.15 cd/m2. Extra cellular recordings were made from single cells in visual cortex by electrolytically sharpened varnished tungsten microelectrodes. An automatic driver advanced the microelectrode in minimum step of 1 micrometer with depth monitoring by a special electronic table. Sig- nals were amplified, sent to window discriminator and monitored through loudspeaker and oscilloscope. The is- olated spikes were stored like sequence of codes to indi- cate the time of occurrence (1 ms resolution). The boun- daries of the RF and light active RF centre for each DS cell encountered were mapped on the screen with a fla- shing and moving light spot of the hand held electric ophthalmoscope. The location of area centralis was deri- ved from the position of the optic disk, plotted on the screen by the mean angular separation [17]. The experiment consisted of two parts. Firstly the directional characteristics of neurons were registered. The stimulus (single light spot) moved across the centre of the RF in 12 directions with step of 30º (in the range from 0 to 360º), in randomized order. The amp- litude of motion always substantially (by 40%) exceeded the RF width. Optimal speed of the spot movement and spot luminance were chosen for each neuron. A post sti- mulus time histogram PSTH (10–20 consecutive pres- entations; bin width 20 ms) was compiled in response to a moving light spot in the single direction (Figure 1(a)). From these 12 PSTHs the directional–tuning diagram was formed (Figure 1(b)). The directional–tuning band- width was calculated as the full width of the tuning curve at 0.63 of its maximum height. The PD was calcu- lated by the method of linear approximation with refer- ence to the value of weights in the adjacent directions. We concerned as DS neurons those cells that had the directional index (DI) higher than 0.5. DI was defined as a relative difference in responses to a preferred direction of movement (Rpref) and opposite to it. DI = (Rpref – Rbackgr)/(Rpref) where Rpref and Rbackgr expressed as maximum firing rates in indicated direction minus the maximum rate of background activity. Further investigation was performed only on DS neu- rons, which rather well responded to moving light spot. Secondly, we investigated how the PDs of the DS neurons depend on the angle between the two moving spots. The angle was changed with a step of 30º ranging from 0 to 180º. Two identical light spots of equal lumi- nance and at the same speed moved simultaneously with Figure 1. (a) – Post–stimulus–time histograms of responses to a light spot stimulus moving across the RF of a simple cell in an indicated direction. Bin width 20 ms, 20 consecutive pres- entations, velocity – 2 deg/s; (b) – Di- rectional tuning diagram. Maximal rate of response – 45 imp/s, PD – 220º, directional–tuning bandwidth – 70º. 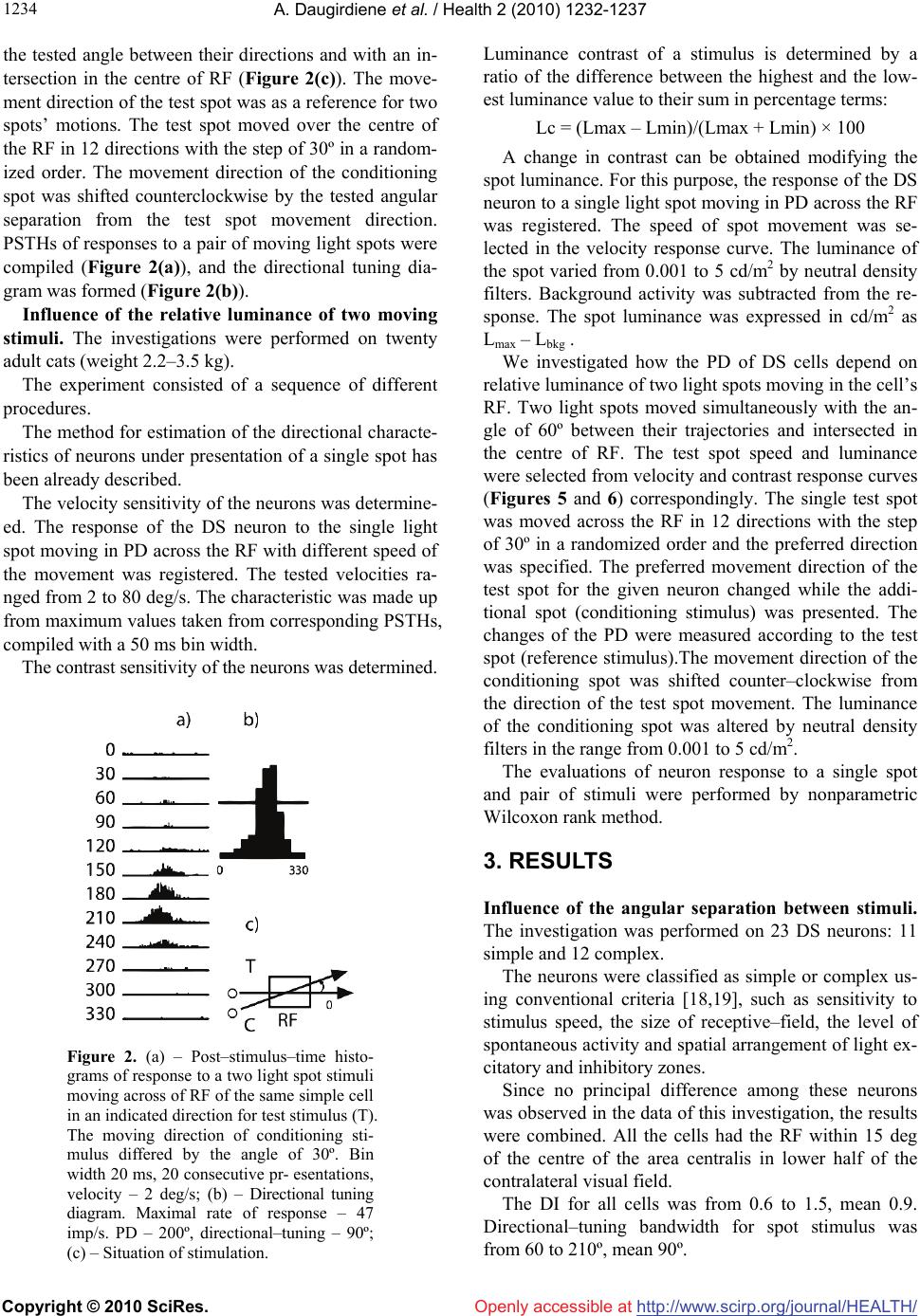 A. Daugirdiene et al. / Health 2 (2010) 1232-1237 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 1234 the tested angle between their directions and with an in- tersection in the centre of RF (Figure 2(c)). The move- ment direction of the test spot was as a reference for two spots’ motions. The test spot moved over the centre of the RF in 12 directions with the step of 30º in a random- ized order. The movement direction of the conditioning spot was shifted counterclockwise by the tested angular separation from the test spot movement direction. PSTHs of responses to a pair of moving light spots were compiled (Figure 2(a)), and the directional tuning dia- gram was formed (Figure 2(b)). Influence of the relative luminance of two moving stimuli. The investigations were performed on twenty adult cats (weight 2.2–3.5 kg). The experiment consisted of a sequence of different procedures. The method for estimation of the directional characte- ristics of neurons under presentation of a single spot has been already described. The velocity sensitivity of the neurons was determine- ed. The response of the DS neuron to the single light spot moving in PD across the RF with different speed of the movement was registered. The tested velocities ra- nged from 2 to 80 deg/s. The characteristic was made up from maximum values taken from corresponding PSTHs, compiled with a 50 ms bin width. The contrast sensitivity of the neurons was determined. Figure 2. (a) – Post–stimulus–time histo- grams of response to a two light spot stimuli moving across of RF of the same simple cell in an indicated direction for test stimulus (T). The moving direction of conditioning sti- mulus differed by the angle of 30º. Bin width 20 ms, 20 consecutive pr- esentations, velocity – 2 deg/s; (b) – Directional tuning diagram. Maximal rate of response – 47 imp/s. PD – 200º, directional–tuning – 90º; (c) – Situation of stimulation. Luminance contrast of a stimulus is determined by a ratio of the difference between the highest and the low- est luminance value to their sum in percentage terms: Lc = (Lmax – Lmin)/(Lmax + Lmin) × 100 A change in contrast can be obtained modifying the spot luminance. For this purpose, the response of the DS neuron to a single light spot moving in PD across the RF was registered. The speed of spot movement was se- lected in the velocity response curve. The luminance of the spot varied from 0.001 to 5 cd/m2 by neutral density filters. Background activity was subtracted from the re- sponse. The spot luminance was expressed in cd/m2 as Lmax – Lbkg . We investigated how the PD of DS cells depend on relative luminance of two light spots moving in the cell’s RF. Two light spots moved simultaneously with the an- gle of 60º between their trajectories and intersected in the centre of RF. The test spot speed and luminance were selected from velocity and contrast response curves (Figures 5 and 6) correspondingly. The single test spot was moved across the RF in 12 directions with the step of 30º in a randomized order and the preferred direction was specified. The preferred movement direction of the test spot for the given neuron changed while the addi- tional spot (conditioning stimulus) was presented. The changes of the PD were measured according to the test spot (reference stimulus).The movement direction of the conditioning spot was shifted counter–clockwise from the direction of the test spot movement. The luminance of the conditioning spot was altered by neutral density filters in the range from 0.001 to 5 cd/m2. The evaluations of neuron response to a single spot and pair of stimuli were performed by nonparametric Wilcoxon rank method. 3. RESULTS Influence of the angular separation between stimuli. The investigation was performed on 23 DS neurons: 11 simple and 12 complex. The neurons were classified as simple or complex us- ing conventional criteria [18,19], such as sensitivity to stimulus speed, the size of receptive–field, the level of spontaneous activity and spatial arrangement of light ex- citatory and inhibitory zones. Since no principal difference among these neurons was observed in the data of this investigation, the results were combined. All the cells had the RF within 15 deg of the centre of the area centralis in lower half of the contralateral visual field. The DI for all cells was from 0.6 to 1.5, mean 0.9. Directional–tuning bandwidth for spot stimulus was from 60 to 210º, mean 90º. 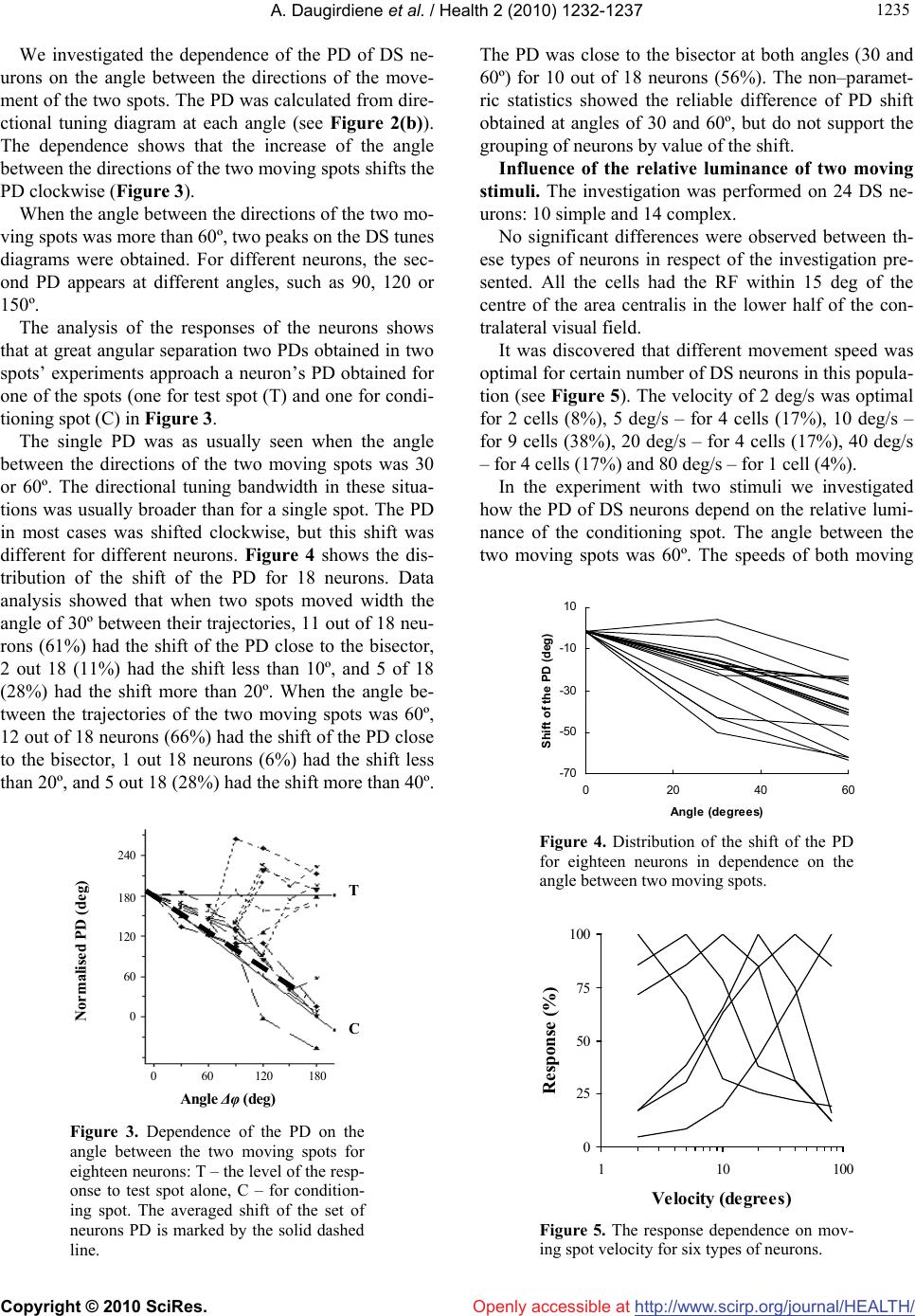 A. Daugirdiene et al. / Health 2 (2010) 1232-1237 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 123 1235 We investigated the dependence of the PD of DS ne- urons on the angle between the directions of the move- ment of the two spots. The PD was calculated from dire- ctional tuning diagram at each angle (see Figure 2(b)). The dependence shows that the increase of the angle between the directions of the two moving spots shifts the PD clockwise (Figure 3). When the angle between the directions of the two mo- ving spots was more than 60º, two peaks on the DS tunes diagrams were obtained. For different neurons, the sec- ond PD appears at different angles, such as 90, 120 or 150º. The analysis of the responses of the neurons shows that at great angular separation two PDs obtained in two spots’ experiments approach a neuron’s PD obtained for one of the spots (one for test spot (T) and one for condi- tioning spot (C) in Figure 3. The single PD was as usually seen when the angle between the directions of the two moving spots was 30 or 60º. The directional tuning bandwidth in these situa- tions was usually broader than for a single spot. The PD in most cases was shifted clockwise, but this shift was different for different neurons. Figure 4 shows the dis- tribution of the shift of the PD for 18 neurons. Data analysis showed that when two spots moved width the angle of 30º between their trajectories, 11 out of 18 neu- rons (61%) had the shift of the PD close to the bisector, 2 out 18 (11%) had the shift less than 10º, and 5 of 18 (28%) had the shift more than 20º. When the angle be- tween the trajectories of the two moving spots was 60º, 12 out of 18 neurons (66%) had the shift of the PD close to the bisector, 1 out 18 neurons (6%) had the shift less than 20º, and 5 out 18 (28%) had the shift more than 40º. Angle Δφ (deg) C Normalised PD (deg) 0 60 120 180 240 180 120 60 0 T Figure 3. Dependence of the PD on the angle between the two moving spots for eighteen neurons: T – the level of the resp- onse to test spot alone, C – for condition- ing spot. The averaged shift of the set of neurons PD is marked by the solid dashed line. The PD was close to the bisector at both angles (30 and 60º) for 10 out of 18 neurons (56%). The non–paramet- ric statistics showed the reliable difference of PD shift obtained at angles of 30 and 60º, but do not support the grouping of neurons by value of the shift. Influence of the relative luminance of two moving stimuli. The investigation was performed on 24 DS ne- urons: 10 simple and 14 complex. No significant differences were observed between th- ese types of neurons in respect of the investigation pre- sented. All the cells had the RF within 15 deg of the centre of the area centralis in the lower half of the con- tralateral visual field. It was discovered that different movement speed was optimal for certain number of DS neurons in this popula- tion (see Figure 5). The velocity of 2 deg/s was optimal for 2 cells (8%), 5 deg/s – for 4 cells (17%), 10 deg/s – for 9 cells (38%), 20 deg/s – for 4 cells (17%), 40 deg/s – for 4 cells (17%) and 80 deg/s – for 1 cell (4%). In the experiment with two stimuli we investigated how the PD of DS neurons depend on the relative lumi- nance of the conditioning spot. The angle between the two moving spots was 60º. The speeds of both moving -70 -50 -30 -10 10 0 204060 Angle (degrees) Shift of the PD (deg) Figure 4. Distribution of the shift of the PD for eighteen neurons in dependence on the angle between two moving spots. 0 25 50 75 100 110100 Velo cit y (de g re es ) Response (%) Figure 5. The response dependence on mov- ing spot velocity for six types of neurons. 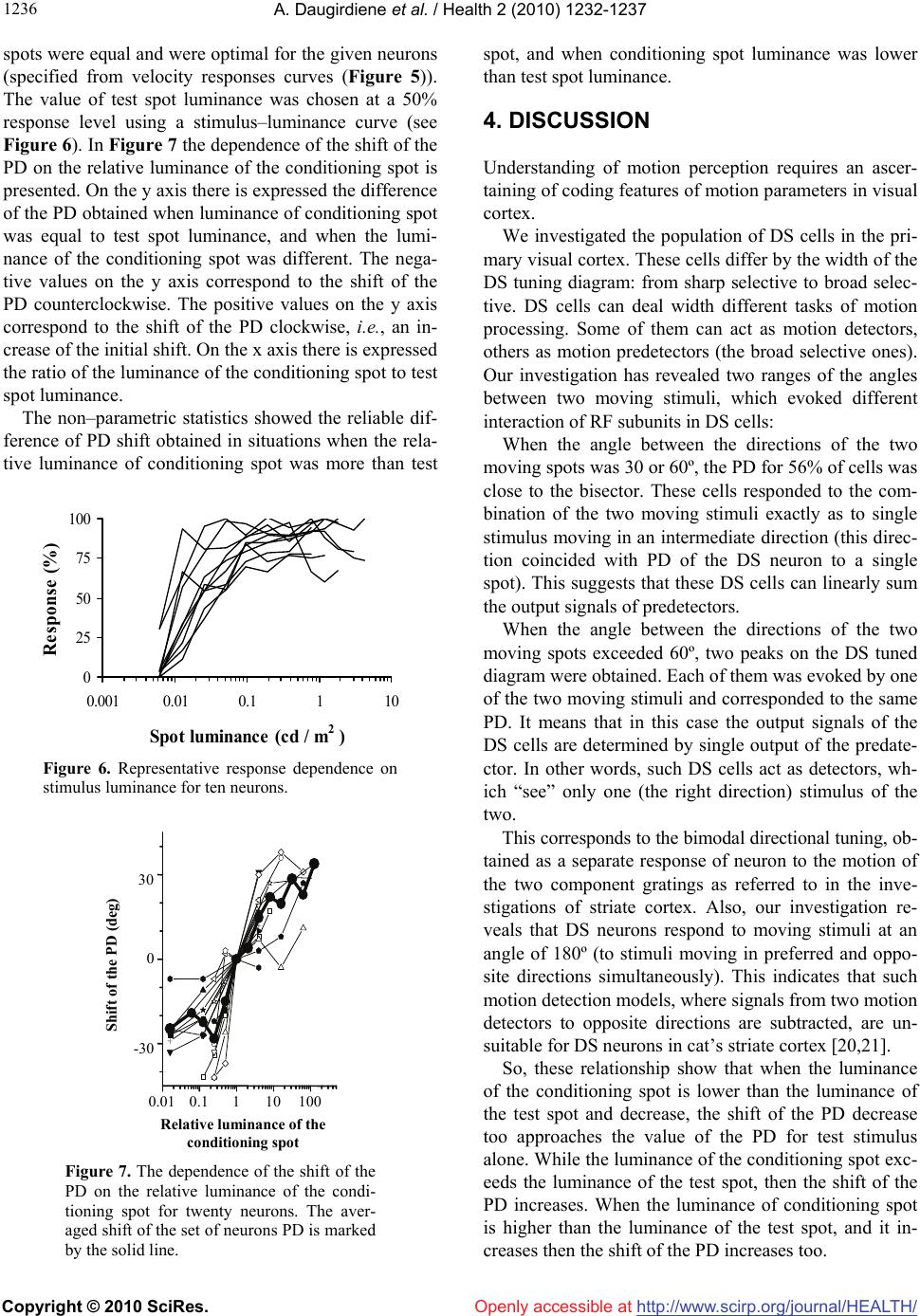 A. Daugirdiene et al. / Health 2 (2010) 1232-1237 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 1236 spots were equal and were optimal for the given neurons (specified from velocity responses curves (Figure 5)). The value of test spot luminance was chosen at a 50% response level using a stimulus–luminance curve (see Figure 6). In Figure 7 the dependence of the shift of the PD on the relative luminance of the conditioning spot is presented. On the y axis there is expressed the difference of the PD obtained when luminance of conditioning spot was equal to test spot luminance, and when the lumi- nance of the conditioning spot was different. The nega- tive values on the y axis correspond to the shift of the PD counterclockwise. The positive values on the y axis correspond to the shift of the PD clockwise, i.e., an in- crease of the initial shift. On the x axis there is expressed the ratio of the luminance of the conditioning spot to test spot luminance. The non–parametric statistics showed the reliable dif- ference of PD shift obtained in situations when the rela- tive luminance of conditioning spot was more than test 0 25 50 75 100 0.001 0.010.1110 Spot luminance (cd / m 2 ) Response (%) Figure 6. Representative response dependence on stimulus luminance for ten neurons. Relative luminance of the conditionin g s p ot Shift of the PD (deg) 0.01 0.1 110 100 -30 0 30 Figure 7. The dependence of the shift of the PD on the relative luminance of the condi- tioning spot for twenty neurons. The aver- aged shift of the set of neurons PD is marked by the solid line. spot, and when conditioning spot luminance was lower than test spot luminance. 4. DISCUSSION Understanding of motion perception requires an ascer- taining of coding features of motion parameters in visual cortex. We investigated the population of DS cells in the pri- mary visual cortex. These cells differ by the width of the DS tuning diagram: from sharp selective to broad selec- tive. DS cells can deal width different tasks of motion processing. Some of them can act as motion detectors, others as motion predetectors (the broad selective ones). Our investigation has revealed two ranges of the angles between two moving stimuli, which evoked different interaction of RF subunits in DS cells: When the angle between the directions of the two moving spots was 30 or 60º, the PD for 56% of cells was close to the bisector. These cells responded to the com- bination of the two moving stimuli exactly as to single stimulus moving in an intermediate direction (this direc- tion coincided with PD of the DS neuron to a single spot). This suggests that these DS cells can linearly sum the output signals of predetectors. When the angle between the directions of the two moving spots exceeded 60º, two peaks on the DS tuned diagram were obtained. Each of them was evoked by one of the two moving stimuli and corresponded to the same PD. It means that in this case the output signals of the DS cells are determined by single output of the predate- ctor. In other words, such DS cells act as detectors, wh- ich “see” only one (the right direction) stimulus of the two. This corresponds to the bimodal directional tuning, ob- tained as a separate response of neuron to the motion of the two component gratings as referred to in the inve- stigations of striate cortex. Also, our investigation re- veals that DS neurons respond to moving stimuli at an angle of 180º (to stimuli moving in preferred and oppo- site directions simultaneously). This indicates that such motion detection models, where signals from two motion detectors to opposite directions are subtracted, are un- suitable for DS neurons in cat’s striate cortex [20,21]. So, these relationship show that when the luminance of the conditioning spot is lower than the luminance of the test spot and decrease, the shift of the PD decrease too approaches the value of the PD for test stimulus alone. While the luminance of the conditioning spot exc- eeds the luminance of the test spot, then the shift of the PD increases. When the luminance of conditioning spot is higher than the luminance of the test spot, and it in- creases then the shift of the PD increases too.  A. Daugirdiene et al. / Health 2 (2010) 1232-1237 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 123 1237 Thus, alteration of the relative luminance of two mo- ving spots by changing the luminance of the condition- ing spot evokes the deviation of the PD of DS neurons towards the direction of the more intensive stimulus in pair. Similar results were obtained in psychophysical experiments on human subjects with apparent motion or with random dot stimuli [22]. Such familiarity can indi- cate that some features of basic principles that are nec- essary for discrimination and perception of motion di- rection are already presented at the level of striate cor- tex. 5. CONCLUSIONS 1) When the two stimuli moving in different directions (angles between movement directions were 30º and 60º) are projected in the receptive field of directional sensi- tive neurons, the preferred direction for 56% of tested neurons was close to the bisector of angles mentioned above. Thus, these neurons selective to movement direc- tion averaged the movement directions of two moving bright spots. 2) When the relative luminosity of two spots moving in different directions was changed, the preferred directi- on of the DS neurons was also changed. The preferred direction was shifted toward the brighter spot. The grea- ter was the difference in luminosity of two spot the lar- ger was the shift of the preferred direction. REFERENCES [1] Cleland, B.G. and Levick, W.R. (1974) Properties of rar- ely encountered types of ganglion cells in the cat’s retina and an overall classification. Journal of Physiology, 240(2), 457-492. [2] Stone, J. and Fukuda, Y. (1974) Properties of cat retinal ganglion cells: a comparison of W-cells with X- and Y-cells. Journal of Neurophysiology, 37, 722-748. [3] Hoffmann, K.P. (1973) Conduction velocity in pathways from retina to superior colliculus in the cat: A correlation with receptive-field properties. Journal of Neurophysi- ology, 36(3), 409-424. [4] Hoffmann, K.P. and Stone, J. (1985) Retinal input to the nucleus of the optic tract of the cat assessed by antid- romic activation of ganglion cells. Experimental Brain Research, 59(2), 395-403. [5] Cleland, B.G., Levick, W.R., Morstyn, R. and Wagner, H.G. (1976) Lateral geniculate relay of slowly conduct- ing retinal afferents to cat visual cortex. Journal of Physiology, 255(1), 299-320. [6] Stone, J. and Dreher, B. (1973) Projection of X- and Y- cells of the cat’s lateral geniculate nucleus to areas 17 and 18 of visual cortex. Journal of Neurophysiology, 36(3), 551-567. [7] Orban, G.A. and Callens, M. (1977) Receptive field types of area 18 neurones in the cat. Experimental Brain Research, 30(1), 107-123. [8] Orban, G.A., Kenedy, H. and Maes, H. (1981) Response to movement of neurons in areas 17 and 18 of the cat: Direction selectivity. Journal of Neurophysiology, 45(6), 1059-1073. [9] Tretter, F., Cynader, M. and Singer, W. (1975) Cat par- astriate cortex: A primary or secondary visual area. Journal of Neurophysiology, 38(5), 109-113. [10] Movshon, J.A., Thompson, I.D. and Tolhurst, D.J. (1978) Receptive field organization of complex cells in the cat’s striate cortex. Journal of Physiology, 283, 79-99. [11] Emerson, R.C., Bergen, J.R. and Adelson, E.H. (1992) Directionally selective complex cells and the computa- tion of motion energy in cat visual cortex. Vision Re- search, 32(2), 203-218. [12] Hammond, P. and MacKay, D.M. (1975) Differential responses of cat visual cortical cells to textured stimuli. Experimental Brain Research, 22(4), 427-430. [13] Jagadeesh, B., Wheat, H.S. and Ferster, D. (1993) Line- arity of summation of synaptic potentials underlying di- rection selectivity in simple cells of the cat visual cortex. Science, 262(5141), 1901-1904. [14] Emerson, R.C. (1997) Quadrature subunits in direction- ally selective simple cells: Spatiotemporal interactions. Visual Neuroscience, 14(2), 357-371. [15] Barlow, H.B. and Levick, W.R. (1965) The mechanism of directionally selective units in rabbit’s retina. Journal of Physiology, 178(3), 477-504. [16] Orban, G.A., Kenedy, H. and Maes, H. (1981) Response to movement of neurons in areas 17 and 18 of the cat: direction selectivity. Journal of Neurophysiology, 45(6), 1059-1073. [17] Bishop, P.O., Kozak, W. and Vakkur, G.J. (1962) Some quantitative aspects of the cat’s eye: Axis and plane of reference, visual field co-ordinates and optics. Journal of Physiology, 163(3), 466-502. [18] Hubel, D.H. and Wiesel, T.N. (1962) Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. Journal of Physiology, 160(1), 106- 154. [19] Henry, G.H. (1977) Receptive field classes of cells in the striate cortex of the cat. Brain Research, 133(1), 1-28. [20] Adelson, E.H. and Berger, J.R. (1985) Spatiotemporal energy models for the perception of motion. Journal of the Optical Society of America A, 2(2), 284-291. [21] Van Santen, J.P. and Sperling, G. (1985) Elaborated Reichardt detectors. Journal of the Optical Society of America A, 2(2), 300-321. [22] Zohary, E., Scase, M.O. and Bradic, O.J. (1996) Integra- tion across directions in dynamic random dot displays: vector summation or winner take all? Vision Research, 36(15), 2321-2331. |

