M. E. LAVOIE, J. E. A. STAUDER
154
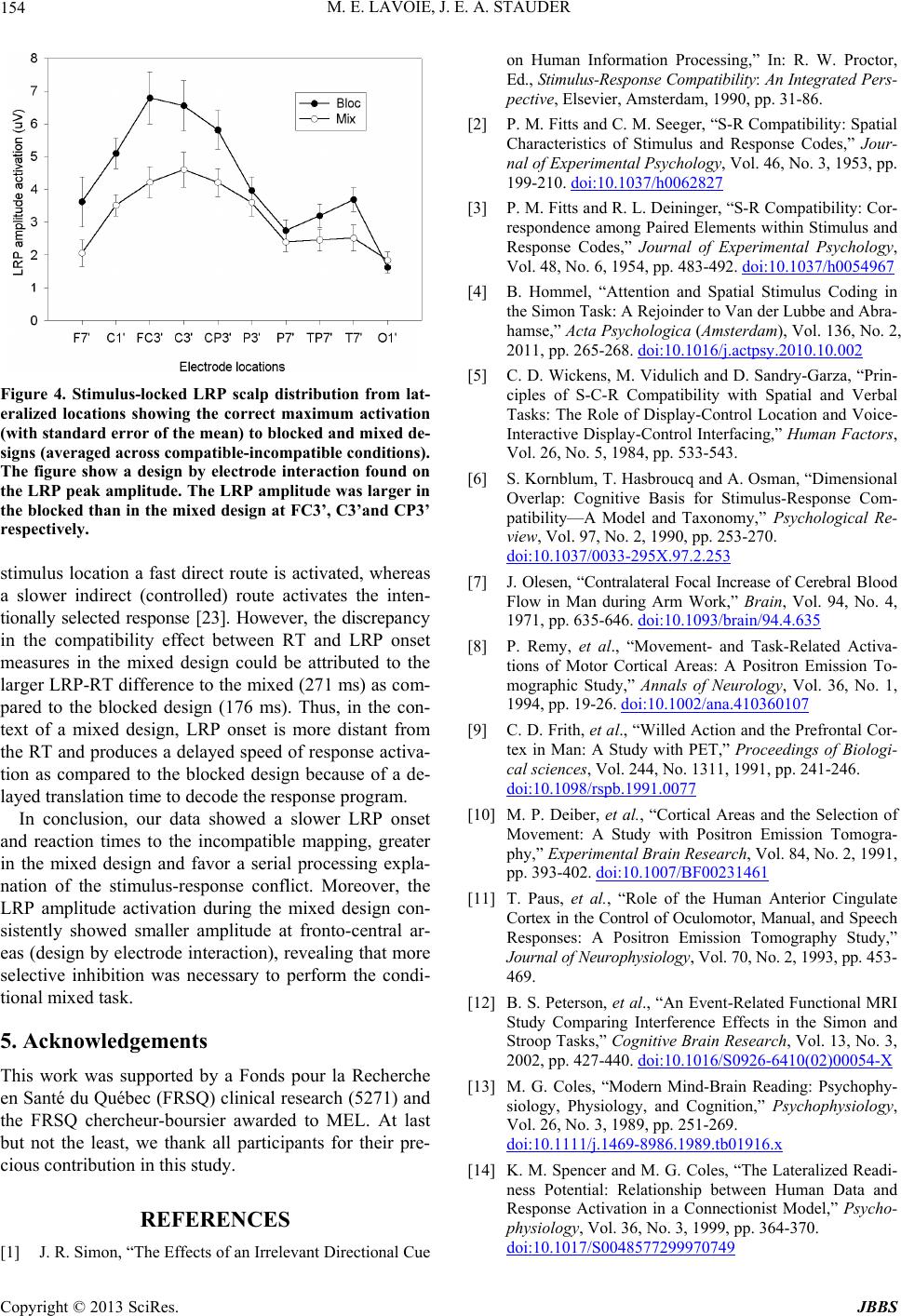
Figure 4. Stimulus-locked LRP scalp distribution from lat-
eralized locations showing the correct maximum activation
(with standard error of the mean) to blocked and mixed de-
signs (averaged across compatible-incompatible conditions).
The figure show a design by electrode interaction found on
the LRP peak amplitude. The LRP amplitude w as larger in
the blocked than in the mixed design at FC3’, C3’and CP3’
respectively.
stimulus location a fast direct route is activated, whereas
a slower indirect (controlled) route activates the inten-
tionally selected response [23]. However, the discrepancy
in the compatibility effect between RT and LRP onset
measures in the mixed design could be attributed to the
larger LRP-RT difference to the mixed (271 ms) as com-
pared to the blocked design (176 ms). Thus, in the con-
text of a mixed design, LRP onset is more distant from
the RT and produces a delayed speed of response activa-
tion as compared to the blocked design because of a de-
layed translation time to decode the response program.
In conclusion, our data showed a slower LRP onset
and reaction times to the incompatible mapping, greater
in the mixed design and favor a serial processing expla-
nation of the stimulus-response conflict. Moreover, the
LRP amplitude activation during the mixed design con-
sistently showed smaller amplitude at fronto-central ar-
eas (design by electrode interaction), revealing that more
selective inhibition was necessary to perform the condi-
tional mixed task.
5. Acknowledgements
This work was supported by a Fonds pour la Recherche
en Santé du Québec (FRSQ) clinical research (5271) and
the FRSQ chercheur-boursier awarded to MEL. At last
but not the least, we thank all participants for their pre-
cious contribution in this study.
REFERENCES
[1] J. R. Simon, “The Effects of an Irrelevant Directional Cue
on Human Information Processing,” In: R. W. Proctor,
Ed., Stimulus-Re sponse Compatibility: An Integrated Pers-
pective, Elsevier, Amsterdam, 1990, pp. 31-86.
[2] P. M. Fitts and C. M. Seeger, “S-R Compatibility: Spatial
Characteristics of Stimulus and Response Codes,” Jour-
nal of Experimental Psychology, Vol. 46, No. 3, 1953, pp.
199-210. doi:10.1037/h0062827
[3] P. M. Fitts and R. L. Deininger, “S-R Compatibility: Cor-
respondence among Paired Elements within Stimulus and
Response Codes,” Journal of Experimental Psychology,
Vol. 48, No. 6, 1954, pp. 483-492. doi:10.1037/h0054967
[4] B. Hommel, “Attention and Spatial Stimulus Coding in
the Simon Task: A Rejoinder to Van der Lubbe and Abra-
hamse,” Acta Psychologica (Amsterdam), Vol. 136, No. 2,
2011, pp. 265-268. doi:10.1016/j.actpsy.2010.10.002
[5] C. D. Wickens, M. Vidulich and D. Sandry-Garza, “Prin-
ciples of S-C-R Compatibility with Spatial and Verbal
Tasks: The Role of Display-Control Location and Voice-
Interactive Display-Control Interfacing,” Human Factors,
Vol. 26, No. 5, 1984, pp. 533-543.
[6] S. Kornblum, T. Hasbroucq and A. Osman, “Dimensional
Overlap: Cognitive Basis for Stimulus-Response Com-
patibility—A Model and Taxonomy,” Psychological Re-
view, Vol. 97, No. 2, 1990, pp. 253-270.
doi:10.1037/0033-295X.97.2.253
[7] J. Olesen, “Contralateral Focal Increase of Cerebral Blood
Flow in Man during Arm Work,” Brain, Vol. 94, No. 4,
1971, pp. 635-646. doi:10.1093/brain/94.4.635
[8] P. Remy, et al., “Movement- and Task-Related Activa-
tions of Motor Cortical Areas: A Positron Emission To-
mographic Study,” Annals of Neurology, Vol. 36, No. 1,
1994, pp. 19-26. doi:10.1002/ana.410360107
[9] C. D. Frith, et al., “Willed Action and the Prefrontal Cor-
tex in Man: A Study with PET,” Proceedings of Biologi-
cal sciences, Vol. 244, No. 1311, 1991, pp. 241-246.
doi:10.1098/rspb.1991.0077
[10] M. P. Deiber, et al., “Cortical Areas and the Selection of
Movement: A Study with Positron Emission Tomogra-
phy,” Experimental Brain Research, Vol. 84, No. 2, 1991,
pp. 393-402. doi:10.1007/BF00231461
[11] T. Paus, et al., “Role of the Human Anterior Cingulate
Cortex in the Control of Oculomotor, Manual, and Speech
Responses: A Positron Emission Tomography Study,”
Journal of Neurophysiology, Vol. 70, No. 2, 1993, pp. 453-
469.
[12] B. S. Peterson, et al., “An Event-Related Functional MRI
Study Comparing Interference Effects in the Simon and
Stroop Tasks,” Cognitive Brain Research, Vol. 13, No. 3,
2002, pp. 427-440. doi:10.1016/S0926-6410(02)00054-X
[13] M. G. Coles, “Modern Mind-Brain Reading: Psychophy-
siology, Physiology, and Cognition,” Psychophysiology,
Vol. 26, No. 3, 1989, pp. 251-269.
doi:10.1111/j.1469-8986.1989.tb01916.x
[14] K. M. Spencer and M. G. Coles, “The Lateralized Readi-
ness Potential: Relationship between Human Data and
Response Activation in a Connectionist Model,” Psycho-
physiology, Vol. 36, No. 3, 1999, pp. 364-370.
doi:10.1017/S0048577299970749
Copyright © 2013 SciRes. JBBS