Paper Menu >>
Journal Menu >>
 American Journal of Plant Sciences, 2010, 1, 24-31 doi:10.4236/ajps.2010.11004 Published Online September 2010 (http://www.SciRP.org/journal/ajps) Copyright © 2010 SciRes. AJPS Effects of Hypoxia Stress and Different Level of Mn2+ on Antioxidant Enzyme of Tomato Seedlings Airong Liu1, Shuangchen Chen1*, Yinfa Mi1, Zhou Zhou1, Golam Jalal Ahammed2 1College of Forestry, Henan University of Science and Technology, Luoyang, P.R. China; 2College of Horticulture, Bangladesh Agricultural niversity, Mymensingh , Bangladesh. Email: *lamont@126.com Received June 2nd, 2010; August 2nd, 2010; September 13th, 2010 ABSTRACT The changes of antioxidant enzyme activities and related genes expression of tomato seedlings were evaluated under hypoxia stress with different levels of Mn2+. Activities of superoxide dismutase (SOD), peroxidase (POD), ascorbate peroxide (APX), glutathione reductase (GR), catalase (CAT), the contents of H2O2, ascorbic (AsA) and malondialde- hyde (MDA) were studied to investigate how active oxygen damaged the membrane lipid under hypoxia stress. With 10-200 μmol·L-1 Mn2+, the activities of SOD, POD, APX, GR and the contents of H2O2, AsA, MDA of leaves and roots increased significantly, which indicated that low Mn2+ could eliminate the active oxygen and protect the membrane lipid from hurt. But the activities of catalase (CAT) decreased evidently in the root. When the concentration of Mn2+ reached 400-600 μmol·L-1 under hypoxia stress, the activities of SOD, POD, APX, GR and ASA content decreased remarkably. However, the contents of H2O2 and MDA increased contrarily. A series of resistance genes level achieved peak value with 10 µmol·L-1 Mn2+. The expression level of SOD, CAT, APX, POD, GR were 6.28, 2.19, 5.66, 5.21 and 6.79 times compared to control respectively. These results illustrated appropriate amount of Mn2+ could reduce the damage of active oxygen under hypoxia stress, but reversely, high level of Mn2+ just aggravated the already serious damage to the tomato seedlings. Keywords: Tomato, Hypoxia Stress, Mn2+, Antioxidant Enzyme 1. Introduction Manganese (Mn) is an essential trace element for plant systems. It is involved in photosynthesis, respiration and activation of several enzymes including superoxide dism- utase, NADPH-specific decarboxylating malate dehydro- genase and nitrate reductase [1]. The availability of manganese (Mn) to plants is governed by redox proc- esses, which depend on soil Mn reserve, pH and the availability of electrons [2]. However, the presence of Mn at higher concentrations retards plant growth and development by interfering with metabolic processes [3,4], and consequently it represents an important factor in environmental contamination with various phytotoxic effects. But during plant cultivation, lack of oxygen or anoxia is a common environmental ch- allenge which plants have to face throughout their life. Winter ice encasement, seed imbibition, spring floods and excess of rainfall are examples of natural conditions lea- ding to root hypoxia or anoxia. Hypoxia occurs when the decomposition gives rise to water oxygen concentrations less than 2 ml·L-1 [5]. Due to lack of oxygen, the reduction potential and pH will become much lower around the root-zoon of plants. On the one hand, under this reducing conditions, Mn4+ will be deoxygenized to Mn2+, which is the most form of manganese absorbed by plants in the soil. If the anoxic soil keep this low reducing and pH conditions for a long time, Mn2+ will be accumulated to a high concentration, which lead to serious acidification of soil and result in increasing available Mn concentration [6] and Mn toxic- ity to plants maybe occur. It has been reported that ex- cess Mn disturbs the metabolism of plants and inhibit the plant growth [7-9]. Mn in excess inhibit respiration, af- fect negatively nitrogen and protein metabolism, cause a reduction of chlorophyll contents and inhibit some pho- tosynthetic functions in leaves [10]. As Mn is essential micronutrients imported actively in the chloroplasts, par- ticipating in the structure of different photosynthetic proteins and enzymes, the excess of Mn seems to be par- ticularly damaging to chloroplasts. Some studies have indicated that excess Mn causes deficiency of Fe, Mg  Effects of Hypoxia Stress and Different Level of Mn2+ on Antioxidant Enzyme of Tomato Seedlings Copyright © 2010 SciRes. AJPS 25 and Ca [11,12] and induces inhibition of chlorophyll bio- synthesis and a decline in the photosynthetic rate [13,14]. Research indicates that the degree of cell damage un- der heavy metal stress depends on the rate of ROS form- ation and on the efficiency and capacity of detoxification and repair mechanisms. ROS were partially reduced fo- rms of atmospheric oxygen and under normal conditions their production in cells was low and tightly controlled [15]. Stress that disrupts the cellular homeostasis, inclu- ding heavy metal toxicity, can enhance the production of ROS and increase the steady-state level of H2O2 up to 30-fold [16]. The degree of cell damage under heavy me- tal stress depends on the rate of ROS formation and on the efficiency and capacity of detoxification and repair mechanisms. Many other proteins require metal ions for their catalytic activities and contain metal binding sites making these enzymes highly susceptible to site specific metal-catalyzed oxidation. Any system capable of produ- cing H2O2 and of reducing Fe3+ or Cu2+ can provoke this type of oxidative modification and convert some amino acid residues to carbonyl derivates [17]. This mechanism of oxidative damage could be relevant with Cu and Mn toxicity. Metal-catalyzed oxidation of proteins has been implicated in the marking of proteins for subsequent proteolytic degradation [18]. Tomato (Solanum lycopersium Mill.) plants are very sensitive to water flooding or excess of rainfall, which can easily produce root-zoon hypoxia stress and reducing conditions. So Mn toxicity is incidental to the tomato pl- ants during growing season. Previous researches had ma- inly focused on plant growth and chlorophyll fluorescen- ce in plants [12]. However, little work has been done to determine the antioxidant enzyme activity and related ge- nes expression. The objectives of this study were to identify changes in activities of SOD, POD, CAT, APX, GR and their gene expressions, along with the contents of ASA, H2O2 and MDA in tomato seedlings leaves and roots under hypoxia stress with different level of Mn2+. 2. Material and Methods 2.1. Plant Materials and Treatment Conditions The experiments were carried out in a greenhouse of He- nan University of Science and Technology. Tomato see- ds (S. lycopersium Mill. cv. Zhongza 9) were sown in gr- owth medium containing a mixture of peat and vermicu- lite (7:3, v:v) in trays. When the first true leaf fully ex- panded, groups of eight seedlings were transplanted into a container (40 cm × 25 cm × 15 cm) filled with Hoagland nutrient solution. When the seedlings reached 5 leaves, they were transferred to 10 L plastic pots con- taining aerated full nutrient solution, six seedlings per pot, pH was maintained close to 6.5 by adding diluted H2SO4 or KOH. After 7 days of pre-culture, the plants were treated with 10 μM Mn2+ concentration (normal Mn2+) and 200 μM, 400 μM, 600 μM Mn2+ concentration (excess Mn2+) (su- pplied with MnSO4) and two level of dissolved oxygen (DO) with about 8.0-8.5 mg·L-1 at a normal level and about 0.9-1.1 mg·L-1 at a low level. The experiments seedlings were subjected to hypoxia by flushing nutrient solution with N2 gas for 10 days. The nutrient solution of control plants was continuously flu- shed with air with an air pump. Oxygen concentration in the vessels was monitored with an oxygen meter. The normal DO (8.0-8.5 mg·L-1) was an optimum in- tensity and the low DO (0.9-1.1 mg·L-1) was suboptim- um intensity for plant growth. The experiment had eight treatments: Mn2+ 10 μM + normal DO; Mn2+ 200 μM + normal DO; Mn2+ 400 μM + normal DO; Mn2+ 600 μM + normal DO; Mn2+ 10 μM + low DO; Mn2+ 200 μM + low DO; Mn2+ 400 μM + low DO; Mn2+ 600 μM + low DO. The experiment was arranged as a randomized, complete block design with four replicates, giving a total 64 pots. The growth conditions were as follows: photoperiod of 14/10 h (day/night), temperature of 25/17˚C (day/night), and photosynthetic photon flux density (PPFD) of 600 µmol m-2 s-1. The youngest fully developed leaves and tender roots were rinsed carefully in water, dried with filter paper and used either immediately or frozen in liquid nitrogen for assays of antioxidant enzymes. ASA, H2O2 and MDA co- ntents were measured as soon as symptoms were extre- mely evident. 2.2. Determination of MDA and H2O2 Contents in Leaves The thiobarbituric acid (TBA) test, which determines ma- lonaldehyde (MDA) as an end-product of lipid peroxida- tion in the leaves, was used to measure MDA. Leaves were homogenized and centrifuged in a potassium phos- phate buffer (pH 7.8) for 20 min at 12,000 g, with 1 ml of the supernatant incubated in boiling water for 30 min. The tubes were placed in an ice bath to stop the reaction, after which the samples were centrifuged at 1500 g for 10 min and the absorption was read at 532 nm. The value for nonspecific absorption at 600 nm was measured sim- ultaneously and then subtracted from OD532. The extinc- tion coefficient of 155 mM cm–1 was used to calculate the amount of the MDA-TBA complex. The concentration of H2O2 in leaves was measured by monitoring the absorbance of the titanium-peroxide com- plex at 415 nm, using the method of Brennan and Frenkel [19]. Absorbance values were quantified using a standard curve generated from known concentrations of H2O2.  Effects of Hypoxia Stress and Different Level of Mn2+ on Antioxidant Enzyme of Tomato Seedlings Copyright © 2010 SciRes. AJPS 26 2.3. Enzyme Extraction and Activity Assay For the enzyme assays, 0.3 g of leaf were ground with 3 ml ice-cold 25 mM HEPES buffer (pH 7.8) containing 0.2 mM EDTA, 2 mM AsA and 2% PVP. The homoge- nates were centrifuged at 4˚C for 20 min at 12,000 g and the resulting supernatants were used for the determina- tion of enzymatic activity. A photochemical method pub- lished by Giannopolitis and Ries [20] was used to deter- mine superoxide dismutase (SOD). One unit of SOD activity was defined as the amount of enzyme required to cause a 50% inhibition in the rate of p-nitro blue tetra- zolium chloride reduction at 560 nm. Catalase (CAT) was measured in a reaction mixture containing 25 mM phosphate buffer (pH 7.0), 10 mM H2O2, and the enzyme. The decomposition of H2O2 was determined at 240 nm (E = 39.4 mM cm–1) [21]. APX activities were measured by a decrease in absor- bance at 290 nm and an increase in absorbance at 265 nm according to Nakano and Asada [22]. Glutathione re- ducetase (GR) activity was measured according to Foyer and Halliwell [23], which depends on the rate of decrease in the absorbance of NADPH at 340 nm. All data presented were the mean values of four replic- ates and were analyzed by Duncan’s multiple new range tests using Origin Pro 7.5 and SAS software. 2.4. RNA Extraction and RT-PCR for Gene Expression Analysis Total RNA was isolated from eggplant leaves with 0.3% using TRIZOL reagent (Invitrogen) according to the ma- nufacturer’s instruction. Genomic DNA was removed with RNeasy Mini Kit (Qiagen). The cDNA used as template for RT-PCR was synthe- sized using a RevertAidTM first strand cDNA Synthesis Kit (Fermentas) from 2 μg purified RNA. On the basis of mRNA or EST sequences, the gene-specific primers we- re shown in Table 1 and used for amplification. Quantitative real time PCR was performed using the iCycler iQTM Real-time PCR Detection System (Bio-Rad, Hercules, CA, USA). PCRs were performed using the SYBR Green PCR Master Mix (Applied Biosystems). The PCR conditions consisted of denaturation at 95˚C for 3 min, followed by 40 cycles of denaturation at 95˚C for 30 s, annealing at 58˚C for 30 s and extension at 72˚C for 30 s. A dissociation curve was generated at the end of each PCR cycle to verify that a single product was am- plified using software provided with the iCycler iQTM Real-time PCR Detection System. The identity of the PCR products was verified by single strand sequencing using MegaBACE 1000 DNA analysis system (Amer- sham Biosciences, USA). To minimize sample variations, mRNA expression of the target gene was normalized relative to the expression of the housekeeping gene actin. All experiments were repeated three times for cDNA prepared for two samples of eggplants leaves. The quan- tification of mRNA levels is based on the method of Li- vak and Schmittgen [24]. The threshold cycle (Ct) value of actin was subtracted from that of the gene of interest to obtain a ∆Ct value. The Ct value of untreated control sample was subtracted from the ∆Ct value to obtain a ∆∆Ct value. The fold changes in expression level relative to the control were expressed as 2-∆∆Ct. Table 1. Primers used for real-time RT-PCR assays. Gene Encoding protein Accession no. Primer pairs SOD Superoxide dismutase AY262025.1 F: GGCTTGCATACAAACCTGAA R: CTGACTGCTTCCCATGACAC CAT catalase M93719.1 F: GTCGATTGGTGTTGAACAGG R: AGGACGACAAGGATCAAACC cAPX cytosolic ascorbate peroxidase DQ099420.1 F: GACTCTTGGAGCCCATTAGG R: AGGGTGAAAGGGAACATCAG POD peroxidase DQ099421.1 F: TTAGGGAGCAGTTTCCCACT R: AGGGTGAAAGGGAACATCAG GR glutathione reductase AW033378 F: TTGGTGGAACGTGTGTTCTT R: TCTCATTCACTTCCCATCCA actin AB199316 F: TGGTCGGAATGGGACAGAAG R: CTCAGTCAGGAGAACAGGGT F: forward primer; R: reverse primer. 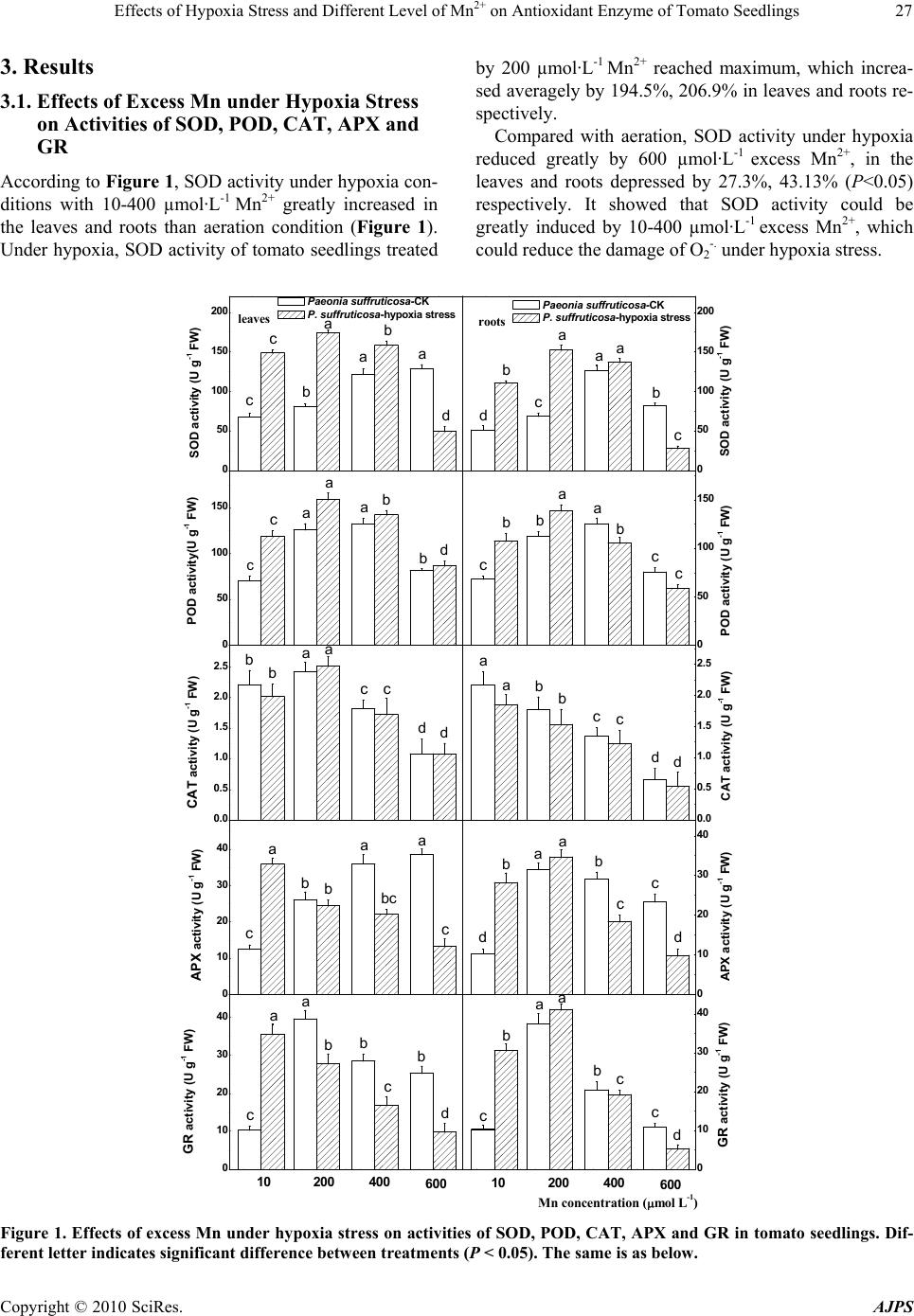 Effects of Hypoxia Stress and Different Level of Mn2+ on Antioxidant Enzyme of Tomato Seedlings Copyright © 2010 SciRes. AJPS 27 3. Results 3.1. Effects of Excess Mn under Hypoxia Stress on Activities of SOD, POD, CAT, APX and GR According to Figure 1, SOD activity under hypoxia con- ditions with 10-400 µmol·L-1 Mn2+ greatly increased in the leaves and roots than aeration condition (Figure 1). Under hypoxia, SOD activity of tomato seedlings treated by 200 µmol·L-1 Mn2+ reached maximum, which increa- sed averagely by 194.5%, 206.9% in leaves and roots re- spectively. Compared with aeration, SOD activity under hypoxia reduced greatly by 600 µmol·L-1 excess Mn2+, in the leaves and roots depressed by 27.3%, 43.13% (P<0.05) respectively. It showed that SOD activity could be greatly induced by 10-400 µmol·L-1 excess Mn2+, which could reduce the damage of O2 -. under hypoxia stress. 0 50 100 150 200 0 50 100 150 0.0 0.5 1.0 1.5 2.0 2.5 0 10 20 30 40 0 10 20 30 40 b b a a a c c d leaves SOD activity (U g-1 FW) Paeon ia suffruticosa-CK P. suffruticosa-hypoxia stress roots 0 50 100 150 ab b a a c cd Paeon ia suffruticosa-CK P. suffruticosa-hypoxia stress POD activity(U g-1 FW) 0 50 100 150 200 d a b a Mn concentration (mol L-1) SOD activity (U g-1 FW) 10 200 400600 10200 400 600 c c b a bbb a a c c c POD activity (U g-1 FW) 0.0 0.5 1.0 1.5 2.0 2.5 b ba a c c dd CAT activity (U g-1 FW) b b a a c c dd CAT acti vity (U g-1 FW) bb aa c c dd APX activity (U g-1 FW) 0 10 20 30 40 b b aaa c c APX activity (U g-1 FW) bc 0 10 20 30 40 bb a a c c d GR activity (U g-1 FW) bb b a a cc c d GR activity (U g-1 FW) Figure 1. Effects of excess Mn under hypoxia stress on activities of SOD, POD, CAT, APX and GR in tomato seedlings. Dif- ferent letter indicates significant difference between treatments (P < 0.05). The same is as below.  Effects of Hypoxia Stress and Different Level of Mn2+ on Antioxidant Enzyme of Tomato Seedlings Copyright © 2010 SciRes. AJPS 28 POD activity in the leaves and roots were increased ev- idently under hypoxia conditions with 10-200 µmol·L-1 Mn2+ (Figure 1). However, POD activity were gradually decreasing with 200 to 600 µmol·L-1 Mn2+ under hypoxia stress. POD activity in the leaves and roots researched the highest point with 400 µmol·L-1 Mn2+ under aeration conditions. POD activity under hypoxia in the roots was gradually lower than those of aeration with the increasing concentration of Mn2+. But in the leaves, POD activity under hypoxia was kept nearly in the same level as those of aeration. Thus, it indicated that 200 µmol·L-1 Mn2+ co- uld eliminate the contents of O2 .- and H2O2 produced by hypoxia stress, which could avoid O2 .- and H2O2 react- ing to form another serious kind of free radical .OH and reduced their harmfulness. According to Figure 1, CAT activity in the leaves in- creased with the increasing Mn2+ concentration from 10 to 200 μmol·L-1. CAT activities enhanced averagely by 11.9%, 11.3% under aeration and hypoxia stress respec- tively. When Mn2+ was higher than 200 μmol·L-1, CAT activity in the leaves and roots both reduced with the increasing Mn2+ concentration. Moreover, with the same Mn2+ concentration, CAT activity in leaves and roots had no significant under aeration and hypoxia. Varying tendency of APX activities in leaves had mu- ch difference from those in roots (Figure 1). Under aera- tion conditions, APX activities in the leaves increased obviously with the increasing of Mn2+. But under hypo- xia conditions, APX activities in the leaves reduced obv- iously with the increasing of Mn2+. APX activity in the leaves under hypoxia were distinctly lower than those of aeration when Mn2+ was higher than 400 μmol·L-1. APX activity in the roots had peak value when Mn2+ was 200 μmol·L-1. When Mn2+ was above 200 μmol·L-1, APX activities in the roots reduced rapidly with the incre- asing of Mn2+ under hypoxia conditions. But under nor- mal aeration conditions, the reducing tendency of APX activity was much gently. When Mn2+ was less than 200 μmol·L-1, APX activi- ties of hypoxia in the roots were higher than those of aer- ation. At the concentration of 200 μmol·L-1 Mn2+, APX activities enhanced averagely by 237.9%, 208.4% in the roots under hypoxia and aeration respectively compared with the control respectively. When Mn2+ was higher th- an 400μmol·L-1, APX activities of hypoxia in the roots were evidently lower than those of aeration. GR activity in the leaves enhanced obviously with the increase of Mn2+ under aeration conditions when Mn2+ was less than 200 μmol·L-1, but it reduced obviously with the increasing of Mn2+ (Figure 1). When Mn2+ was 10 μmol·L-1, GR activities in the leaves of hypoxia increased averagely by 243.9%, which were much higher than those of aeration. When Mn2+ was less than 200 μmol·L-1, GR activity under aeration or hypoxia conditions incre- ased obviously with the increase of Mn2+ in the roots. When Mn2+ was higher than 200 μmol·L-1, GR activity under aeration or hypoxia conditions reduced obviously with the increase of Mn2+. It could come to the conclu- sion that 200 μmol·L-1 Mn2+ could effectively eliminate the harmfulness to the seedlings of free radical. 3.2. Effects of Excess Mn under Hypoxia Stress on H2O2, MDA and ASA Content AsA activity in the leaves and roots increased obviously with the increase of Mn under aeration or hypoxia condi- tions when Mn2+ was less than 200 μmol·L-1, and AsA activity in the leaves and roots of hypoxia were much higher than those of aeration (Figure 2). The AsA activ- ity in the leaves of hypoxia and aeration enhanced 262.1%, 226.9% respectively. AsA activity in the roots of hypoxia and aeration enhanced 312.1%, 261.2% re- spectively. However, when Mn2+ was higher than 400 μmol·L-1, AsA activity in the leaves and roots of hypoxia were obviously lower than those of aeration. AsA activ- ity in the leaves of hypoxia and aeration were reduced averagely by 10.2%, 57.5% respectively compared with the control and AsA activity in the roots of hypoxia and aeration were reduced averagely by 121.8%, 81.7% re- spectively compared with the control. Therefore, low level of Mn2+ could relieve the harmfulness from hypoxia stress, on the contrary, high level of Mn2+ could only make more serious. As shown in Figure 2, contents of H2O2 in the leaves and roots reduced when Mn2+ was less than 200 μmol·L-1 and then increased obviously when Mn2+ was higher than 200 μmol·L-1 under hypoxia conditions. However, con- tents of H2O2 in the roots raised all the way under aera- tion condition. Under hypoxia, contents of MDA in the leaves and roots reduced at the critical point of 200 μmol·L-1 Mn2+ and then increased (Figure 2). Under the conditions of other things being equal, the hypoxia contents of MDA in leaves and roots were much higher than those of aera- tion. Contents of MDA in the hypoxia leaves and roots, aeration leaves and roots increased by 246.1%, 157.7%, 396.7%, and 320.2% respectively. In term of hypoxia stress, 200-400 μmol·L- 1 Mn2+ could reduce the con- tents of MDA evidently and the contents of MDA in hy- poxia leaves and roots were lowered by 57.9%, 52.2% than those of control. It indicated appropriate amount of Mn2+ could effectively alleviate the over-oxidation to the membrane lipid of cell under hypoxia. 3.3. Changes in Gene Expressions in Response to Mn2+ Levels under Hypoxia Stress To analyze the underlying molecular mechanisms for 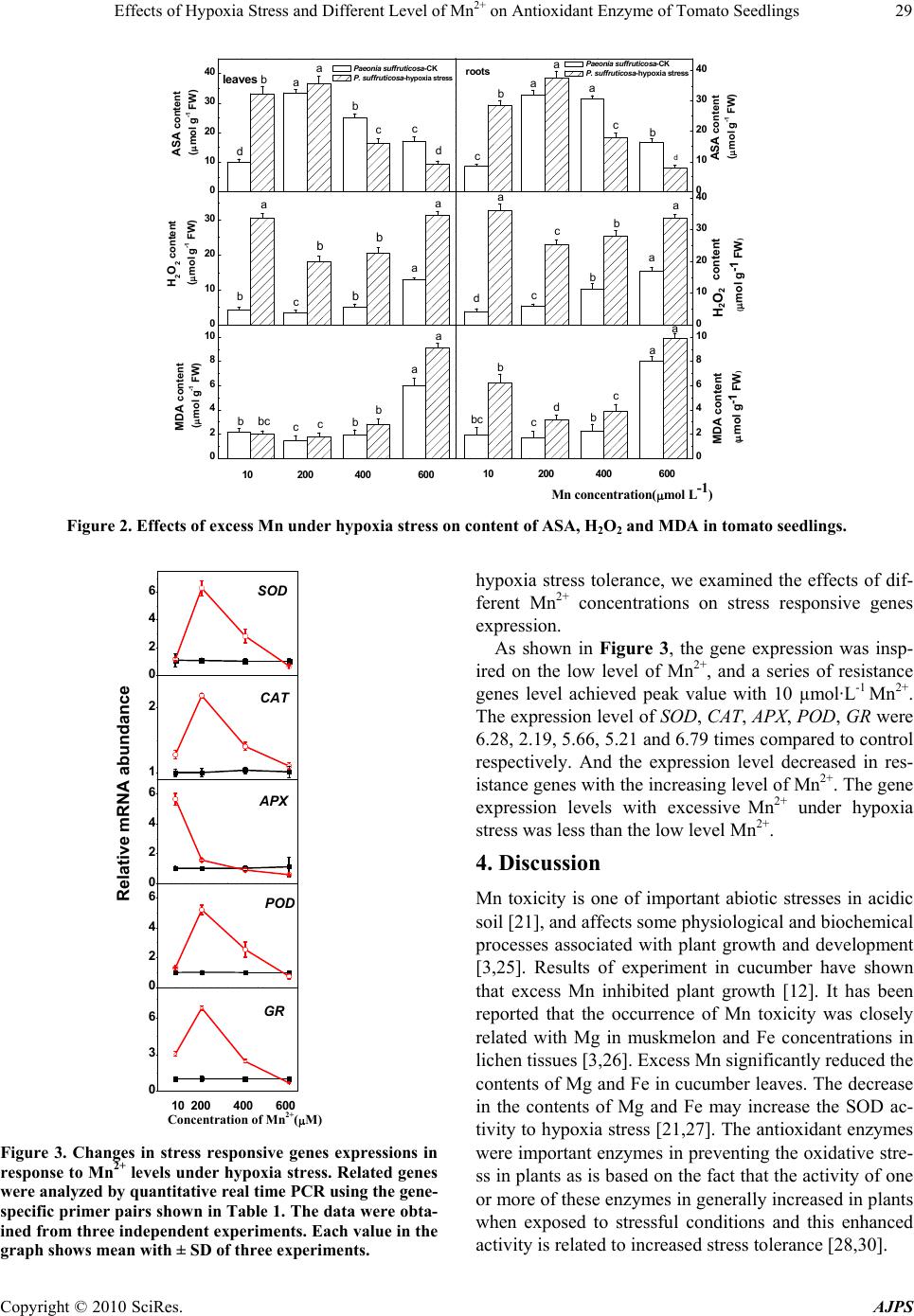 Effects of Hypoxia Stress and Different Level of Mn2+ on Antioxidant Enzyme of Tomato Seedlings Copyright © 2010 SciRes. AJPS 29 0 10 20 30 40 0 10 20 30 40 0 2 4 6 8 10 0 10 20 30 40 10 200 4 00 600 c b a ASA content (mol g-1 FW) Pae onia suffruticosa-CK P . su ffru ti c o s a-hypoxia stress Paeonia s uffruticosa-CK P . suffr uti cos a-hypoxia stress d ba c d leaves 10 200 400 60 0 c c b b a a a a ASA content (mol g-1 FW) d roots 0 10 20 30 H2O2 content (mol g-1 FW) b a c b b b a a c c b b a H2O2 content (mol g-1 FW) d a 0 2 4 6 8 10 c c bbb a a MDA content (mol g-1 FW) Mn conc en tration(mol L-1 ) bc c c b b a a MDA content (mol g-1 FW) bc d H 2 O 2 Figure 2. Effects of excess Mn under hypoxia stress on content of ASA, H2O2 and MDA in tomato seedlings. 0 2 4 6 0 2 4 6 0 2 4 6 1 2 0 3 6 SOD Concentration of Mn2+(M) 10 2 00 4 00 6 00 POD APX Relative mRNA abundance CAT GR Figure 3. Changes in stress responsive genes expressions in response to Mn2+ levels under hypoxia stress. Related genes were analyzed by quantitative real time PCR using the gene- specific primer pairs show n in Table 1. The data were obta- ined from three independent experi ments. Each value in the graph shows mean wi th ± SD of thr ee experiments. hypoxia stress tolerance, we examined the effects of dif- ferent Mn2+ concentrations on stress responsive genes expression. As shown in Figure 3, the gene expression was insp- ired on the low level of Mn2+, and a series of resistance genes level achieved peak value with 10 µmol·L-1 Mn2+. The expression level of SOD, CAT, APX, POD, GR were 6.28, 2.19, 5.66, 5.21 and 6.79 times compared to control respectively. And the expression level decreased in res- istance genes with the increasing level of Mn2+. The gene expression levels with excessive Mn2+ under hypoxia stress was less than the low level Mn2+. 4. Discussion Mn toxicity is one of important abiotic stresses in acidic soil [21], and affects some physiological and biochemical processes associated with plant growth and development [3,25]. Results of experiment in cucumber have shown that excess Mn inhibited plant growth [12]. It has been reported that the occurrence of Mn toxicity was closely related with Mg in muskmelon and Fe concentrations in lichen tissues [3,26]. Excess Mn significantly reduced the contents of Mg and Fe in cucumber leaves. The decrease in the contents of Mg and Fe may increase the SOD ac- tivity to hypoxia stress [21,27]. The antioxidant enzymes were important enzymes in preventing the oxidative stre- ss in plants as is based on the fact that the activity of one or more of these enzymes in generally increased in plants when exposed to stressful conditions and this enhanced activity is related to increased stress tolerance [28,30].  Effects of Hypoxia Stress and Different Level of Mn2+ on Antioxidant Enzyme of Tomato Seedlings Copyright © 2010 SciRes. AJPS 30 The oxidative stress is a key component of environ- mental stress [31]. Various environmental stresses cause accumulation of H2O2 in different tissue segments and the regulation of H2O2 levels is of utmost importance in plant cell metabolism [32]. The enhancement of antioxid- ant enzymes activities was correlated with increased pro- tection from damage associated with oxidative stress [33]. In the present experiment, excess Mn increased the ac- tivities of SOD, APX and GR, particularly under hypoxia stress. It may be concluded that appropriate amount of Mn2+could reduce the damage of active oxygen under hy- poxia stress, but reversely, excessive level of Mn2+ were just to aggravate the already serious damage to the to- mato seedlings. REFERENCES [1] M. J. Mukaopadhyay and A. Sharma, “Manganese in Cell Metabolism of Higher Plants,” Botanical Review, Vol. 51, No. 2, April 1991, pp.117-149. [2] C. Negra, D. S. Ross and A. Lanzirotti, “Oxidizing Be- havior of Soil Manganese: Interactions among Abundance, Oxidation State, and pH,” Soil Science Society of America Journal, Vol. 69, No. 1, January 2005, pp. 87-95. [3] M. Hauck, A. Paul, S. Gross and M. Raubuch, “Manga- nese Toxicity in Epiphytic Lichens: Chlorophyll Degra- dation and Interaction with Iron and Phosphorus,” Envi- ronmental and Experimental Botany, Vol. 49, No. 2, April 2003, pp. 181-191. [4] C. O. Dimkpa, D. Merten, A. Svatoš, G. Büchel and E. Kothe, “Metal-Induced Oxidative Stress Impacting Plant Growth in Contaminated Soil is Alleviated by Microbial Siderophores,” Soil Biology & Biochemistry, Vol. 41, No. 1, January 2009, pp. 154-162. [5] R. V. Tyson and T. H. Pearson, “Modern and Ancient Continental Shelf Anoxia: An Overview,” In: R. V. Ty- son, T. H. Pearson, Ed., Modern and ancient continental shelf anoxia, London, Geological Society Special Publi- cation, 1991, pp. 1-24. [6] L. Blake and K. W. T. Goulding, “Effects of Atmospheric Deposition, Soil pH and Acidification on Heavy Metal Contents in Soils and Vegetation of Semi-Natural Eco- systems at Rothamsted Experimental Station, UK,” Plant Soil, Vol. 240, No. 2, March 2002, pp. 235-251. [7] F. C. Vlidon and M. G. Teixeira, “Oxygen Radical Pro- duction and Control in the Chloroplast of Mn-Treated Rice,” Plant Science, Vol. 152, No. 1, January 2000, pp. 7-15. [8] H. Markus, P. Alexander, G. Shana and R. Markus, “Manganese Toxicity in Epiphytic Lichens: Chlorophyll Degradation and Interaction with Iron and Phosphorus,” Environmental and Experimental Botany, Vol. 49, No. 2, April 2003, pp. 181-191. [9] Q. H. Shi, Z. Y. Bao, Z. J. Zhu, Q. S. Ying and Q. Q. Qian, “Effects of Different Treatments of Salicylic Acid on Heat Tolerance, Chlorophyll Fluorescence, and Anti- oxidant Enzyme Activity in Seedlings of Cucumis Sativa L.,” Plant Growth Regulation, Vol. 48, No. 2, February 2006, pp. 127-135. [10] T. El-Jaoual and D. A. Cox, “Manganese Toxicity in Plants,” Journal of Plant Nutrition, Vol. 24, No. 2, Feb- ruary 1998, pp. 353-386. [11] J. Le Bot, E. A. Kirkby and M. L. Van Beusichem, “Manganese Toxicity in Tomato Plants: Effects on Cation Uptake and Distribution,” Journal of Plant Nutrition, Vol. 13, No. 5, May 1990, pp. 513- 525. [12] Q. H. Shi, Z. J. Zhu, Y. He, Q. Q. Qian and J. Q. Yu, “Silicon-Mediated Alleviation of Mn Toxicity in Cucumis Sativus in Relation to Activities of Superoxide Dismutase and Ascorbate Peroxidase,” Phytochemistry, Vol. 66, No. 13, July 2005, pp. 1551-1559. [13] S. M. Macfie and G. J. Taylor, “The Effects of Excess Manganese on Photosynthetic Rate and Concentration of Chlorophyll in Triticum Aestivum Grown in Solution Culture,” Physiologia Plantarum, Vol. 85, No. 3, June 2008, pp. 467-475. [14] M. Hauck, A. Paul, C. Mulack, E. Fritz and M. Runge, “Effects of Manganese on the Viability of Vegetative Di- aspores of the Epiphytic Lichen Hypogymnia Physodes,” Environmental and Experimental Botany, Vol. 47, No. 2, March 2002, pp. 127-142. [15] S. S. Sharma and K. J. Dietz, “The Relationship between Metal Toxicity and Cellular Redox Imbalance,” Trends in Plant Science, Vol. 14, No. 1, January 2009, pp. 43-50. [16] R. Mittler, “Oxidative Stress, Antioxidants and Stress Tolerance,” Trends in Plant Science, Vol. 7, No. 9, Sep- tember 2002, pp. 405-410. [17] E. R. Stadtman and C. N. Oliver, “Metal-Catalyzed Oxi- dation of Proteins. Physiological Consequences,” Journal of Biological Chemistry, Vol. 266, No. 4, February 1991, pp. 2005-2008. [18] J. A. Harrigan, J. Piotrowski, L. D. Noto, R. L. Levine and V. A. Bohr, “Metal-Catalyzed Oxidation of the Werner Syndrome Protein Causes Loss of Catalytic Activities and Impaired Protein-Protein Interactions,” Journal of Bio- logical Chemistry, Vol. 282, No. 50, December 2007, pp. 36403-36411. [19] T. Brennan and C. Frenkel, “Involvement of Hydrogen Peroxide in the Regulation of Senescence in Pear,” Plant Physiology, Vol. 59, No. 3, March 1977, pp. 411-416. [20] C. N. Giannopolitis and S. K. Ries, “Superoxide Dismu- tases I. Occurrence in Higher Plants,” Plant Physiology, Vol. 59, No. 2, 1977, pp. 309-314. [21] I. Cakmak and H. Marschner, “Magnesium Deficiency and High Light Intensity Enhance Activities of Superox- ide Dismutase, Ascorbate Peroxidase and Glutathione Reductase in Bean Leaves,” Plant Physiology, Vol. 98, No. 4, April 1992, pp. 1222-1227. [22] Y. Nakano and K. Asada, “Purification of Ascorbate Peroxidase in Spinach Chloroplasts; its Inactivation in Ascorbate-Depleted Medium and Reactivation by Monode- hydroascorbate Radical,” Plant and Cell Physiology, Vol. 28, No. 1, January 1987, pp. 131-140.  Effects of Hypoxia Stress and Different Level of Mn2+ on Antioxidant Enzyme of Tomato Seedlings Copyright © 2010 SciRes. AJPS 31 [23] C. H. Foyer and B. Halliwell, “The Presence of Glu- tathione and Glutathione Reductase in Chloroplasts: A Proposed Role in Ascorbic Acid Metabolism,” Planta, Vol. 133, No. 1, January 1976, pp. 21-25. [24] K. J. Livak and T. D. Schmittgen, “Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2-ΔΔCT Method,” Methods, Vol. 25, No. 4, December 2001, pp. 402-408. [25] A. Paul, M. Hauck and E. Fritz, “Effects of Manganese on Element Distribution and Structure in Thalli of the Epiphytic Lichens Hypogymnia Physodes and Lecanora Conizaeoides,” Environmental and Experimental Botany, Vol. 50, No. 2, October 2003, pp. 113-124. [26] O. M. Elamin and G. E. Wilcox, “Effects of Magnesium and Manganese Nutrition on Muskmelon Growth and Manganese Toxicity,” Journal of the American Society for Horticultural Science, Vol. 111, No. 4, April 1986, pp. 582-587. [27] I. Cakmak, B. Erenoglu, K. Y. Gulut, R. Derici and V. Romheld, “Light-Mediated Release of Phytosiderophores in Wheat and Barley under Iron or Zinc Deficiency,” Plant Soil, Vol. 202, No. 2, May 1998, pp. 309-315. [28] G. Santandrea, T. Pandolfini and A. Bennici, “A Physio- logical Characterization of Mn-Tolerant Tobacco Plants Selected by In Vitro Culture,” Plant Science, Vol. 150, No. 2, January 2000, pp. 163-177. [29] M. M. Fecht-Christoffers, P. Maier and W. J. Horst, “Apoplastic Peroxidases and Ascorbate are Involved in Manganese Toxicity and Tolerance of Vigna Unguicu- lata,” Physiologia Plantarum, Vol. 117, No. 2, March 2003, pp. 237-244. [30] A. Hameed, S. Naseer, T. Iqbal, H. Syed and M. A. Haq, “Effects of NaCl Salinity on Seedling Growth, Senes- cence, Catalase and Protease Activities in Two Wheat Genotypes Differing in Salt Tolerance,” Pakistan Journal of Botany, Vol. 40, No. 3, March 2008, pp. 1043-1051. [31] A. C. F. de Vasconcelos, X. Z. Zhang, E. H. Ervin and K. J. de Castro, “Enzymatic Antioxidant Responses to Bio- stimulants in Maize and Soybean Subjected to Drought,” Scientia Agricola, Vol. 66, No. 3, May 2009, pp. 395- 402. [32] T. S. Gechev and J. Hille, “Hydrogen Peroxide as a Sig- nal Controlling Plant Programmed Cell Death,” Journal of Cell Biology, Vol. 168, No. 1, January 2005, pp. 17-20. [33] H. H. Abd El-Baky, F. K. El Baz and G. S. El-Baroty, “Enhancement of Antioxidant Production in Spirulina Platensis under Oxidative Stress,” Acta Physiologiae Plan- tarum, Vol. 31, No. 3, May 2009, pp. 623-631. |

