Paper Menu >>
Journal Menu >>
 American Journal of Plant Sciences, 2010, 1, 1-11 doi:10.4236/ajps.2010.11001 Published Online September 2010 (http://www.SciRP.org/journal/ajps) Copyright © 2010 SciRes. AJPS 1 Photosynthesis-Dependent Extracellular Ca2+ Influx Triggers an Asexual Reproductive Cycle in the Marine Red Macroalga Porphyra yezoensis Megumu Takahashi1, Naotsune Saga2, Koji Mikami2* 1Graduate School of Fisheries Sciences, Hokkaido University, Hakodate, Japan; 2Faculty of Fisheries Sciences, Hokkaido University, Hakodate, Japan. *Email: komikami@fish.hokudai.ac.jp Received August 16th, 2010; revised September 6th, 2010; accepted September 9th, 2010 ABSTRACT Asexual propagation to increase the number of gametophytic clones via the growth of asexual haploid spores is a unique survival strategy found in marine multicellular algae. However, the mechanisms regulating the asexual life cycle are largely unknown. Here, factors involved in the regulation of production and discharge of asexual spores, so-called monospores, are identified in the marine red macroalga Porphyra yezoensis. First, enhanced discharge of monospores was found by incubation of gametophytes in ASPMT1, a modified version of the previously established synthetic me- dium ASP12. Comparison of the compositions of ASPMT1 and our standard medium, ESL, indicated that the Ca2+ con- centration in ASPMT1 was three times lower than that in ESL medium. Thus, we modified ASPMT1 by increasing its Ca2+ concentration, resulting in reduction of monospore discharge. These findings demonstrate the role of reduced Ca2+ concentrations in enhancing monospore production and release. Moreover, it was also observed that initiation of asexual life cycle required illumination, was repressed by DCMU, and was induced by a Ca2+ ionophore in the dark. Taken together, these results indicate that photosynthesis-dependent Ca2+ influx triggers the asexual life cycle by pro- moting the production and discharge of monospores in P. yezoensis. Keywords: Asexual Life Cycle, Bangiophyceae, Ca2+ Influx, Monospore, Photosynthesis, Porphyra yezoensis, Rhodophyta, Synthetic Medium 1. Introduction Asexual life cycle occurs in multicellular eukaryotes inc- luding algae and fungi [1-3]. In the sexual haploid-dip- loid life cycle of multicellular plants, meiosis in the dipl- oid sporophyte produces a haploid gametophyte and syn- gamy (fertilization) of male and female gametes restores the diploid sporophytic genome [3-5]. By contrast, free- living haploid gametophytes of marine macroalgae often produce asexual spores that develop into haploid gamet- ophytic clones by mitotic cell division without ploidy ch- ange [6]. Extensive analysis of plant haploid-diploid life cycles has progressed with recent genomic and genetic studies in Arabidopsis thaliana, which identified candi- date genes involved in the regulation of the sexual life cycle [7-9]. In contrast, despite an accumulation of mor- phological and cytological observations, mechanisms of regulation of the asexual life cycle are still largely un- known, due to the lack of a model plant for investigation of the asexual life cycle. Porphyra yezoensis, a red macroalga included in Ban- giophycideae of Rhodophyta, has recently received con- siderable attention as a promising model macroalga for physiological and molecular biological studies of marine red algae, which largely depends on the establishment of laboratory cultures in which the haploid-diploid life cycle can be completed in a short period [10]. As for most of Bangiophycideae red algae, asexual spores, so-called mo- nospores, are produced in monosporangia typically occu- rring at the marginal region of the gametophyte in P. ye- zoensis [11], although a biphasic sexual life cycle, which consists of morphologically distinct macroscopic gamet- ophytic blades in winter and microscopic sporophytic fil- aments in summer, is predominant [11,12]. Thus, it is important to analyze the production and development of monospores in P. yezoensis to elucidate the biological significance and regulatory mechanisms of asexual pro- pagation in multicellular red algae.  Photosynthesis-Dependent Extracellular Ca2+ Influx Triggers an Asexual Reproductive Cycle in the Marine Red Macroalga Porphyra yezoensis Copyright © 2010 SciRes. AJPS 2 In the asexual life cycle of P. yezoensis, monospores are released from monosporangia and migrate while un- dergoing morphological changes, then adhere to the sub- stratum and divide asymmetrically to produce two dif- ferent vegetative and rhizoid cells [13-17]. The early development of monospores has been extensively studied, revealing the critical involvement of photosynthsis-depe- ndent extracellular Ca2+ influx and phosphoinositide sig- naling, including phosphatidylinositol 3-kinase and phos- pholipase C, in the establishment of cell polarity that di- rects migration and adherence of monospores [16-19]. In contrast, there is only a few information about monospo- re production and discharge. Gametophytes may be ind- uced to release monospores by changing water tempera- ture and light conditions [20] and by irradiance with str- ong light [21]. Also, high yields of monospores from gametophytes treated with allantoin have been reported [22]. A mutant showing high levels of monospore pro- duction has also been isolated and characterized [23]; ho- wever, this mutant has not yet been analyzed to determ- ine the mechanisms regulating the production and disch- arge of monospores in P. yezoensis. Therefore, knowl- edge of the mechanisms regulating production and dis- charge of monospores from gametophytes in P. yezoensis is not complete. Here, we identified factors involved in regulation of the asexual life cycle in P. yezoensis. Our establishment of an artificial synthetic medium for the culture of P. yezoensis clearly demonstrated that extracellular Ca2+ influx plays a critical role in the production and dis- charge of monospores from gametophytes, and that these processes depend completely on photosynthetic activity. These findings could provide new insights into the regu- lation of the eukaryotic asexual life cycle. 2. Materials and Methods 2.1. Growth of Gametophytes and Discharge of Monospores The cultivation of gametophytic blades of P. yezoensis strain TU-1 was performed as previously reported [16]. Briefly, the ESL (enriched SEALIFE) medium, which is made by dissolving commercially available SEALIFE powder (Marintech Co. Ltd., Tokyo, Japan) in distilled water (DW) with the addition of ESS2 (enriched seawater Saga2) solution, was renewed weekly until gametophytes were about 3 mm long. Subsequently, gametophytes we- re grown in ESL or various synthetic media as mentioned in the text. Two pieces of 1 cm polyvinyl alcohol (PVA) monofilaments to which gametophytic blades (ca. 3 mm long) attached were transferred to 100 mL of culture me- dium. The culture media were continuously bubbled with filter-sterilized air under 60 μmol / m 2/s irradiance with a photocycle of 10 h light and 14 h dark at 15˚C, with we- ekly renewal of culture medium. The length of a total of 10 gametophytic blades was measured to calculate their growth rate. Observation of monospore discharge was performed by three different ways as below. First is com- parison of accumulation of monospores on the bottom of culture flask, for which flasks were placed on white pap- er to make photographs taken using a digital camera (Ca- non PowerShot G10). Second is observation of monos- pores attached to PVA monofilaments that photographed using the stereomicroscopic (Leica S8AP0) equipped with Nikon DigitalSight (DS-L2). The last is direct counting the number of monospores, for which 6 pieces gametophytes (ca. 3 mm long) were transferred to 35 mm tissue culture dishes (Iwaki Scitech Div., Asahi Techno Glass) containing ESL or various synthetic media and then the number of discharged monospores was counted under the inverted microscopic (Leica DMIL) during 7 days. 2.2. Quantification of Pigment Contents Four-week-old gametophytes cultured in ESL or synthe- tic media were used for calculation of photosynthetic pi- gments (chlorophyll a, Chl a; phycoerythrin, PE; phyco- cyanin, PC) and carotenoids (Car). Chl a and Car were measured using a method described previously [24], while extraction and measurement of PE and PC were carried out according to the method Beer and Eshel [25]. 2.3. Calculation of Magnesium, Potassium and Calcium ion Contents Concentrations of magnesium, potassium and calcium ions were determined for ESL and ASPMT1 with a po- larized Zeeman atomic absorption spectrophotometer (Hitachi Z-6100) according to the manufacture’s proto- cols. 2.4. Treatment of Gametophytic Blades with Pharmacological Reagents The photosynthesis inhibitor 3-(3,4-dichlorophenyl)-1,1- dimethylurea (DCMU) (Sigma, USA) was dissolved in dimethyl sulfoxide (DMSO) to prepare a 100 mM stock solution. The calcium ion-specific chelator ethylene gly- col tetraacetic acid (EGTA) (Dojindo Laboratories, Japan) was dissolved in distilled water (DW) to create a 0.5 M stock solution adjusted to pH 8.0 with NaOH. The cal- cium ionophore A23187 (Sigma, USA) was dissolved in DW to prepare a 1.0 M stock solution. The stocks pre- pared above were added to the each medium to treat ga- metophytic blades at the working concentrations indi- cated below. The pH of medium was adjusted at 8.0 after adding of these regents. The concentration of the solvents 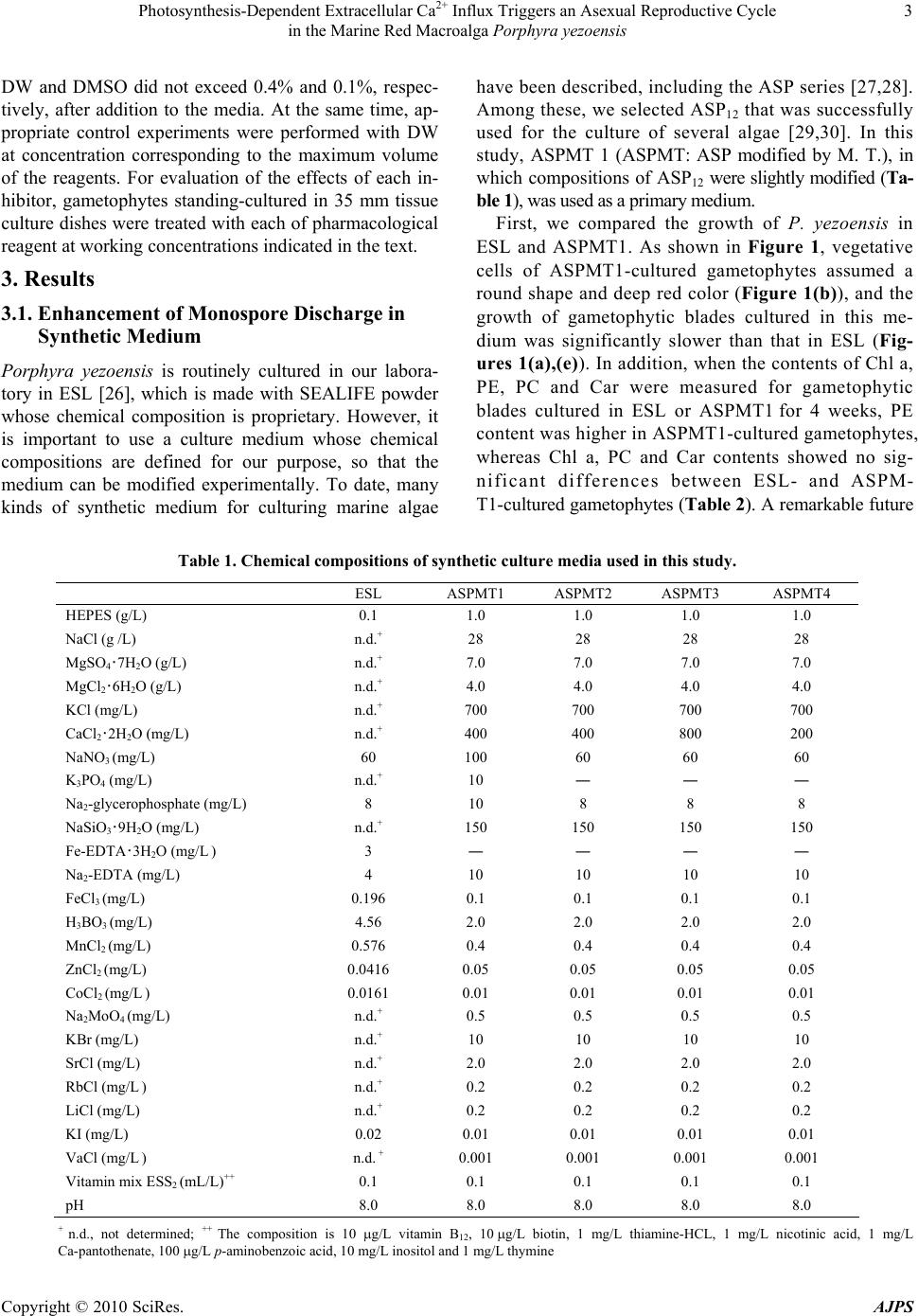 Photosynthesis-Dependent Extracellular Ca2+ Influx Triggers an Asexual Reproductive Cycle in the Marine Red Macroalga Porphyra yezoensis Copyright © 2010 SciRes. AJPS 3 DW and DMSO did not exceed 0.4% and 0.1%, respec- tively, after addition to the media. At the same time, ap- propriate control experiments were performed with DW at concentration corresponding to the maximum volume of the reagents. For evaluation of the effects of each in- hibitor, gametophytes standing-cultured in 35 mm tissue culture dishes were treated with each of pharmacological reagent at working concentrations indicated in the text. 3. Results 3.1. Enhancement of Monospore Discharge in Synthetic Medium Porphyra yezoensis is routinely cultured in our labora- tory in ESL [26], which is made with SEALIFE powder whose chemical composition is proprietary. However, it is important to use a culture medium whose chemical compositions are defined for our purpose, so that the medium can be modified experimentally. To date, many kinds of synthetic medium for culturing marine algae have been described, including the ASP series [27,28]. Among these, we selected ASP12 that was successfully used for the culture of several algae [29,30]. In this study, ASPMT 1 (ASPMT: ASP modified by M. T.), in which compositions of ASP12 were slightly modified (Ta- ble 1), was used as a primary medium. First, we compared the growth of P. yezoensis in ESL and ASPMT1. As shown in Figure 1, vegetative cells of ASPMT1-cultured gametophytes assumed a round shape and deep red color (Figure 1(b)), and the growth of gametophytic blades cultured in this me- dium was significantly slower than that in ESL (Fig- ures 1(a),(e)). In addition, when the contents of Chl a, PE, PC and Car were measured for gametophytic blades cultured in ESL or ASPMT1 for 4 weeks, PE content was higher in ASPMT1-cultured gametophytes, whereas Chl a, PC and Car contents showed no sig- nificant differences between ESL- and ASPM- T1-cultured gametophytes (Table 2). A remarkable future Table 1. Chemical compositions of synthetic culture media used in this study. ESL ASPMT1 ASPMT2 ASPMT3 ASPMT4 HEPES (g/L) 0.1 1.0 1.0 1.0 1.0 NaCl (g /L) n.d.+ 28 28 28 28 MgSO4・7H2O (g/L) n.d.+ 7.0 7.0 7.0 7.0 MgCl2・6H2O (g/L) n.d.+ 4.0 4.0 4.0 4.0 KCl (mg/L) n.d.+ 700 700 700 700 CaCl2・2H2O (mg/L) n.d.+ 400 400 800 200 NaNO3 (mg/L) 60 100 60 60 60 K3PO4 (mg/L) n.d.+ 10 ― ― ― Na2-glycerophosphate (mg/L) 8 10 8 8 8 NaSiO3・9H2O (mg/L) n.d.+ 150 150 150 150 Fe-EDTA・3H2O (mg/L ) 3 ― ― ― ― Na2-EDTA (mg/L) 4 10 10 10 10 FeCl3 (mg/L) 0.196 0.1 0.1 0.1 0.1 H3BO3 (mg/L) 4.56 2.0 2.0 2.0 2.0 MnCl2 (mg/L) 0.576 0.4 0.4 0.4 0.4 ZnCl2 (mg/L) 0.0416 0.05 0.05 0.05 0.05 CoCl2 (mg/L ) 0.0161 0.01 0.01 0.01 0.01 Na2MoO4 (mg/L) n.d.+ 0.5 0.5 0.5 0.5 KBr (mg/L) n.d.+ 10 10 10 10 SrCl (mg/L) n.d.+ 2.0 2.0 2.0 2.0 RbCl (mg/L ) n.d.+ 0.2 0.2 0.2 0.2 LiCl (mg/L) n.d.+ 0.2 0.2 0.2 0.2 KI (mg/L) 0.02 0.01 0.01 0.01 0.01 VaCl (mg/L ) n.d. + 0.001 0.001 0.001 0.001 Vitamin mix ESS2 (mL/L)++ 0.1 0.1 0.1 0.1 0.1 pH 8.0 8.0 8.0 8.0 8.0 + n.d., not determined; ++ The composition is 10 g/L vitamin B12, 10 g/L biotin, 1 mg/L thiamine-HCL, 1 mg/L nicotinic acid, 1 mg/L Ca-pantothenate, 100 g/L p-aminobenzoic acid, 10 mg/L inositol and 1 mg/L thymine 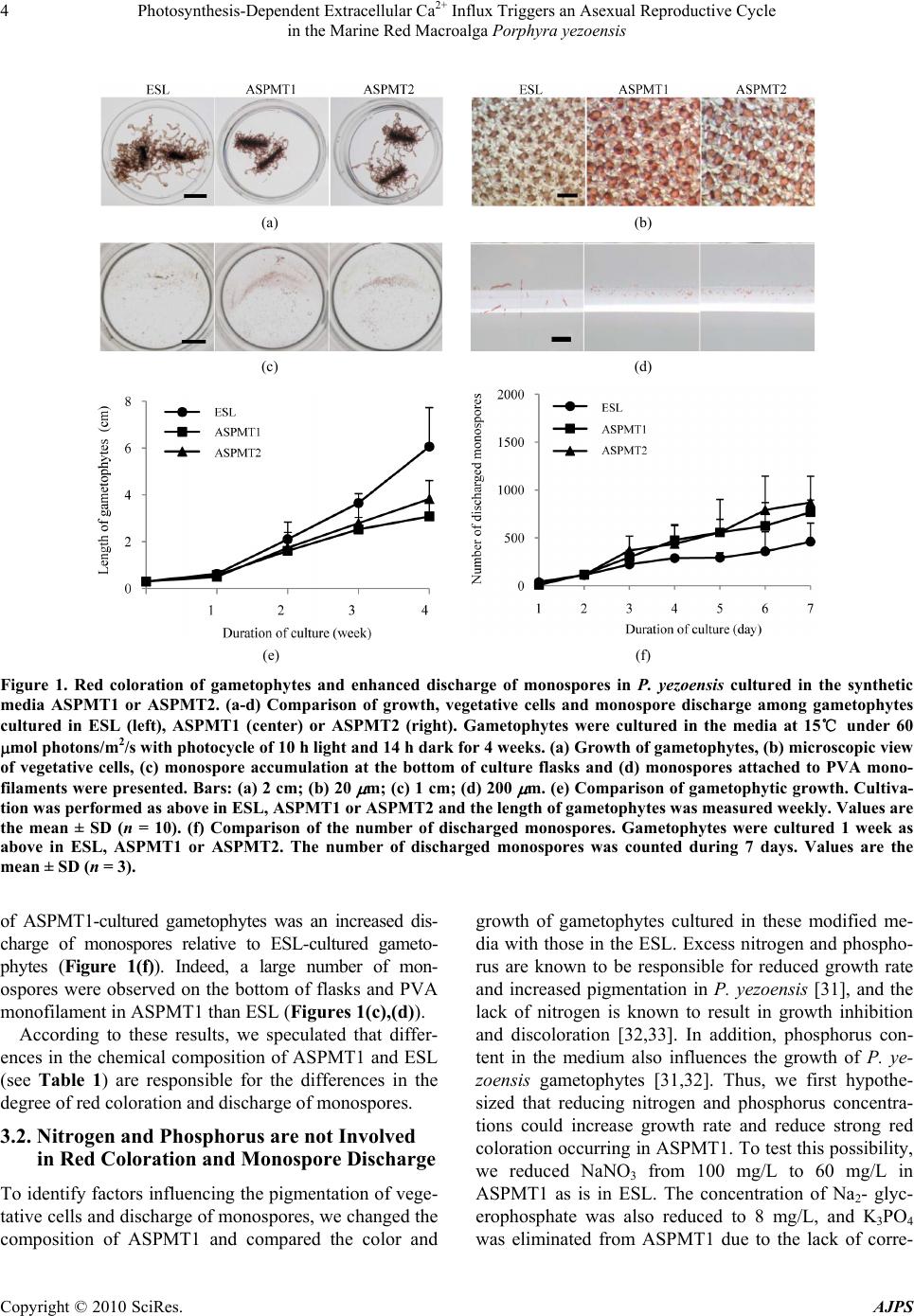 Photosynthesis-Dependent Extracellular Ca2+ Influx Triggers an Asexual Reproductive Cycle in the Marine Red Macroalga Porphyra yezoensis Copyright © 2010 SciRes. AJPS 4 (a) (b) (c) (d) (e) (f) Figure 1. Red coloration of gametophytes and enhanced discharge of monospores in P. yezoensis cultured in the synthetic media ASPMT1 or ASPMT2. (a-d) Comparison of growth, vegetative cells and monospore discharge among gametophytes cultured in ESL (left), ASPMT1 (center) or ASPMT2 (right). Gametophytes were cultured in the media at 15℃ under 60 mol photons/m2/s with photocycle of 10 h light and 14 h dark for 4 week s. (a) Growth of gametophytes, (b) microscopic view of vegetative cells, (c) monospore accumulation at the bottom of culture flasks and (d) monospores attached to PVA mono- filaments were presented. Bars: (a) 2 cm; (b) 20 m; (c) 1 cm; (d) 200 m. (e) Comparison of gametophytic growth. Cultiva- tion was performed as above in ESL, ASPMT1 or ASPMT2 and the length of gametophytes was measured weekly. Values are the mean ± SD (n = 10). (f) Comparison of the number of discharged monospores. Gametophytes were cultured 1 week as above in ESL, ASPMT1 or ASPMT2. The number of discharged monospores was counted during 7 days. Values are the mean ± SD (n = 3). of ASPMT1-cultured gametophytes was an increased dis- charge of monospores relative to ESL-cultured gameto- phytes (Figure 1(f)). Indeed, a large number of mon- ospores were observed on the bottom of flasks and PVA monofilament in ASPMT1 than ESL (Figures 1(c),(d)). According to these results, we speculated that differ- ences in the chemical composition of ASPMT1 and ESL (see Table 1) are responsible for the differences in the degree of red coloration and discharge of monospores. 3.2. Nitrogen and Phosphorus are not Involved in Red Coloration and Monospore Discharge To identify factors influencing the pigmentation of vege- tative cells and discharge of monospores, we changed the composition of ASPMT1 and compared the color and growth of gametophytes cultured in these modified me- dia with those in the ESL. Excess nitrogen and phospho- rus are known to be responsible for reduced growth rate and increased pigmentation in P. yezoensis [31], and the lack of nitrogen is known to result in growth inhibition and discoloration [32,33]. In addition, phosphorus con- tent in the medium also influences the growth of P. ye- zoensis gametophytes [31,32]. Thus, we first hypothe- sized that reducing nitrogen and phosphorus concentra- tions could increase growth rate and reduce strong red coloration occurring in ASPMT1. To test this possibility, we reduced NaNO3 from 100 mg/L to 60 mg/L in ASPMT1 as is in ESL. The concentration of Na2- glyc- erophosphate was also reduced to 8 mg/L, and K3PO4 was eliminated from ASPMT1 due to the lack of corre-  Photosynthesis-Dependent Extracellular Ca2+ Influx Triggers an Asexual Reproductive Cycle in the Marine Red Macroalga Porphyra yezoensis Copyright © 2010 SciRes. AJPS 5 sponding information about the K3PO4. Concentration in the ESL medium. The modified med- ium was designated ASPMT2 (Table 1 ). Following culture of P. yezoensis gametophytes in ASPMT2 for 4 weeks, a small increase in the growth of gametophytes was observed (Figures 1(a),(e)), which was due to a small increase in the size of vegetative cells attributable to an increase in the cytoplasmic chloroplast-free space compared with ASPMT1 -cultured gametophytes (Figure 1(b)). However, there was no significant change in the degree of monospore discharge (Figure 1(f)), because consistently larger quantities of monospores were observed on the bottom of flasks and PVA monofilaments in ASPMT1 or ASPMT2 compared with ESL (Figures 1(c),(d)). In addition, cul- ture in ASPMT2 for 4 weeks did not affect red coloration (Figure 1(b)) and there were no significant changes in the contents of photosynthetic pigments compared with ASPMT1-cultured gametophytes (Table 2). These re- sults indicate that differences in the concentration of ni- trogen and phosphorus between ASPMT1 and ESL are not responsible for the red coloration of gametophytes and enhanced discharge of asexual monospores. 3.3. Decrease in the Extracellular Ca2+ Concentration Results in Red Coloration of Vegetative Cells and Enhancement of Monospore Discharge As shown in Table 1, the concentrations of MgCl2, Mg- SO4, KCl, K3PO4 and CaCl2 are unknown in ESL. Thus, the concentration of Mg2+, K+ and Ca2+ in both ESL and ASPMT1 were measured by determining atomic weight using the polarized Zeeman atomic absorption spectro- photometer. We found that although Mg+ and K+ con- centrations were similar in these two media, remarkable differences in the Ca2+ concentration was observed; that is, ESL contains Ca2+ concentration three times greater than that in ASPMT1 (Figure 2), suggesting that the de- creased concentration of Ca2+ in ASPMT1 and ASPMT2 may be responsible for the observed growth and red pigmentation effects on P. yezoensis gametophytes. To address this possibility, we made two modified versions of ASPMT2 with CaCl2 concentrations two tim- es greater (800 mg/L) (ASPMT3) or half (200 mg/L) (AS- PMT4) (Table 1). Gametophytes cultured in ASPMT3 for 4 weeks exhibited the same rate of growth as those grown in ESL (Figures 3(a),(e)), whereas gametophytes grown in ASPMT2 or ASPMT4 (Figures 1(a) and 3(a)) exhibited negative growth effects relative to growth in ESL. In addition, although ASPMT4 had no effect on the recovery of color and cell shape, vegetative cells of gametophytes cultured in ASPMT3 showed normal shape and color as those grown in ESL (Figure 3(b)). Although cultivation in ASPMT3 resulted in reduced concentration of Chl a and PE compared with ESL-cultured, PE/Chl a, PC/Chl a and PE/PC were similar between ASPMT3 and ESL-cultured gametophytes (Table 2). On the other hand, culture in ASPMT4, whose Ca2+ concentration is approximately 6 times lower than that in ESL (Table 1 and Figure 2), Chl a and PE contents were higher than those in ESL-cultured gametophytes (Table 2). Moreover, large quantities of dis- charged monospores were observed in ASPMT4, while the number of monospores discharged from gameto- phytes grown in ESL and ASPMT3 was similar to each other (Figure 3(f)). Furthermore, large numbers of monospores adhered to the bottom of flasks and PVA Figure 2. Quantification of magnesium, potassium and cal- cium ions in ESL and ASPMT1. The co ncentration of Mg2+, K+ and Ca2+ in both ESL and ASPMT1 was measured as atomic weight by the polarized Zeeman atomic absorption spectrophotometer. Columns and vertical bars represent the mean ± SD, respectively (n = 3). Table 2. Comparison of concentration of photosynthetic pigments in P. yezoensis cultured in ESL and modified media. Chl a* PE* PC* Car* PE/Chl a PC/Chl a PE/PC ESL 1.56 ± 0.11 6.40 ± 1.22 1.5 ± 0.38 0.03 ± 0.01 4.11 0.97 4.26 ASPMT1 1.31 ± 0.24 8.09 ± 0.43 1.41 ± 0.10 0.04 ± 0.02 6.18 1.08 5.73 ASPMT2 1.45 ± 0.07 8.43 ± 0.72 1.70 ± 0.34 0.03 ± 0.01 5.80 1.17 4.96 ASPMT3 1.36 ± 0.12 5.59 ± 1.56 1.28 ± 0.44 0.02 ± 0.01 4.12 0.94 4.38 ASPMT4 1.92 ± 0.35 11.18 ± 0.40 1.47 ± 0.23 0.05 ± 0.01 5.82 0.77 7.60 Concentrations of chlorophyll a (Chl a), phycoerythrin (PE), and phycocyanin (PC) carotenoids (Car) were measured using gametophytes cultured in each medium for 4 weeks. *mg/g fresh wt ± SD  Photosynthesis-Dependent Extracellular Ca2+ Influx Triggers an Asexual Reproductive Cycle in the Marine Red Macroalga Porphyra yezoensis Copyright © 2010 SciRes. AJPS 6 (a) (b) (c) (d) (e) (f) Figure 3. Effects of extracellular Ca2+ contents on growth, color and monospore discharge. (a-d) Comparison of growth, vegetative cells and monospore discharge among gametophytes cultured in ESL, ASPMT3 or ASPMT4. Gametophytes were cultured in the media at 15℃ under 60 μmol photons/m2/s with photocycle of 10 h light and 14 h dark for 4 weeks. (a) Growth of gametophytes, (b) microscopic view of vegetative cells, (c) monospore accumulation at the bottom of culture flasks and (d) monospores attached to PVA monofilaments were presented. Bars: (a) 2 cm; (b) 20 μm; (c) 1 cm; (d) 200 μm. (e) Comparison of gametophytic growth. Cultivation was performed as above in ESL, ASPMT3 or ASPMT4 and the length of gametophytes was measured weekly. Values are the mean ± SD (n = 10). (f) Comparison of the number of discharged mono- spores. Gametophytes were cultured as above in ESL, ASPMT3 or ASPMT4. The number of discharged monospores was counted during 7 days. Values are the me an and SD ( n = 3). monofilaments in ASPMT4, while a smaller number of monospores was observed in ESL and ASPMT3 (Fig- ures 3(c),(d)). We therefore concluded that the concentration of Ca2+ in the medium is an important factor responsible for the negative effects of ASPMT1 on growth, coloration and monospore discharge in gametophytes, indicating that the extracellular concentration of Ca2+ has an important in- fluence on the asexual reproductive life cycle in P. ye- zoensis. 3.4. Photosynthetic Activity Regulates Discharge of Monospores Consistent with previous reports showing that discharge of monospores is light-dependent in P. yezoensis and Bangia atropurpurea [21,34], strong inhibition of the discharge of monospores was observed under dark cond- itions (Figure 4(a)). To understand the role of the light, we tested whether photosynthetic activity is required for the discharge of monospores using DCMU, an inhibitor of electron transport on the acceptor side of Photosystem II. When gametophytes were treated with 0.1, 1 or 10 μM DCMU for 3 days, after which photosynthesis was grad- ually inhibited but not showed the cell death (data not sh- own), the number of discharged monospores decreased in a concentration-dependent manner (Figure 4(b)). These results indicate that photosynthesis is another important factor regulating the asexual life cycle in P. yezoensis. Thus, enhancement of monospore discharge is regulated by both a decrease in extracellular Ca2+ concentration and illumination resulting in photosynthesis (Figures 3 and 4).  Photosynthesis-Dependent Extracellular Ca2+ Influx Triggers an Asexual Reproductive Cycle in the Marine Red Macroalga Porphyra yezoensis Copyright © 2010 SciRes. AJPS 7 (a) (b) Figure 4. Critical involvement of photosynthetic activity in production and discharge of monospores. (a) Comparison of gametophytic vegetative cells and monospore discharge among gametophytes cultured in ESL (left), ASPMT3 (mid- dle) or ASPMT4 (left). Gametophytes were cultured in me- dia at 15℃ under 60 μmol photons/m2/s with photocycle of 10 h light and 14 h dark or consecutively dark for 1 weeks. Under light conditions (upper part), monosporangia were observed only at the edge of the gametophytes in ESL and ASPMT3 but spread to the mid region of gametophytes in ASPMT4. Microscopic view of vegetative cells and monosp- ore accumulation at the bottom of culture flasks were com- binationally represented for light and dark conditions. Bars = 20 μm in photos for vegetative cells; 1 cm in photos for bottom of culture flasks. (b) Comparison of the number of discharged monospores. Gametophytes were cultured as ab- ove for 3 days in ESL or ASPMT3 with or without DCMU (0.1, 1, 10 μM), and then the number of discharged mono- spores was counted. Columns and vertical bars represent the mean ± SD, respectively (n = 3). 3.5. Extracellular Ca2+ Influx is Required for Discharge of Monospores We next addressed the interaction between extracellular Ca2+ concentration and photosynthesis. First, extracellular Ca2+ was reduced by the addition of EGTA to ESL and modified media ASPMT3. After 3 days of treatment of ga- metophytes with 0.5 or 1.0 mM EGTA, which did not bring cell death (data not shown), the discharge of monospores was enhanced by EGTA in both ESL and ASPMT3 (Fig- ure 5(a)). Thus, it is possible that a decrease in extracel- lular Ca2+ may affect the influx of Ca2+ into (a) (b) Figure 5. Critical involvement of Ca2+ influx in production and discharge of monospores. (a) Effects of EGTA on dis- charge of monospores. Gametophytes were cultured in ESL (left), or ASPMT3 (right) with or without EGTA (0.5, 1.0, 2.0, 5.0 mM) at 15℃ under 60 μmol photons/m2/s with photocycleof 10 h light and 14 h dark for 3 days. Then, the number of discharged monospores was counted. Columns and vertical bars represent the mean ± SD, respectively (n = 3). (b) Effects of artificial Ca2+ influx on discharge of mon- ospores. Gametophytes were cultured as above except for under continual dark with or without the calcium ionopho- re A23187 (1 μM) and the number of discharged monosp- ores was counted. Columns and vertical bars represent the mean ± SD, respect i v ely (n = 3).  Photosynthesis-Dependent Extracellular Ca2+ Influx Triggers an Asexual Reproductive Cycle in the Marine Red Macroalga Porphyra yezoensis Copyright © 2010 SciRes. AJPS 8 vegetative cells. Based on these findings, we then examined the effects of the artificial influx of Ca2+ on monospore dis- charge in the dark using the calcium ionophore A23187. As shown in Figure 5(b), although gametophytes cultured in the dark did not produce monospores, as mentioned in Figure 4(a), a number of monospores were released from gametophytes treated with 1 μM A23187 in the absence of illumination, in which no cell death was observed (data not shown), although effects of A23187 treatment on monospore discharge werenot observed under the illumination because of strong influence of photosynthe- sis on monospore discharge as shown Figure 4. These above-mentioned results clearly indicate that Ca2+ influx is photosynthesis-dependent and a critical factor direct- ing the asexual life cycle in P. yezoensis. Figure 5(b) shows that the effects of A23187 on monospore discharge were different between ESL and ASPMT3. Since Ca2+ concentration of ASPMT3 is less than that of ESL (Table 1 and Figure 2), we propose that gametophytes cultured in ASPMT3 may acquire the po- tential of monospore discharge during cultivation. In addition, it was also observed that extensive deficiency of extracellular Ca2+ caused by the addition of 2.0 and 5.0 mM EGTA in ASPMT3 and ESL inhibited the dis- charge of monospores (Figure 5(a)). The viability of vegetative cells under these conditions were quite low (data not shown), suggesting that inhibition of mono- spore discharge by EGTA treatment may be due to cell death before or after the formation of monosporangia. 4. Discussion The presence of asexual reproduction via monospores is a remarkable strategy for survival in Bangiophycideae red algae because of its absence in land plants. Because the origin of asexual monospores can be traced back to 1,200 MYA based on fossil records of monospores of an ancient Bangiophycean alga [35], research into the asex- ual life cycle of modern red algae might provide novel information regarding the origin and evolution of mech- anisms regulating the eukaryotic life cycle. To date, it has been known that artificial changes in culture systems provided information about production and discharge of monospores [20,21]; however, regulatory mechanisms of this asexual reproduction mode have not yet been exten- sively analyzed. In the present study, we demonstrated that photosynthesis-dependent extracellular Ca2+ influx triggers the production and discharge of monospores from gametophytes of P. yezoensis via the establishment of artificial synthetic medium for the laboratory culture of this organism. The importance of extracellular Ca2+ influx in the pro- duction of monospores (Figures 3 and 5) indicates the close relationship between enhanced monospore production and changes in both morphology and color of vegetative cells in gametophytes (Figures 1 and 3). We have previ- ously reported that monospore production requires the formation of monosporangia in which vegetative cells become small, red-pigmented monospores [16]. Thus, the characteristics of cells found in gametophytes cultured in ASPMT1 and 2 (see Figure 1(b)) resemble those natu- rally occurring in monosporangia. Accordingly, we pro- posed that the decrease in extracellular Ca2+ due to incu- bation in ASPMT4 resulted in the extensive formation of monosporangia throughout the entire gametophyte fol- lowed by enhanced discharge of monospores due to the resulting greater area devoted to the production of monosporangia compared with ESL-cultured gameto- phytes. Based on this prediction, we hypothesized a mechanism for the formation and discharge of mono- spores (Figure 6). Briefly, the reduction of extracellular Ca2+ concentration stimulates the formation of monospo- rangia at the edge of gametophytes in which photosyn- thesis-dependent extracellular Ca2+ influx is indispensa- ble. Monosporangia then release monospores autono- mously or in a Ca2+ influx-dependent manner (Figure 6 upper). Thus, enhanced formation of monosporangia in gametophytes cultured in ASPMT4 (Figures 3 and 4) appears to have resulted in the development of nearly all vegetative cells into monospores, causing an extensive discharge of monospores (Figure 6 lower). Another significant result of the present study is the establishment of the novel synthetic medium ASPMT3 for P. yezoensis culture (Figure 3). At present, there are three types of media for red algal cultivation: filter-ster- ilized natural seawater with addition of a mineral mixture, ESL using commercially released SEALIFE powder with ESS2 [26], and synthetic medium such as the ASP series [27,28]. The composition of the first medium usually varies greatly depending on location and season, thus it is not fit for physiological studies due to difficulties re- garding control conditions and reproducibility. The sec- ond medium is a standard in our laboratory; however, the composition of the SEALIFE powder is proprietary, and therefore not obtainable. As mentioned in Figure 1, the third synthetic medium, the ASP series, has negative eff- ects on the growth of P. yezoensis, although its chemical composition is well known. Considering the previous lack of availability of a good synthetic medium for P. yezoensis in physiological experiments, the establishment of ASPMT3 is therefore an important technological im- provement for the study of P. yezoensis. Because appli- cability of ASPMT3 to the culture of other Bangiophy- cean red algae is unknown, it is important to test the growth of Porphyra and Bangia species of Bangiophy-  Photosynthesis-Dependent Extracellular Ca2+ Influx Triggers an Asexual Reproductive Cycle in the Marine Red Macroalga Porphyra yezoensis Copyright © 2010 SciRes. AJPS 9 cideae in compared with ESL medium to be able to gen- eralize the utility of ASPMT3 for Bangiophycean red algal research. Our aim is to understand the survival advantage of the asexual life cycle in red algae compared with other algae performing only sexual life cycle. The present study de- monstrates the importance of photosynthesis-dependent extracellular Ca2+ influx in monospore production (Fig- ures 4 and 5). However, the relative significance of ase- xual life cycle compared with the sexual life cycle in P. yezoensis and other Bangiophycean red algae still re- mains to be settled. Due to the dominance of the sexual life cycle in red algae, we propose that the advantage of asexual life cycle relates to the relatively low success rate of syngamy between male and female gametes. Red algal cells are aflagellate during their entire life cycle [36,37], thus success of syngamy depends completely on the number of free discharged male gametes compared with the number of female gametes located in gametophytes. The probability of syngamy may be enhanced by incr- easing the number of free male gametes and increasing the total area producing female gametes in gametophytes. Thus, increasing the density of gametophytic clones pro- duced by asexual propagation of monospores could in- crease both the number of male gametes released in sea water and the probability of fertilizing female gametes existing in gametophytes. In fact, similar explanation has been given for the tri-phasic life cycle observed in the Figure 6. A model of the involvement of Ca2+ and photosynthesis in production and discharge of asexual monospores in P. yezoensis gametophytes. In ESL and ASPMT3, if extracellular Ca2+ content is decreased, the formation of monosporangia is stimulated at the tip region of gametophytes, followed by discharge of monospores, for which photosynthesis activated by illumination accelerates extracellular Ca2+ influx to promote the formation of monosporangia (upper part). According to this hypothesis, the strong effects of ASPMT4 on growth of gametophytes and monospore discharge are explained by enhance- ment of monosporangium formation throughout the body of gametophytes after severe limitation of extracellular Ca2+, re- sulting in enhanced extracellular Ca2+ influx and monospore discharge, which reflects growth inhibition (lower part). Area containing monosporangia is schematically indicated by highlighted gray region. Photos show monosporangia in gameto- phytes cultured in ESL (upper ) and ASPM T4 (lower).  Photosynthesis-Dependent Extracellular Ca2+ Influx Triggers an Asexual Reproductive Cycle in the Marine Red Macroalga Porphyra yezoensis Copyright © 2010 SciRes. AJPS 10 more advanced red algae, classified as Florideophyceae, in which an extra diploid generation, so-called a tetraspo- rophyte, is found between the gametophyte and sporo- phyte phases [38]. The increase in the number of ga- metophytes via production of haploid tetraspores in tet- rasporophytes also makes on advantage for successful syngamy in Florideophyceae. However, regulation mech- anism producing spores are completely different between Bangiophyceae and Florideophyceae. To determine the relative advantages of asexual life cycle against sexual one in P. yezoensis, it is necessary to elucidate the molecular mechanisms regulating formation of monosporangia, sporulation of vegetative cells to mo- nospores, and discharge of monospores from gameto- phytes. Since the importance of extracellular Ca2+ influx (Figure 5) suggested the involvement of pumps and/or transporters of Ca2+ in monosporangia formation, it is essential to identify such molecules to understand how photosynthesis regulates extracellular Ca2+ influx for the promotion of monosporangia formation. In addition, it is necessary to elucidate how a decrease of extracellular Ca2+ concentration stimulates the intracellular Ca2+ influx, since there is no other example for the promotion of ex- tracellular Ca2+ influx by decrease in the Ca2+ concentra- tion outside of cells. Moreover, to understand the effects of the transfer of Ca2+ into the cytoplasm, it is important to identify targets of Ca2+ that participate in cellular sig- nal transduction pathways for monosporangia formation, sporulation and monospore discharge. We have also observed a similar regulatory system in the early development of P. yezoensis monospores, in which photosynthesis-dependent extracellular Ca2+ influx triggers initiation of the directional migration of mono- spores via the activation of phosphoinositide signaling components such as phosphatidylinositol 3-kinase and phospholipase C [17-19]. Thus, extracellular Ca2+ influx plays roles in different stages of the early development of monospores in this asexual life cycle. Such complexity related to multiple functions of Ca2+ should be resolved by focusing on whether extracellular Ca2+ influx is regu- lated by the same or different Ca2+ pumps and/or trans- porters during the formation of monosporangia and mi- gration of monospores. Therefore, identification of genes involved in the Ca2+ dependence of the asexual life cycle is necessary for progress in molecular biological and genetic studies on the regulation of the asexual life cycle in eukaryotic red algae. 5. Acknowledgements We are grateful to Drs. Yasuaki Takagi and Hajime Ya- sui (Hokkaido University, Japan) for kindly providing the polarized Zeeman atomic absorption spectropho- tometer and microscopes, respectively. This study was supported in part by Grants-in-Aid for Scientific Re- search (21580213) and the Regional Innovation Cluster Program (Global Type) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan. REFERENCES [1] T. H. Adams, J. K. Wieser and J. H. Yu, “Asexual Sporu- lation in Aspergillus nidulans,” Microbiology and Mo- lecular Biology Reviews, Vol. 62, No. 1, March 1998, pp. 35-54. [2] R. M. McCourt, C. F. Delwiche and K. G. Karol, “Ch- Arophyte Algae and Land Plant Origins,” Trends in Ecol- ogy and Evolution, Vol. 19, No. 12, December 2004, pp. 661-666. [3] K. J. Niklas and U. Kutschera, “The Evolution of the Land Plant Life Cycle,” New Phytologist, Vol. 185, No. 1, January 2010, pp. 27-41. [4] S. M. Coelho, A. F. Peters, B. Charrier, D. Roze, C. Destombe, M. Valero and J. M. Cock, “Complex Life Cycles of Multicellular Eukaryotes: New Approaches Based on the Use of Model Organisms,” Gene, Vol. 406, No. 1-2, December 2007, pp. 152-170. [5] K. J. Niklas and U. Kutschera, “The Evolutionary Devel- opment of Plant Body Plans,” Functional Plant Biology, Vol. 36, No. 8, August 2009, pp. 682-695. [6] F. Magne, “Classification and Phylogeny in the Lower Rhodophyta: A New Proposal,” Journal of Phycology, Vol. 27, No. s3, June 1991, p. 46. [7] L. Goosey and R. Sharrock, “The Arabidopsis Compact Inflorescence Genes: Phase-Specific Growth Regulation and the Determination of Inflorescence Architecture,” Plant Journal, Vol. 26, No. 5, June 2001, pp. 549-559. [8] G. C. K. Chiang, D. Barua, E. M. Kramer, R. M. Ama- sino and K. Donohue, “Major Flowering Time Gene, FL- OWERING LOCUS C, Regulates Seed Germination in Arabidopsis Thaliana,” Proceeding of the National Acad- emy of Sciences of the USA, Vol. 106, No. 28, July 2009, pp. 11661-11666. [9] K. E. Hubbard, F. C. Robertson, N. Dalchau and A. A. R. Webb, “Systems Analyses of Circadian Networks,” Mo- lecular Biosystems, Vol. 5, No. 12, December 2009, pp. 1502-1511. [10] N. Saga and Y. Kitade, “Porphyra: A Model Plant in Marine Sciences,” Fisheries Science, vol. 68, Supplement II, November 2002, pp. 1075-1078. [11] A. Miura, “Genetic Analysis of the Variant Color Types of Light Red, Light Green and Light Yellow Phenotypes of Porphyra yezoensis (Rhodophyta, Bangiaceae),” In: H. Hara, ed., Origin and Evolution of Diversity in Plants and Plant Communities, Academic Scientific Book Inc, To- kyo, 1985, pp. 270-284. [12] M. Kurogi, “Species of Cultivated Porphyra and Their Life Histories,” Bulletin of Tohoku Regional Fisheries Resear- ch Laboratory, Vol. 18, March 1961, pp. 91-115. [13] M. D. Guiry, “The Life-History of Liagora harveyana (Nemaliales, Rhodophyta) from SOUTH-EASTERN AUSTRALIA,” European Journal of Phycology, Vol. 25,  Photosynthesis-Dependent Extracellular Ca2+ Influx Triggers an Asexual Reproductive Cycle in the Marine Red Macroalga Porphyra yezoensis Copyright © 2010 SciRes. AJPS 11 No. 4, December 1990, pp. 353-362. [14] J. D. Pickett-Heaps, J. A. West, S. M. Wilson and D. L. McBride, “Time-Lapse Videomicroscopy of Cell (Spore) Movement in Red Algae,” European Journal of Phycol- ogy, Vol. 36, No. 1, February 2001, pp. 9-22. [15] J. C. Ackland, J. A. West and J. Pickett-Heaps, “Actin and Myosin Regulate Pseudopodia of Porphyra pulchella (Rhodophyta) Archeospores,” Journal of Phycology, Vol. 43, No. 1, February 2007, pp. 129-138. [16] L. Li, N. Saga and K. Mikami, “Phosphatidylinositol 3- Kinase Activity and Asymmetrical Accumulation of F- Ac- tin are Necessary for Establishment of Cell Polarity in the Early Development of Monospores from the Marine Red Alga Porphyra yezoensis,” Journal of Experimental Botany, Vol. 59, No. 13, October 2008, pp. 3575- 3586. [17] K. Mikami, “Migrating Plant Cell: F-Actin Asymmetry Directed by Phosphoinositide Signaling,” In: S. Lansing and T. Rousseau, eds., Cytoskeleton: Cell Movement, Cy- tokinesis and Organelles Organization, Nova Science Publishers, New York, 2010, pp. 205-218. [18] L. Li, N. Saga and K. Mikami, “Ca2+ Influx and Phos- phoinositide Signalling Are Essential for the Establish- ment and Maintenance of Cell Polarity in Monospores from the Red Alga Porphyra yezoensis,” Journal of Ex- perimental Botany, Vol. 60, No. 12, August 2009, pp. 3477-3489. [19] K. Mikami, L. Li, M. Takahashi and N. Saga, “Photosyn- thesis-Dependent Ca2+ Influx and Functional Diversity between Phospholipases in Formation of Cell Polarity in Migrating Cells of Red Algae,” Plant Signaling and Be- havior, Vol. 4, No. 9, September 2009, pp. 911-913. [20] Y. Kitade, G. Taguchi, J. A. Shin and N. Saga, “Porphyra Monospore System (Bangiales, Rhodophyta): A Model for the Developmental Biology of Marine Plans,” Phycological Research, Vol. 46, No. 1, March 1998, pp. 17-20. [21] S. Y. Li, “The Ecological Characteristics of Monospores of Porphyra yezoensis Ueda and their Use in Cultiva- tion,” Hydrobiologia, Vol. 116/117, No. 1, September 1984, pp. 255-258. [22] H. Mizuta, H. Yasui and N. Saga, “A Simple Method to Mass Monospores in the Thallus of Porphyra yezoensis Ueda,” Journal of Applied Phycology, Vol. 15, No. 4, July 2003, pp. 345-349. [23] X. H. Yan, Y. Fujita and Y. Aruga, “High Monospore- Producing Mutants Obtained by Treatment with MNNG in Porphyra yezoensis Ueda (Bangiales, Rhodophyta),” Hydrobiologia, Vol. 512, No. 1-3, January 2004, pp. 133- 140. [24] G. R. Seely, W. E. Vidaver and M. J. Duncan, “Prepara- tive and Analytical Extraction of Pigments from Brown Algae with Dimethyl Sulfoxide,” Marine Biology, Vol. 12, No. 2, January 1972, pp. 184-188. [25] S. Beer and A. Eshel, “Determining Phycoerythrin and Phycocyanin Concentrations in Aqueous Crude Extracts of Red Algae,” Australian Journal of Marine and Fresh- water Research, Vol. 36, No. 6, September 1985, pp. 785-792. [26] Y. Kitade, S. Fukuda, M. Nakajima, T. Watanabe and N. Saga, “Isolation of a cDNA Encoding a Homolog of Ac- tin from Porphyra yezoensis (Rhodophyta),” Journal of Applied Phycology, Vol. 14, No. 2, April 2002, pp. 135-141. [27] L. Provasoli, J. J. A. McLaughlin and M. R. Droop, “The Development of Artificial Media for Marine Algae,” Ar- chiv für Mikrobiologie, Vol. 25, No. 4, December 1957, pp. 392-428. [28] L. Provasoli, “Growing Marine Seaweeds,” In: A. D. De Virville and J. Feldman, eds., Proceedings of the Fourth International Seaweed Symposium, Pergamon Press, Ox- ford, 1963, pp.9-17. [29] N. Saga, T. Motomura and Y. Sakai, “Induction of Callus from the Marine Brown Alga Dictyosiphon foenicu- laceus,” Plant and Cell Physiology, Vol. 23, No. 4, June 1982, pp. 727-730. [30] N. S. Yokoya, “Apical Callus Formation and Plant Re- generation Controlled by Plant Growth Regulators on Axenic Culture of Red Alga Gracilariopsis tenuifrons (Gracilariales, Rhodophyta),” Phycological Research, Vol. 48, No. 3, September 2000, pp. 133-142. [31] S. Shen, L. He, P. Xu and J. Zhu, “Effect of Nitrogen and Phosphorus on the Development and Differentiation of Vegetative Cells of Porphyra yezoensis on Solid Agar Medium,” Botanica Marina, Vol. 49, No. 5/6, December 2006, pp. 372-378. [32] J. T. Hafting, “Effect of Tissue Nitrogen and Phosphorus Quota on Growth of Porphyra yezoensis Blades in Sus- pension Cultures,” Hydrobiologia, Vol. 398/399, No. 0, April 1999, pp. 305-314. [33] M. Kakinuma, D. Coury, C. Nakamoto, K. Sakaguchi and H. Amano, “Molecular Analysis of Physiological Re- sponses to Changes in Nitrogen in a Marine Macroalga, Porphyra yezoensis (Rhodophyta),” Cell Biology and Toxicology, Vol. 24, No. 6, December 2008, pp. 629-639. [34] K. Charnofsky, L. R. Towill and M. R. Sommerfeld, “Li- Ght Requirements for Monospore Germination in Bangia atropurpurea (Rhodophyta),” Journal of Phycology, Vol. 18, No. 3, September 1982, pp. 417-422. [35] N. J. Butterfield, “Bangiomorpha pubescens n. gen., n. sp.: Implications for the Evolution of Sex, Multicellular- ity, and the Mesoproterozoic/Neoproterozoic Radiation of Eukaryotes,” Paleobiology, Vol. 26, No. 3, September 2000, pp. 386-404. [36] G. Tripodi and F. de Masi, “Unusual Structure in the Spermatial Vesicles of the Red Alga Erythrocystis mon- tagnei,” Plant Systematics and Evolution, Vol. 143, No. 3, September 1983, pp. 197-206. [37] M. N. Clayton, “Propagules of Marine Macroalgae: Stru- Cture and Development,” European Journal of Phycology, Vol. 27, No. 3, September 1992, pp. 219-232. [38] C. S. Thornber, “Functional Properties of the Isomorphic Biphasic Algal Life Cycle,” Integrative and Comparative Biology, Vol. 46, No. 5, October 2006, pp. 605-614. |

