Paper Menu >>
Journal Menu >>
 Journal of Environmental Protec tion, 2013, 4, 70-73 doi:10.4236/jep.2013.41b013 Published Online January 2013 (http://www.SciRP.org/journal/jep) Copyright © 2013 SciRes. JEP Studies On Extracting Microcystin-Lr From Microcystis Aeruginosa By Water Bath* Fang Li, Wenqing Liu, Nanjing Zhao#, Jingbo Duan, Zhigang Wang, Yujun Zhang, Xue Xiao, Jing Liu, Gaofang Yin, Chaoyi Shi Key Laboratory of Environmental Optics and Technology, Anhui Institute of Optics and Fine Mechanics, Chinese Academy of Sciences, 350 Shu Shan Hu Road, Hefei, Anhui, China. Email: #njzhao@aiofm.ac.cn Received 2013 ABSTRACT Different temper atures of water bath was used to extract the intracellular microcystin-LR(MC-LR) of M icro cystis a eru- ginosa. Researching the extraction efficiency under the suitable temperature, so that it could find o ut the best te mpera- ture a nd ti me for extracting MC-LR from Microcystis aeruginosa cells. Five equal Microcystis aeruginosa was used to find out the best temperature, extracting at 60˚C, 70˚C, 80˚C, 90˚C and 100˚C for 15 minutes, respectively. Results sho wed that t he co ntent o f MC-LR extracted with the water under 100˚C wa s the highest. But mean whil e, the type and the content of impurities was the highest, too. In addition, an o ther six equal Microcystis aeruginosa was extract with the water under 100˚C for 5min, 10 min, 15 min, 20 min, 25 min and 30 min respectively. It was proved that 20 minutes was enough for extracting MC-LR from Microcystis aeruginosa, no long time was needed. Keywords: Boiling Wate r Bath; Extract; Micro cystin-LR 1. Introduction Cyanobacteria bloom happens more and more frequently worldwide because of growing pollution, causing many kind s of mic roc ysti ns(MC s) whic h have dra wn muc h pub- lic attenti on. M Cs, whic h coul d be detected in 80 percent water bloom according to the survey, can inhibit the ac- tivity of protein phosphatase(1, 2Aand3), promote onco- genesis, or even lead to death. MCs are ring peptide compounds composition of sev- en amino acid. They are very stable and release into wa- ter body after the cellular ruptured, causing serious harm to the water quality and aquatic. There’re more than 80 different microcystins according to the data has been already reported. At present, the research of MCs has bee n focuse s on t he toxicolog y, environmental c hemistry, preparation of standard toxins used for analysis and the applications in biochemistry, etc. It can’t obtain MCs from chemical synthesis. So that extracting MCs from toxigenic strains efficiently becomes the basis and pre- requisite of the study of MCs. At pre se nt t her e ’re many methods to extract MCs from cellular. ZHANG Ming-ming [1] used 40% methanol solution as the extract solvent, thawing and refreezing cells repeatly to extract microcystin-LR(MC-LR). REN Jing’s results showed that it was efficient to use 75% methanol as extract solvent, freezing and thawing the cells as well as ultrasonication. Because cyanobacteria belongs to prokaryotes, the ce ll wall is mainly mucop ep- tide, so that both cryogenically freezing and boiling can destroy cell wall and then MCs released. But freezing and thawing resulted in low toxin and time consuming. The research of Jarkko Rapala [3] showed that after the first freezing and thawing only a small number of cya- nobacteria colonies had b een dispersed. Sonication for 60 min in the water bath disrupted only the outermost cells of the colonies. After the second freezing and thawing and sonication for 15 min a high number of unbroken cells was still seen. Using methanol to extract MCs, it must dilute the extract solvent by water or heat and vola- tile the extract solvent to reduce the concentratio n of the methanol before solid phase extraction. This increases the time of solid phase extraction, and the use of organic regents is bad to the health of the researchers. In addition, many organics such as phycobiliprotein in cyanobacteria cells, which could be extracted with MCs, may pollute the column of the chromatographic. Some reported that using guard columns to remove the protein, and then *Foundation item: The national “863” project grants program (2009A- A063005); Nat u ra l s cienc e fund projects in anhui province (11040606- M26); Anhui institute of optics and fine mechanics, director of the project fund (Y03AG31144). #Corresponding author.  Studies On Extracting Microcystin-Lr From Microcystis Aeruginosa By Water Bath Copyright © 2013 SciRes. JEP 71 elute MCs. So me adjusted the PH value to 4 with diluted solution of sulfuric acid, so that the protein was denatu- ration and precipitated and the column was defended from pollution. All these methods was time consuming and increased the cost. Metcalf et al. [5] first extracted MCs with boilin g water, usin g deionized water as extrac- tion so l vent. T he re sult sho wed that one mi nute was ver y good for 150 μL extraction solvent. The use of boiling water based upon the heat resisitance of MCs. MCs would not lose activity after heating in the water of 100˚C for 30 min, and was hard to resolve under 300˚C [6]. This method avoided the use of metha nol during the extraction process which was unsafe to the analyst. In addition, it lowered the cost, and shortened the time of extraction. LEI La-mei et al [7] also studied extracting MCs with boiling water, and contrasted with the tradi- tional method using methanol, found that the relative error was between 0.2% and 16.59%. 12 min of heating time was enough in their study and deionized water was better than disti lle d water for the extra c tion of MC s. In conclusion, the use of boiling water to extract MCs from cyanobacteria cells has a good application prospect. But at present this method is not very mature as there are not many related reports. This study systematically re- searched extracting MC-LR within the cells o f Microcys- tis aeruginosa, obtaining the best temperature and time. And good results had been achieved with the recovery of 89.3%. A great quantity of organic solvent was avoid during the extraction process. The entire analysis could be finishe d i n one day. 2. Material and Method 2.1. Instruments The main instruments needed in this experiment were as follows: Visiprep SPE Vacuum Manifold-DL, 24-port model; Supelclean ENVI-18 cartridges(500 mg/3 mL); Agilent 120 0 HPLC (Eclipse XDS-C18 chromato grap hic column); HP400G incubator(Wuhan Ruihua Instrument & Equipment Co.Ltd); Molecular ultra-pure water ma- chine; sterilizer; The Labnet 6Liter Water Bath; H-1650 Ultracentrifuge; NMT-2800 Pressure Blowing Concen- trator. 2.2. Reagent The main reagents are as follows: methanol (chromato- graphic grade), trifluoroacetic acid (TFA, chromato- graphic grade); deionized water; phosphoric acid; 0.05 mol/L KH2PO4 (chromatographic grade); Microcystis aeru ginosa(FACHB-905) was purchased from institute of hydrobiology, Chinese academy of sciences and cul- tured in BG-11 medium. 2.3. Extraction of MC-LR 2.3.1. The Experiment of Selecting the Best Temperature Added 10 equal parts of Microcystis aeruginosa which were in logarithmic growth phase into 10 Erlenmeyer flasks, centrifuging and removing the supernatant. The cells were transferred into other 10 Erlenmeyer flasks with deionized water. Then put them into water bath at 60˚C, 70˚C, 80˚C, 90˚C and 100˚C, respectively. Two parallel samples were managed for each temperature. After 15 minutes took them out and centrifuged, the su- pernatant was transferred into 10 clean Erlenmeyer flasks, respectively. Washing the residue with deionized water and centrifuged again, combining the supernatant. The supernatant were loaded into SPE cartriege, rinsed with 10 mL deionized water and 20% methanol, and then the MC-LR was eluted by 90% methanol which containing 0.1% TFA). The eluents were dried under nitrogen in 40˚C water bath, the rest substances was dissolved in 0.5 mL methanol(two times) and transferred into autosamp- ler vial, dried again with strea m of nitrogen, d iluted wit h 50% methanol to 200μL, and detected by HPLC. 2.3.2. The Extracting Time under 100˚C Added 12 equal parts of Microcystis aeruginosa which were in logarithmic growth phase into 12 Erlenmeyer flasks. The isolation of cells was the same as the above. The flasks were put into 100˚C for 5min, 10min, 15min, 20 min, 25 min and 30 min, respectively. Two parallel samples for each gradient. The crude extract was centri- fuged and loaded into SPE as above. 2.4. HPLC Conditions Column temperature at 40˚C, using methanol and the buffer solution of phosphoric acid (PH = 3) at 57:43(V/V) as the mobile phases, velocity of flow was 1mL/min, wavel ength of the U V-vis detectors was 380 nm. 3. Results and Discussion The contents of MC-LR extracted under different tem- perature were showed in Figure 1. The algae cells were extracted 15 minutes under each temperature. As it turned out, t he higher the tem peratur e, the muc h MC-LR was extracted. In the range of 70˚C to 100˚C, the co ntent of MC-LR and the temperature had a good linear rela- tionship. The data showed that every 10˚C degree tem- perature rise would increase the content of MC-LR about 15 μg/L. Thus it could be seen that the appropriate tem- perature for extracting MC-LR from Microcystis aerugi- nosa was 100˚C. But the disadvantage was that the pig- ments and other impurities in the algae cells would ex- tracted out simultaneously. These impurities would in- terfere with the detection of MC-LR. Research about eliminating influe nce from these impur ities is c ontinuing. 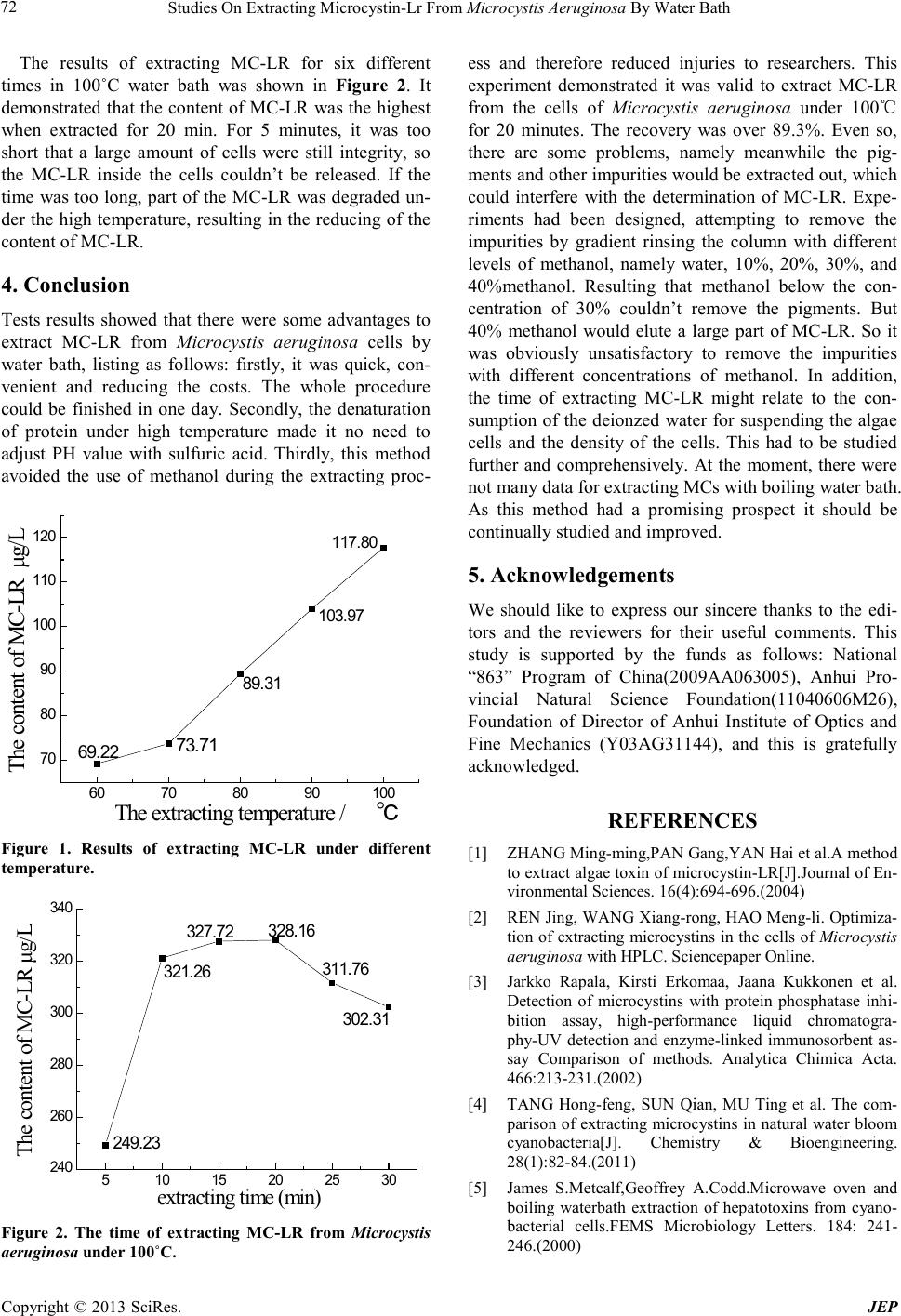 Studies On Extracting Microcystin-Lr From Microcystis Aeruginosa By Water Bath Copyright © 2013 SciRes. JEP 72 The results of extracting MC-LR for six different times in 100˚C water bath was shown in Figure 2. It demonstrated that the content of MC-LR was the highest when extracted for 20 min. For 5 minutes, it was too short that a large amount of cells were still integrity, so the MC-LR inside the cells couldn’t be released. If the time was too long, part of the MC-LR was degraded un- der the high temperat ure, resulting in the reducing of the content of MC-LR. 4. Conclusion Tests results showed that there were some advantages to extract MC-LR from Microcystis aeruginosa cells by water bath, listing as follows: firstly, it was quick, con- venient and reducing the costs. The whole procedure could be finished in one day. Secondly, the denaturation of protein under high temperature made it no need to adjust PH value with sulfuric acid. Thirdly, this method avoided the use of methanol during the extracting proc- 60 70 80 90100 70 80 90 100 110 120 The content of MC-LR μg/L Th e extracting tem perature /℃ 69. 22 73.71 89. 31 103.97 117.80 Figure 1. Results of extracting MC-LR under different temperature. 510 15 20 25 30 240 260 280 300 320 340 The content of MC-LR μg/L e x tra ctin g time (min) 249. 23 321. 26 327.72 328. 16 311. 76 302. 31 Figure 2. The time of extracting MC-LR from Microcystis aeruginosa under 100˚C. ess and therefore reduced injuries to researchers. This experiment demonstrated it was valid to extract MC-LR from the cells of Microcystis aeruginosa under 100℃ for 20 minutes. The recovery was over 89.3%. Even so, there are some problems, namely meanwhile the pig- ments and other i mpurities would b e extrac ted o ut, which could interfere with the determination of MC-LR. Expe- riments had been designed, attempting to remove the impurities by gradient rinsing the column with different levels of methanol, namely water, 10%, 20%, 30%, and 40%methanol. Resulting that methanol below the con- centration of 30% couldn’t remove the pigments. But 40% methanol would elute a large part of MC-LR. So it was obviously unsatisfactory to remove the impurities with different concentrations of methanol. In addition, the time of extracting MC-LR might relate to the con- sumption of the deionzed water for suspending the algae cells and the density of the cells. This had to be studied further and co mprehensively. At the moment, there were not many data for e xtra c ting M Cs with boili ng water bath. As this method had a promising prospect it should be continually studied and improved. 5. Acknowledgements We should like to express our sincere thanks to the edi- tors and the reviewers for their useful comments. This study is supported by the funds as follows: National “863” Program of China(2009AA063005), Anhui Pro- vincial Natural Science Foundation(11040606M26), Foundation of Director of Anhui Institute of Optics and Fine Mechanics (Y03AG31144), and this is gratefully acknowledged. REFERENCES [1] Z H AN G M i n g -ming,PAN Gang, YAN Hai et al .A met ho d to extract algae toxin of microcystin-LR[J].Journal of En- vironmental Sciences. 16(4):694-696.(2004) [2] REN Jin g, WANG Xiang-rong, HAO Meng-li. Optimiza- tion of extracting microcystins in the cells of Microcystis aeruginosa with HPLC. Sciencepaper Online. [3] Jarkko Rapala, Kirsti Erkomaa, Jaana Kukkonen et al. Detection of microcystins with protein phosphatase inhi- bition assay, high-performance liquid chromatogra- phy-UV detection and enzyme-linked immunosorbent as- say Comparison of methods. Analytica Chimica Acta. 466:213-231.(2002) [4] TANG Hong-feng, SUN Qian, MU Ting et al. The com- parison of extracting microcystins in natural water bloom cyanob acteria[J]. Chemistry & Bioengineering. 28(1):82-84.(2011) [5] James S.Metcalf,Geoffrey A.Codd.Microwave oven and boiling waterbath extraction of hepatotoxins from cyano- bacterial cells.FEMS Microbiology Letters. 184: 241- 246.(2000)  Studies On Extracting Microcystin-Lr From Microcystis Aeruginosa By Water Bath Copyright © 2013 SciRes. JEP 73 [6] LI Qiu-xia, CAI Chao-hai, XU Gui-lan. Rapid detection of microcystins-LR in drinking water with super fast per- formance liquid chromatography-tandem mass spectro- metry. Chin J Health Lab Technol. 20(8): 1875- 1877. (2010) [7] LEI La-mei, Gan Nan-qin, SONG Li-rong. A rapid me- thod to extract and analyse microcystins. ACTA Hydro- bio lo gi ca Sini ca. 27(5):468-471.(2003) [8] National Standards of Peoples Republic of China. GB/T 20466-2006. The detection of microcystins in water. |

