Paper Menu >>
Journal Menu >>
 Advances in Ma terials Physics and Che mist ry, 2012, 2, 248-252 doi:10.4236/ampc.2012.24B063 Published Online December 2012 (htt p://www.SciRP.org/journal/ampc) Copyright © 2012 SciRes. AM PC Resolving a Challenge i n t he Modeling of Hydrogen Production Using Steam Reforming of Methane in Monolith React ors Using CFD Methods Mohammad Irani Research Institute of Petroleum Industry (RIPI), Tehran, Iran Email: iranim@ripi.ir Received 2012 ABSTRACT Reaction modeling of SMR (Steam Methane Reforming) process inside monolith reactors using two approaches were investigated and compared with each other . In the first ap proach, the reaction s were assu med to take p lace exactly on the wall su rfaces, while in the seco nd approach th ey considered in side a thin thickness n ear the walls. Experi ments of SMR were carried o ut in a lab-scale mo- nolith reactor. A single-channel was considered and CFD model were developed for each of aforementioned approaches. Compari- sons b etween model ing resu lts an d experi mental d ata sho wed that t he first appr oach (su rface model) give s better result s. P erforming reaction s are d if ficul t an d exp ensi ve, CF D si mulat io ns ar e con sid ered as n umeric al e xper imen ts in man y cases. It was co ncluded that obtained results from CFD analysis gives precise guidelines for further studies on optimization of SMR monolithic reactor perfor- mance. Keywords: Hydrogen Production; Monolith ic Reactor; CFD; SMR; Surface-model; Volume-model 1. Introduction Hydrogen is one o f the cl eanest fuels whi ch can b e used inst ead of fossil fuels. There are variety of applications for hydrogen in industries such as: fuel cells, green cars, metal production and fabrication, Petroleum recovery and refinery, chemical processing, power generation, etc. The mentioned applications made the Hydrogen a strategic product. The most famous process for hydrogen production is Steam Reforming of Methane (SMR) which converts Methane and other hydrocarbons presented in natural gas into Hydrogen in large centralized industrial plants. Researchers are being done in order to develop small-scale SMR technologies to enable the development of distributed hydrogen production and delivery infrastructure [1,2]. There- fore due to attaining this goal small-scale SMR technologies should be modeled and optimized [3-7]. Monolith catalysts can be widely used in many applications particularly for their high geometric su rface are a, low pr essure d rop and good mechan ical strength and durability [8]. In addition using monolithic reactors have significant advantages such as reduced capital cost, smaller footprints and potentially easier transportation [1, 9-11]. These advantages can be particularly valuable when considering the exploration of remote resources such as offshore reserves of natural gas[12]. In the present work, hydrogen production in a bench-scale SMR monol ith reacto r was stud ied. Two different approaches were presented in the literature for implementing reaction rat es in monolith react or models , namely surface approach and volumetric approach. In the first approach, the diffusion into the thin catalytic layers (washcoat) in modeling of monolithic reactors is neglected and the reactions are assumed to take place at the surface of the washcoat. Case studies for this approach include steam Methane reforming (SMR) [14], steam reforming of methanol [15-17], ethanol steam reforming [18] and autothermal reforming of n-hexadecane [19]. Hartmann et al. [20,21] studied Hydrogen production by catalytic p artial o xidation of iso-octane at varying flow rate an d fuel/oxygen ratio. Massing et al. [24] studied the catalytic conversion of propene and demonstrated that diffusional limitations within the washcoat limit the propene conversion. Stutz and Poulikakos [25] considered diffusion and reaction inside the washcoat of a monolithic reformer. In the present study, the surface and volumetric approaches were used to model SMR in a single-channel monolith reactor. The obtained results from each model were compared with experimental dat a. 2. Mathematical Model In order to model the problem, five sets of equations should be solved; continuity equation, momentum balance, energy and species transport equations. .() 0v ρ ∇= (1) ( ) .( ). T vvPvvg S ρ µρ ∇=−∇+∇∇ +∇++ (2) ()( ) 1 .().. n ii R i vH PhJqS ρ = ∇++ ∇=−∇+ ∑ (3) where, ρ represents mi xture den sity, v is veloci ty vector, µ is the mixture viscosity, H and hi are total enthalpy and enth alpy of species, respecti vely and Ci stands for concentration of chemical sp ecies . P i s the static pr essur e and SR is the heat of reaction. 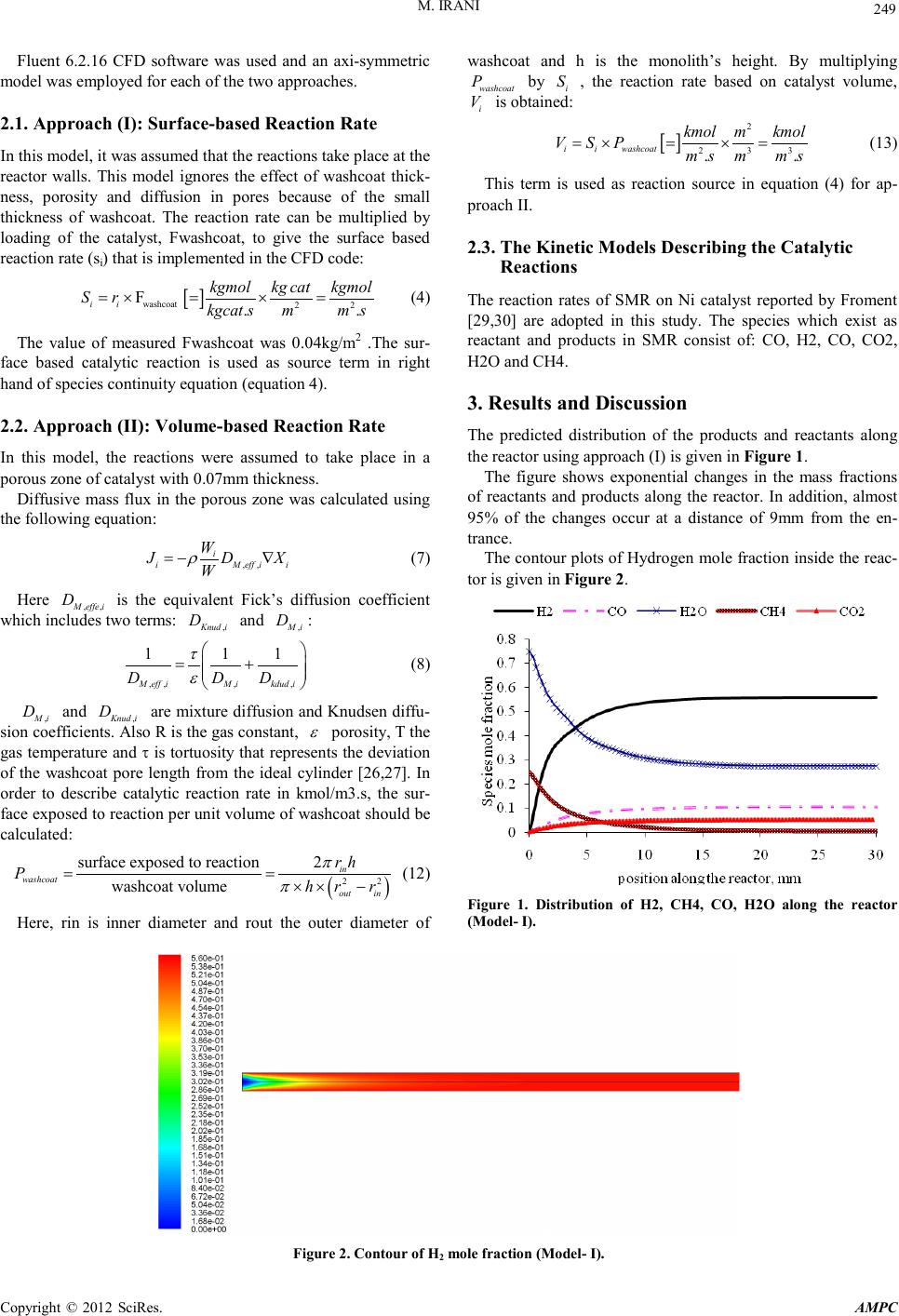 M. IRANI Copyright © 2012 SciRes. AM PC 249 Fluent 6.2.16 CFD software was used and an axi-symmetric model was employed for each of the two approaches. 2.1. Approach (I): Surfa c e-based Reaction Rate In this model, it was assumed that the reacti ons t ake place at the reactor walls. This model ignores the effect of washcoat thick- ness, porosity and diffusion in pores because of the small thickness of washcoat. The reaction rate can be multiplied by loading of the catalyst, Fwashcoat, to give the surface based reaction rate (si) that is implemented in the CFD code: [ ] washcoat 22 F.. ii kgmolkg catkgmol Sr kgcat smms =× =×= (4) The value of measured Fwashcoat was 0.04kg/m2 .The sur- face based catalytic reaction is used as source term in right hand of species continuity equation (equation 4). 2.2. Approach (II) : Volume-based Reaction Rate In this model, the reactions were assumed to take place in a porous zone of catalyst with 0.07mm thickness. Diffusive mass flux in the porous zone was calculated using the following equation: ,, i iM eff ii W J DX W ρ =−∇ (7) Here ,, Meffe i D is the equivalent Fick’s diffusion coefficient which includes two terms: ,Knud i D and ,Mi D : ,,, , 1 11 M effiM ikdud i D DD τ ε = + (8) ,Mi D and ,Knud i D are mixture diffusion and Knudsen diffu- sion coefficients. Also R is the gas constant, ε porosity, T the gas temperatu re and τ is tortuosity that represents the deviation of the washcoat pore length from the ideal cylinder [26,27]. In order to describe catalytic reaction rate in kmol/m3.s, the sur- face exposed to reaction per unit volume of washcoat should be calculat ed: ( ) 22 surface exposed to reaction2 washcoat volumein washcoat out in rh Phr r π π == ×× − (12) Here, rin is inner diameter and rout the outer diameter of washcoat and h is the monolith’s height. By multiplying washcoat P by i S , the reaction rate based on catalyst volume, i V is obtained: [ ] 2 2 33 .. ii washcoat kmol mkmol VSP ms mms =×=×= (13) This term is used as reaction source in equation (4) for ap- proach II. 2.3. The Kinetic Models Describing the Catalytic Reactions The reaction rates of SMR on Ni catalyst reported by Froment [29,30] are adopted in this study. The species which exist as reactant and products in SMR consist of: CO, H2, CO, CO2, H2O and CH4. 3. Results and Discussion The predicted distribution of the products and reactants along the reactor usin g approach (I) is given in Figure 1. The figure shows exponential changes in the mass fractions of reactants and products along the reactor. In addition, almost 95% of the changes occur at a distance of 9mm from the en- trance. The contour plots of Hydrogen mole fractio n inside the reac- tor is given in Figure 2 . Figure 1. Distribution of H2, CH4, CO, H2O along the reactor (Model- I). Figure 2. Contour of H2 mole fr ac tion (Mo del- I). 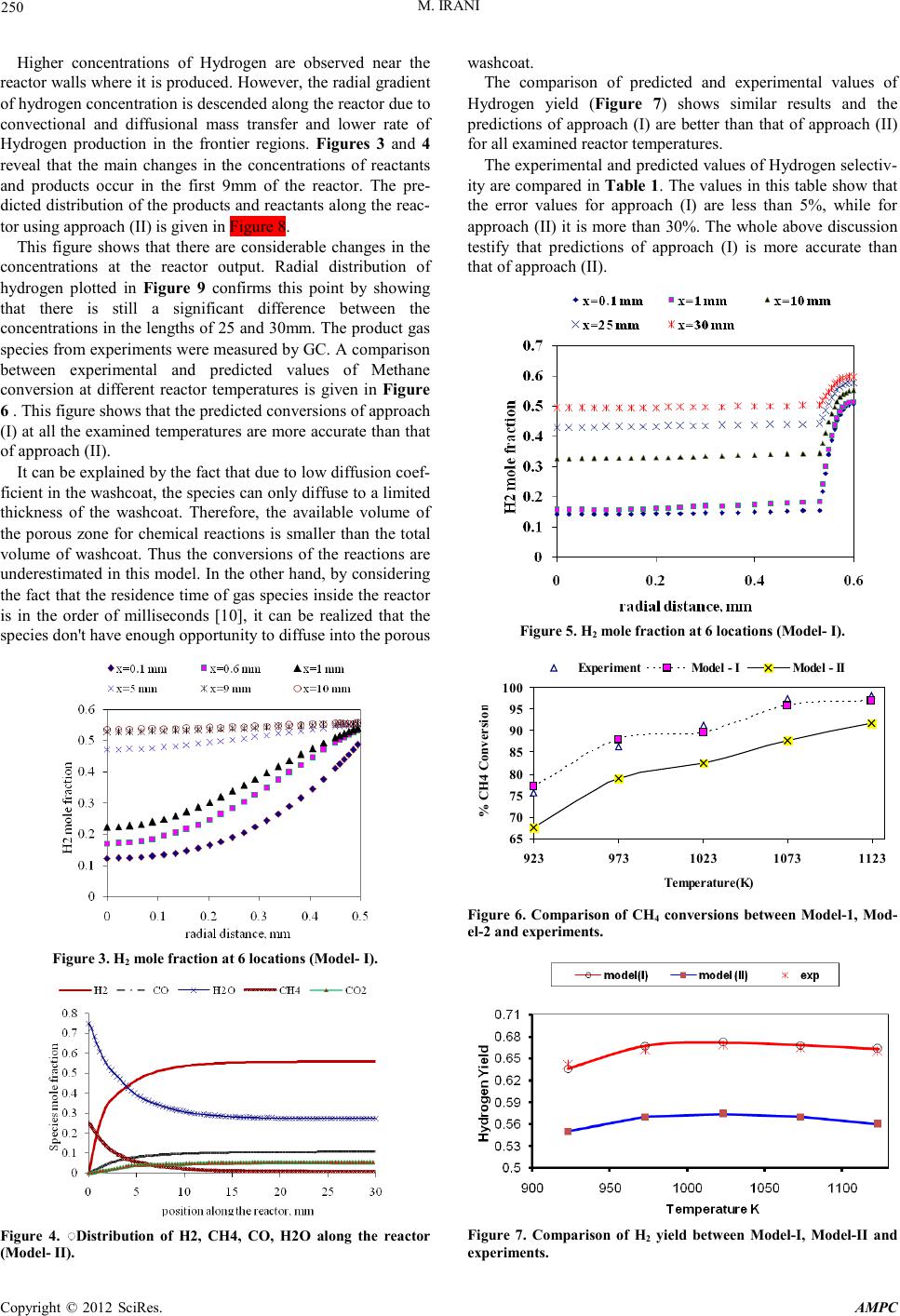 M. IRANI Copyright © 2012 SciRes. AM PC 250 Higher concentrations of Hydrogen are observed near the reactor walls wher e it is produ ced. Ho wever, the rad ial gradien t of hydrogen concentration is descended along the reactor due to convectional and diffusional mass transfer and lower rate of Hydrogen production in the frontier regions. Figures 3 and 4 reveal that the main changes in the concentrations of reactants and products occur in the first 9mm of the reactor. The pre- dicted distribution of the products and reactants along the reac- tor using approach (II) is given in Figure 8. This figure shows that there are considerable changes in the concentrations at the reactor output. Radial distribution of hydrogen plotted in Figure 9 confirms this point by showing that there is still a significant difference between the concentrations in the lengths of 25 and 30mm. The product gas species from experi ment s were measured by GC. A compariso n between experimental and predicted values of Methane conversion at different reactor temperatures is given in Figure 6 . This figure shows that the predicted conversions of approach (I) at all the examined temperatu res are more accurat e than that of approach (II). It can be explained by the fact that due to low diffusion coef- ficient in the washco at, the sp ecies can o nly diffuse to a limited thickness of the washcoat. Therefore, the available volume of the porous zone for chemical reactions is smaller than the total volume of washcoat. Thus the conversions of the reactions are underestimated in this model. In the other hand, by considering the fact th at the resid ence time of gas species in side the reacto r is in the order of milliseconds [10], it can be realized that the species don't have enough opportunity to diffuse into the porous Figure 3. H2 m ole fraction at 6 locat ion s (Mode l- I). Figure 4. ِ◌Distribution of H2, CH4, CO, H2O along the reactor (Model- II). washcoat. The comparison of predicted and experimental values of Hydrogen yield (Figure 7) shows similar results and the predictions of approach (I) are better than that of approach (II) for all exa min ed reacto r temperatures . The experi mental an d p redicted values of Hydrogen selectiv- ity are compared in Table 1. The values in this table show that the error values for approach (I) are less than 5%, while for approach (II) it is more than 30%. The whole above discussion testify that predictions of approach (I) is more accurate than that of approach (II). Figure 5. H2 m ole fraction at 6 locat ion s (Mode l- I). 65 70 75 80 85 90 95 100 9239731023 10731123 Temperature(K) % CH4 Co nversion Experiment Model - I Model - II Figure 6. Comparison of CH4 conversions between Model-1, Mod- el-2 and experiments. Figure 7. Comparison of H2 yield between Model-I, Model-II and experiments.  M. IRANI Copyright © 2012 SciRes. AM PC 251 Table 1. Comparisons between the two models and experimental results. Model 1 Model 2 Exp Model 1 Error (%) Model 2 Error (%) H2 selectivity* (T=700 ºC) 71.26 51.83 73.78 3 .41 2 9 .7 H2 selectivity (T=750 ºC) 75.65 55.37 77.81 2.775 2 8.8 H2 selecti vity (T=800 ºC) 7 8 .1 58.36 79.11 1 .27 2 6 .2 4. Conclusion The current study presents two approaches for numerical mod- eling and implementation of reaction rates in simulation of heat and mass transfer in monolithic reactors. The chemical conver- sion on the Ni-catalyst is modeled using general kinet ic models for SMR and Water–Gas-Shift (WGS) reaction rates based on Langmuir–Hinshelwood type. Monolithic reactor was simu- lated using mentioned two approaches under steady-state con- dition. The results of two approaches were compared to cor- responding experimental data and a comprehensive evaluation was carried out. The results showed that the predictions of sur- fac e-based approach are more accurate than that of vo- lume-based approach. The volume-based mod el underest imates the conversion of reactions. Small values of effective diffusion coefficien t in po rous washcoat layer and low resid ence ti me are the main reasons of discrepancy between volume-based ap- proach and experiment results. In total, despite of its ease of implementation, the first approach (surface reactions) gives better r esults both in generality and accuracy. Also. It was con- cluded that obtained results from CFD analysis gives precise guidelines for further studies on optimization of SMR mono- lithic reactor performance. REFERENCES [1] A.Y. Tonkovich , S. Perry, Y. Wang, D. Qiu, T. LaPlante and W.A. Rogers , “Microchanel process technology for compact Methane steam reforming,” Chem. Eng .Sci , vol. 59, pp. 4819–48 24, 2004. [2] D. Hickman and L.D. Schmidt, “ Production of syngas by direct catalytic oxidation of Methane”, Science , vol. 259, pp. 343–346,1993. [3] E.E. Gonzo, “ Hydrogen from methanol-steam reforming. Isothermal and adiabatic monolith reactors’ simulation.” ,Int J Hydrogen Energy, vol.33,pp. 3511 – 3516, 2008. [4] E. Lo´pez, A. Irigoyen, T. Trifonov, A. Rodrı´guez, J. Llorca, “ A million-cha nnel reformer on a fin gertip: Moving d own the scale in hydrogen production”, Int.J. Hydrogen Energy, vol.35 ,pp. 3472 – 3479, 2010. [5] L. Shi, “ Bayless D J and Prud ich M E. A CFD model of auto- therma l reformi n g”, Int J Hydrogen E n ergy ,vol .35 ,pp . 3472 – 3479 , 201 0. [6] W. Cai, F. Wang, A.V. Veen, C. Descorme, Y. Schuurman and W. Shen , “ Mirodatos C. Hydrogen production from ethanol steam reforming in a micro-channel reactor”, Int J Hydrogen Energy, vol. 35,pp. 1152 – 1159, 2010. [7] X. Zhai, S. Ding, Y. Cheng, Y. Jin and Y. Cheng, “ CFD si- mulation with detailed chemistry of steam reforming of Methane for hydrogen production in an integrated micro-reactor” , Int J Hydrogen Energy , vol. 35, pp. 5383 – 5392, 2010. [8] T. Boger, “ Monolithic catalysts for the chemical Industry”, Ind Eng Ch em Res, vol.43 ,pp. 4602-4611, 2004. [9] R.S. Benson and J .W. Ponton., “ Process Miniaturisation-A Route to Total Environmental Acceptability”, Chem. Eng. Res. Des. ,vol. 71,pp . 160–168,1993. [10] A.Y. Tonkovich, B. Yang , S.T. Perry, S.P. Fitzgerald, Y. Wang , “ From seconds to milliseconds to microseconds through tai- lored mic rocha nn el rea ct or d esi gn of a stea m Met h aner efor m er” , Catal Today , vol.120 ,pp. 21-29, 2007. [11] R. J. Bradley, L.C. Nathan, N.T. Diana, A.D. Robert, D.H. Jamelyn, Y. Wang , “ Engineered SMR catalysts based on hy- drothermally stable, porous, ceramic supports for microchannel reactors” ,Catal Today,vol. 120 , pp. 54-62, 2007. [12] A.K. Avci, D.L. Trimm, M. Karakaya, “ Microreactor catalytic combustion for chemicals processing”, Catal Today,vol.155 ,pp. 66-74 ,2010. [13] G. Arzamendi, P.M. Diéguez, M. Montes, J.A. Odriozola, E. Falabella, E. Sousa-Aguiar and L.M .Gandía, “Methane steam reforming in a microchannel reactor for GTL intensifica- tion: A computational fluid dynamics simulation study ”, Chem- ica l Engineering Journa l , vol.168 ,pp. 168-173, 2009. [14] A. Fazeli, M. Behnam, “ Hydrogen production in a zigzag and straight catalytic wall coated micro channel reactor by CFD modeling”, Int J Hydrogen Energy, vol.35 ,pp. 9496-9503, 2009.. [15] G. Arzamendi, P.M. Diéguez, M. Montes, M.A . Centeno, J.A. Odriozola, L.M . Gandía, “In tegration of methanol steam re- forming and combustion in a microchannel reactor for H2 pro- ducti on: A CF D sim ulati on s tudy ”, Catalysis Today, vol.143, pp. 25-31,2009. [16] S. Fukahori, H. Koga, T . Kitaoka, M. Nakamura, H. Wa- riis hi , “ Steam reforming behavior of methanol using pa- per-structured catalysts: experimental and computational fluid dynamic analysis”, Int J Hydrogen Energy, vol. 33, pp. 1661-70¸2008. [17] I.Uriz, G.Arzamendi, E.,López,J. Llorca, L.M. Gandía , “Com- putational fluid dynamics simulation of etha nol steam reformi ng in catalytic wall microchannels ”, Chemical Engineering Jour- nal, vol.167 ,pp. 536-544 ,2011. [18] L. Shi, D.J. Bayless and M.E. Prudich , “ A CFD model of autothermal reforming” , Int J Hydrogen Energy, vol.34 ,pp. 7666-7675, 2009. [19] M. Hartmanna, L . Maierb, O. Deutschmann, “ Hydrogen produc tion by catalytic partial oxidation of iso-octan e at varyin g flow rate and fuel/oxygen ratio: From detailed kinetics to reactor behavior”, Applied CatalysisA:General , vol.391, pp. 144–152, 2011. [20] M . Hartmann, L. Maier and H.D .Minh, “ Deutschmann O. Catalytic partial oxidation of iso-octane over rhodium catalysts: An experimental, modeling, and simulation study”, Combustion and Flame , vol.157 , pp. 1771–1782,2010. [21] K. Zygourakis and R. Aris, “ Multiple oxidation reactions and diffusion in the catalytic layer of monolith reactors”, Chem. Eng Sci ,vol.32, pp. 733-744 ,1983. [22] Influence of the propene diffusion inside the catalytic layer”, Chemical Engineering Sc ience, vol. 55,p p. 1707-1716,2000. [23] M.J. Stutz, D. Poulikakos, “ Optimum washcoat thickness of a monolith reactor for syngas production by partial oxidation of Methane” ,Chemical Engineering Science ,vol.63 ,pp. 1761 – 1770,2008. [24] N. Mladenov, J. Koop, S. Tischer, O. Deutschmann “Modeling of transport and chemistry in channel flows of automotive cata- lytic converters ”, Chem.Eng.Sci, v ol.65, pp. 812-826, 2010.  M. IRANI Copyright © 2012 SciRes. AM PC 252 [25] Hoseini N, Msc thesis, Tarbiat Modares University: Tehr an- Iran, 2010. [26] J.P. Du Plessis, W.G. Gray, “ Pore-scale modeling of interstitial transport phenomena. In: Fluid Transport in Porous media”, Computational Mechanics Publication Southampton, pp. 61–104,1997. [27] H.K. Plummer, R.J. Jr, R.H. Baird, A.A. Hammerle and Adamc- zyk JD, “Measurement of Automotive Catalyst Washcoat Load- ing Parameters by Microscopy Techniques”, Microscopy and Microanalysis, vol.5 , pp. 267-281,1999. |

