Paper Menu >>
Journal Menu >>
 Engineering, 2012, 5, 80-83 doi:10.4236/eng.2012.410B021 Published Online October 2012 (http://www.SciRP.org/journal/eng) Copyright © 2012 SciRes. ENG Conjugation of the Recombinant Third Domain of Human Alpha-fetoprotein with Doxorubicin Using Poly(amidoamine) Dendrimers and Study of Its Cytotoxic Activity S. I. Kisil1, V. A. Chernikov2, M. I. Danilevskiy2, L. V. Savvateeva3, N. V. Gorokhovets4 1Biochemistry department, I.M. Sechenov First Moscow State Medical University, Moscow, Russia 2Research Institute of Uronephrology and Reproducti v e H ealth Care of the I. M. Sechenov First MSMUk, Moscow, Russia 3National Research Cen tre “Kurchatov Institute”, Moscow, Russia 4Research Institute of Molecular Medicine of the I. M. Sechenov First MSMU, Moscow, Russia Email: ksi-5826@mail.ru Received 2012 ABSTRACT The recombinant third domain of alpha-fetoprotein (AFP3D) is one of the promising vectors for targeted delivery of anticancer d ru gs to tumor cells. AFP3D-based conjugat es with vario us anti-tumor agents have shown specific anti-tumor activity. The content of an- ti-tumor drugs in the preparation could be increased by including dendrimers - highly branched nanoparticles - into a targeted deli- very system. In this study, we synthesized the AFP3D conjugate with doxorubicin-loaded poly(amidoamine) (PAMAM) dendrimer nanoparticles and investigated its cytotoxic activity. Keywords: Alpha-fetoprotein; Dendri mer; Doxorubicin; Targeted Delivery 1. Introduction Cancer tar get ed therapy repr es ent s one of the most rapidly de- veloping areas in pre-clinical and clinical cancer research. The recombinant third domain of alpha-fetoprotein (AFP3D) is one of the promising vectors for targeted delivery of anticancer drugs to tumor cells. Alpha-fetoprotein (AFP) is a glycoprotein, found in embryonic and fetal serum [1,2]. The biological role of AFP is not fully elucidated, but the protein has an affinity for certain types of tumors, is a carrier of hydrophobic molecules and metabolites in the body [3]. The ability to accumulate in tumors makes AFP a potential vector for the creation of tar- geted anticancer drugs. Isolation of AFP from fetal material is associated with significant complications [4], and therefore a recombinant AFP and the third functional domain of AFP were expressed in E. coli [5,6]. Constructs, based on AFP3D, conjugated with various an- ti-tumor agents, have shown specific anti-tumor activity [7], but the content of the cytotoxic sub stance in t hese pr eparation s was too low, as a rule. While carrying out the conjugation of protein with an antibiotic directly, the recommended degree of conju- gation is no more than 1.5-2 molecules of antibiotic per mole- cule of protein, otherwise the conformation of the protein mo- lecule ch anges considerably and won’t be recognized by a spe- cific recep tor. However, t o heighten treat ment effectiveness t he proportion of the antibiotic must be increased. This can be done by including dendrimers - highly branched nanoparti cles - into a targeted delivery system. The accession of antibiotic mole- cules to the protein through the dendrimer particle can signifi- cantly increase the content of antibiotic drug without affecting the protein itself. 2. Materials and Methods Reagents: Doxorubicin hydrochloride was purchased from Ferein (Russia). Dendrimer PAMAM G-3.5 was purchased from Sigma-Aldrich (USA). Bacterial strain: E.coli BL21 (DE3) strain containing plas- mid pAFP28D3 was kindly provided by the Institute of Mole- cular Medicine, I. M. Sechenov First Moscow State Medical Universit y. 2.1. Expression of AFP3D in E. Co li. E. coli strain- producer of recombinant AFP3D- was grown in LB medium with kanamycin (50 µg/mL). Bacterial cells were grown in aerobic conditions for 12 hours at 37°C in a thermos- tatic shaker “Orbi-safe” (“San jo”, UK), then diluted 50 times and raised for 3-4 hours until the A600 reached 0.6-0.8 units. Synthesis of recombinant protein was induced by adding iso- propylthio-β-galactoside (I P TG; 1 mM) and the culture was left for 3 hours at 37°C with shaking. Cells were harvested by cen- trifugation (10,000 g, 15 min, +4°C) and cell pellet was stored at -30°C. Production of recombinant protein was tested using polyacrylamide gel electrophoresis (PAGE) under denaturing conditions. 2.2. Isolation, Refolding and Purification of Recombinant AFP3D Biomass from 150 ml of culture medium was dissolved in 10 ml of buffer A (0.02 M Tris-HCl, 0.3 M NaCl, 2 mM PMSF (phenylmethanesulfonylfluoride), pH 8.0) and homogenized by ultrasound disintegrator High Intensity Ultrasonic Processor 750 Watt Series (“Cole Parmer”, USA) for 1 min on ice. Then the suspension was harvested by centrifugation for 10 min (12,000 g, +4°C). The cell pellet was resuspended in 5 mL of  YU. F. MIGAL ET AL. Copyright © 2012 SciRes. E NG 81 buffer A and centrifuged under the above conditions to clean the inclusion bodies. This procedure was repeated five times. Then the inclusion bodies were dissolved in 4 mL of buffer B (0.1 M Tris-HCl, 6M guanidine chloride, pH 8.0), sonicated to solubilize proteins and centrifuged for 30 min (12000g, +4°C). The supernatant was applied to the column with 2 mL imino- diacetate-Sepharose saturated with nickel ions (Chelating Se- pharose Cl-6B) and equilibrated with buffer B. The column was washed with 10 mL of buffer B, and then proteins were eluted with 6 mL of buffer C (0.05 M Tris-HCl, 6 M urea, 0.4 M N aCl and 0.3 M imidazole, pH 8.0). β-Mercaptoethanol (0.1 M) was added to the eluate with subsequent incubation for 1 hour at room temperature with stirring. Solution of the reduced protein was added dropwise to 60 mL of buffer E (0.1 M Tris-HCl, 0.5 M NaCl, 2 mM EDTA, 0.4 M arginine, pH 8.0), chilled to +4°C. After incubation for 24 hours at +4°C with stirring, solu- tion was dialyzed against 3 L of buffer F (0.05 Tris-HCl, 0.15 NaCl, 2 mM EDTA, pH 8.0), then against 3 L of buffer G (0.01 M Tris-HCl, 0.15 M NaCl, pH 8.0) for 24 hours each. The dialyzed protein was concentrated on the cell membrane Amicon PM-10 ("Millipore", USA) to 400 mL. The concentrate was fractionated on a column Toyopearl TM DEAE ("Tosoh Bioscience LLC") by elution with a gradient of 0-0.5 M NaCl with a flow rate of 1 mL/min and detection at 280 nm. The target fracti on ( monomer) was concen trated on the cell Amico n (PM-10) to 1.5 mL and stored at -70°C. Protein concentration was determined by the BCA assay (Bicinchoninic Acid Kit, Sigma). 2.3. Investigation of AFP 3D Binding and Endocytosis by Flow Cytofluorometry 1) Synthesis of fluorescein isothiocyanate (FITC)-labeled AFP3D: 6.5 µL (4-fold molar excess) of FITC solution in DMSO (dimethyl sulfoxide) (10 mg/mL) was added to AFP3D solution (1 mg in 0.1 M bicarbonate buffer, pH 8.5) and the mixture was stirred in the dark for 1 hour at room temperature, then for 18 hours at +4°C. FITC-labeled protein was applied to a column with Sephadex G-25 equilibrated with phosphate buffered saline (PBS), pH 7.4. Protein fraction was eluted with the same buf fer at a flow rate o f 1 mL/min and detection at 280 nm in the void volume and concentrated on an Amicon cell with PM-10 membrane to 300 µL. The protein concentration was measured using the BCA kit. Concentration of FITC was determined by spectrophotometry assuming the molar extinc- tion coefficient of FITC epsilon 498 = 68,000 M-1•cm-1. The protein/FITC ratio was 1: 1.4. 2) Cell cultivation: Breast adenocarcinoma cells MCF-7 were cultured in plastic bottles ("Corning-Costar", USA) in DMEM medium containing 10% FBS (fetal bovine serum) and gentamicin (50 µg/ml) in incubator at 37 °C and humidified atmosphere containing 5% CO2. 3) Flow cytometry: One day before the experiment, cells were transferred to 6-well plates ("Corning-Costar", USA). Before starting the experiment, cells were incubated for 2 hours in DMEM medium without FBS. To analyze the binding, AFP3D -F ITC was added to a suspension of cells in the concen- tratio n range 30-4000 nM and incubated at 4 °C for 1 hour. To analyze the endocytosis, incubation was performed at 37 °C, and t hen cells were w ashed three times with cold PBS and fixed with 2% paraformaldehyde (PF) in PBS. F luorescence in tensi ty was measured using an EPICS-C flow cytofluorometer ("Coul- ter Electronics", USA). Fluorescence was excited by an argon laser (wavelength 488 nm, emission bandwidth 520 nm). For every sample (10^5 cells) the median fluorescence intensity (MFI) and percentage o f cells that exceeded the flu orescen ce of autofluorescence were determined. The specificity of AFP3D- FITC binding to AFP receptor was determined by incubating the cells with AFP3D-FITC i n the presence of a 30-fold excess of AFP. 2.4. Conjugation of AFP3D with PAMAM Dendrimer and Doxor ubicin 1) Conjugation in the aqueous medium: 172 mg of a con- densing agent, EDC (1-Ethyl-3 -[3-dimethylaminopropyl] car- bodiimide hydrochloride) was dissolved in 3 ml of PBS to ob- tain a co ncent ration of 0.3 M (buffer A). To this buffer 3 mg of dendrimer PAMAM-COOH 3.5 G (37 ml of 10% methanol solution) was added and solution was stirred for 30 min at room temperature. Sulfo-NHS (Sulfo-N-hydroxysuccinimide) was added in catalytic amounts. Protein solution, concentrated to 4 mg in 500 ml, was then added dropwise, with constant stirring. After 10 min of incubation at room temperature, 2 mL of dox- orubicin (Dox) with a concentration of 1mg/ml and buffer A up to concentration of 0.2 M EDC were added. The mixture was incubated for 1 hour at room temperature with stirring. 2) Conjugation in non-aqueous medium: To PAMAM den- drimer (1.8 mg), dissolved in 500 mL of DMFA (dimethylfor- mamide) with 10% of D-MAP (dimethylaminopyridine), a 14-fold excess of EDC (1 mg) was added. Mixture was incu- bated for 30 min at room temperature. Then catalytic amounts of the Sulfo-NHS and 2 mg of AFP3D concentrated in 500 mL were added. Then 4 mL of 0.1% doxorubicin solution was added dropwise. The mixture was incubated for 2 hours with stirring. The solution was clarified by centrifugation. Unbound doxorubicin and dendrimer were removed by dialysis against 3 L of buffer 0.05 M NaHCO3, pH 8.7, for 2 days. Resulting solution was centrifuged at 14,000 rev/min for 30 min at 15°C. Supernatant was collected, filtered through a sterilizing filter Chromafil-Pet-20/15 MS with a pore diameter of 0.20 µm and frozen at -70°C. 2.5. Determination of Antiproliferative Activity of AFP3D-PAMAM-Dox Using the MTT-test Breast carcin oma cells MC F-7 were cultured in plastic flasks in RPMI medium containing 10% of FBS, 100 u/mL penicillin and 100 µg/mL streptomycin in a humidified atmosphere of 5% CO2 at 37°C. Cells were transferred into 96-well plates by 10 000 cell s per well. C onjugates b eing in vestigated were add ed at various concentrations and cells were incubated for 72 h. Four hours before the incubation finish, 50 µL of MTT solution at concentration of 1 mg/mL in the culture medium was added to each well. After color d evelopment , the medium was re moved, the sedimented formazan crystals were dissolved in 150 µL of DMSO, and the color intensity was measured by absorption at 540 nm on a plate spectrophotometer (Labsystem). The cell survivab ility was est imated as percentage of untr eated control. 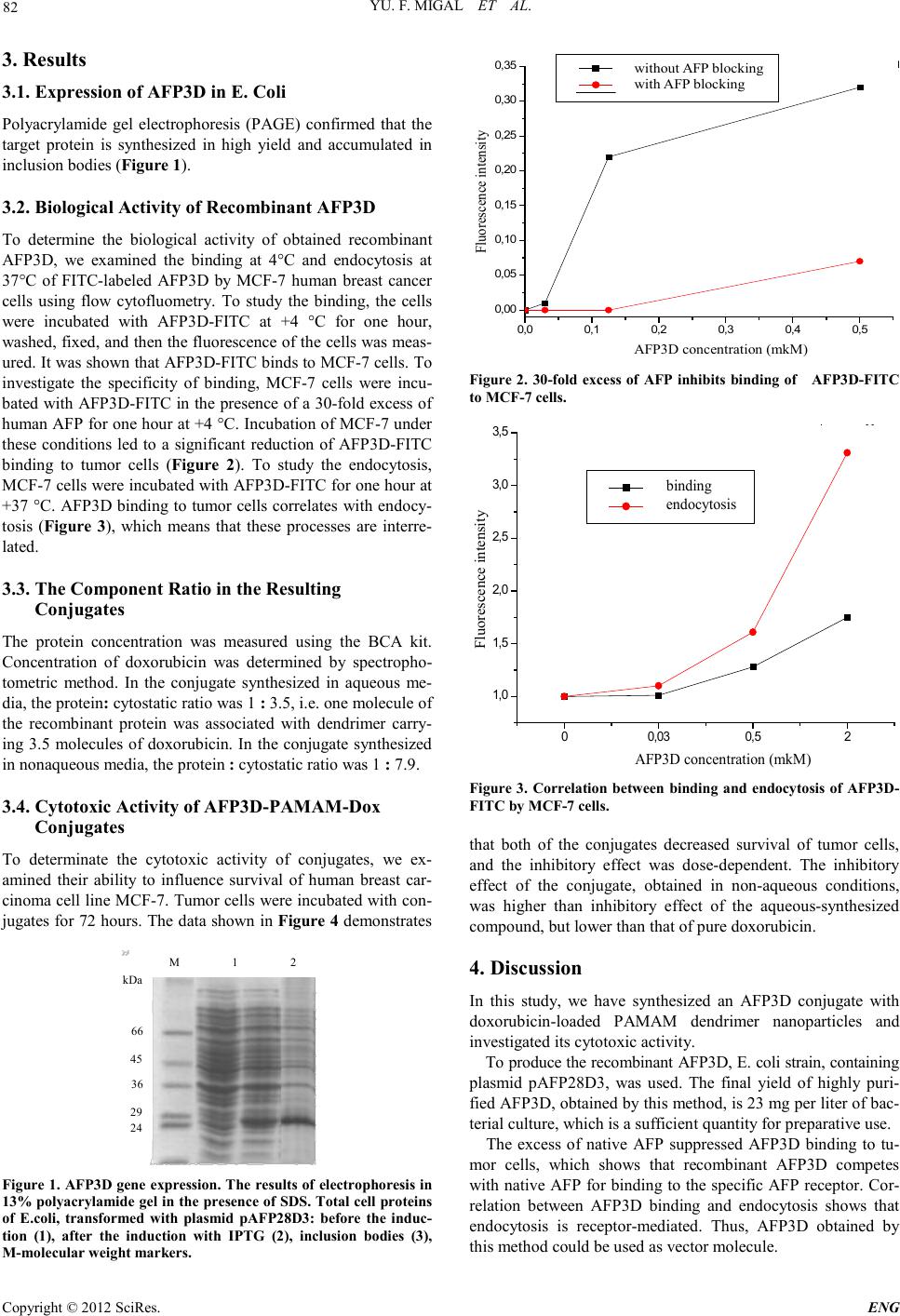 YU. F. MIGAL ET AL. Copyright © 2012 SciRes. ENG 82 3. Results 3.1. Expression of AFP3D in E. Coli Polyacrylamide gel electrophoresis (PAGE) confirmed that the target protein is synthesized in high yield and accumulated in inclus ion bodi e s (Figure 1). 3.2. Biological Activity of Recombinant AFP3D To determine the biological activity of obtained recombinant AFP3D, we examined the binding at 4°C and endocytosis at 37°C of FITC-labeled AFP3D by MCF-7 human breast cancer cells using flow cytofluometry. To study the binding, the cells were incubated with AFP3D-FITC at +4 °C for one hour, washed, fixed, and then the fluo rescence of the cells was meas- ured. It was shown that AFP3D-FITC binds to MCF-7 cells. To investigate the specificity of binding, MCF-7 cells were incu- bated with AFP3D-FITC in the presence of a 30-fold excess of human AFP for one hour at +4 °C. Incubation of MCF-7 under these conditions led to a significant reduction of AFP3D-FITC binding to tumor cells (Figure 2). To study the endocytosis, MCF-7 cells were in cubated with AFP3D -FITC for one hour at +37 °C. AFP3D binding to tumor cells correlates with endocy- tosis (Figure 3), which means that these processes are interre- lated. 3.3. The Co mponent Ratio in the Resulting Conjugates The protein concentration was measured using the BCA kit. Concentration of doxorubicin was determined by spectropho- tometric method. In the conjugate synthesized in aqueous me- dia, the protein: cytostatic ratio was 1 : 3.5, i.e. one molecul e of the recombinant protein was associated with dendrimer carry- ing 3.5 molecules of doxorubicin. In the conjugate synthesized in nonaqueous media, the protein : cytostatic ratio was 1 : 7.9. 3.4. Cytotoxic Activity of AFP3D-PAMAM-Dox Conjugates To determinate the cytotoxic activity of conjugates, we ex- amined their ability to influence survival of human breast car- cinoma cell li ne MCF-7 . Tumor cells were in cubated with con- jugates for 72 hours. The data shown in Figure 4 demonstrates M 1 2 kDa 66 45 36 24 29 Figure 1. AFP3D gene expression. The results of electrophore si s i n 13% polyacrylamide gel in the presence of SDS. Total cell proteins of E.coli, transformed with plasmid pAFP28D3: before the induc- tion (1), after the induction with IPTG (2), inclusion bodies (3), M-molecular weigh t markers. 0,0 0,1 0,2 0,3 0,4 0,5 0,00 0,05 0,10 0,15 0,20 0,25 0,30 0,35 Сблок AFP3D concentration (mkM) Fluorescence intensity without AFP blocking with AFP blocki ng Fig ure 2. 30-fold excess of AFP inhibits binding of AFP3D-FITC to MCF-7 cells . AFP3D concentration (mkM) Fluorescence intensity 00,03 0,52 1,0 1,5 2,0 2,5 3,0 3,5 Эндоц binding endocytosis Fig ure 3. Correlation between binding and endocytosis of AFP3D- FITC by MCF -7 cells. that both of the conjugates decreased survival of tumor cells, and the inhibitory effect was dose-dependent. The inhibitory effect of the conjugate, obtained in non-aqueous conditions, was higher than inhibitory effect of the aqueous-synthesized compound, but lower than that of pure doxorubicin. 4. Discussion In this study, we have synthesized an AFP3D conjugate with doxorubicin-loaded PAMAM dendrimer nanoparticles and investigated its cytotoxic activity. To produce the recombinant AFP3D, E. coli strain, containing plasmid pAFP28D3, was used. The final yield of highly puri- fied AFP3D, obtained by this method, is 23 mg per liter of bac- terial culture, which is a sufficient quantity for preparative use. The excess of native AFP suppressed AFP3D binding to tu- mor cells, which shows that recombinant AFP3D competes with native AFP for binding to the specific AFP recep tor. Cor- relation between AFP3D binding and endocytosis shows that endocytosis is receptor-mediated. Thus, AFP3D obtained by this method could be used as vector molecule. 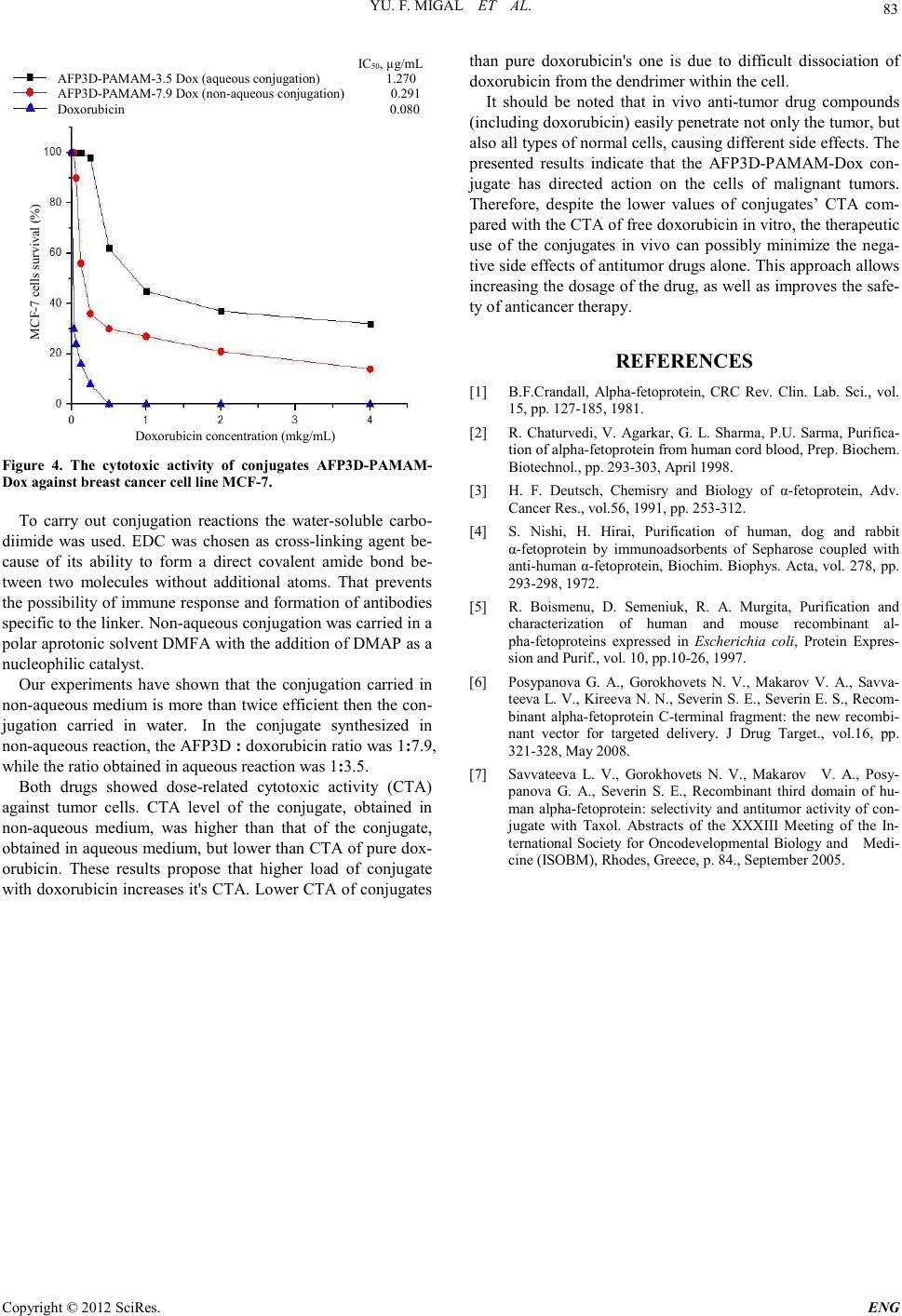 YU. F. MIGAL ET AL. Copyright © 2012 SciRes. E NG 83 IC 50 , µg/mL AFP3D-PAMAM-3.5 Dox (aqueous conjugation) 1.270 AFP3D-PAMAM-7.9 Dox (non-aqueous conjugation) 0.291 Doxorubicin 0.080 Dox orubicin co ncentr ation (mkg/mL) MCF-7 cells sur vival (%) Figure 4. The cytotoxic activity of conjugates AFP3D-PAMAM- Dox agains t brea s t cancer cell lin e M C F-7. To carry out conjugation reactions the water-soluble carbo- diimide was used. EDC was chosen as cross-linking agent be- cause of its ability to form a direct covalent amide bond be- tween two molecules without additional atoms. That prevents the possibility of immune response and formation of antibodies specific to th e linker. No n-aqu eous co nju gatio n was carried in a polar aprotonic solvent DMFA with the addition of DMAP as a nucl eophilic catalyst. Our experiments have shown that the conjugation carried in non-aqueous mediu m is more than t wice efficient th en the con- jugation carried in water. In the conjugate synthesized in non-aqueous reacti on, the AFP3D : doxorubicin ratio was 1:7.9, while the ratio obtained in aqueo us r eaction was 1:3.5. Both drugs showed dose-related cytotoxic activity (CTA) against tumor cells. CTA level of the conjugate, obtained in non-aqueous medium, was higher than that of the conjugate, obtained in aqueous medium, but lower than CTA of pure dox- orubicin. These results propose that higher load of conjugate with doxorubicin increases it's CTA. Lower CTA of conjugates than pure doxorubicin's one is due to difficult dissociation of doxorubicin from the dendrimer within the cell. It should be noted that in vivo anti-tumor drug compounds (including doxorubicin) easily penetrate not only the tumor, but also all types o f nor mal cells, cau sin g d ifferen t sid e effects. The presented results indicate that the AFP3D-PAMAM-Dox con- jugate has directed action on the cells of malignant tumors. Therefore, despite the lower values of conjugates’ CTA com- pared with the CTA of free doxorubicin in vitro, the therapeutic use of the conjugates in vivo can possibly minimize the nega- tive side effects of antitumor drugs alone. This approach allows increasing the dosage of the drug, as well as improves the safe- ty of anti cancer th er apy. REFERENCES [1] B.F.Crandall, Alpha-fetoprotein, CRC Rev. Clin. Lab. Sci., vol. 15, pp. 127-185, 1981. [2] R. Chaturvedi, V. Agarkar, G. L. Sharma, P.U. Sarma, Purifica- tion of alpha-fetoprotein f rom human cord blood , Prep. Biochem. Biotechnol., pp. 293-303, April 1998. [3] H. F. Deutsch, Chemisry and Biology of α-fetoprotein, Adv. Canc er Res., vol.56, 1991, pp. 253-312. [4] S. Nishi, H. Hirai, Purification of human, dog and rabbit α-fetoprotein by immunoadsorbents of Sepharose coupled with anti-human α-fet oprotei n, Biochim. Biophys. Acta, vol. 278, pp. 293-298, 1972. [5] R. Boismenu, D. Semeniuk, R. A. Murgita, Purification and characterization of human and mouse recombinant al- pha-fetoproteins expressed in Escherichia coli, Protein Expres- sion and Purif., vol. 10, pp.10-26, 1997. [6] Posypanova G. A., Gorokhovets N. V., Makarov V. A., Savva- teeva L. V., Kireeva N. N., Severin S. E., Severin E. S., Recom- binant alpha-fetoprotein C-terminal fragment: the new recombi- nant vector for targeted delivery. J Drug Target., vol.16, pp. 321-328, May 2008. [7] Savvateeva L. V., Gorokhovets N. V., Makarov V. A., Posy- panova G. A., Severin S. E., Recombinant third domain of hu- man alpha-fetoprotein: selectivity and antitumor activity of con- jugate with Taxol. Abstracts of the XXXIII Meeting of the In- ternational Society for Oncodevelopmental Biology and Medi- cine (ISOBM), Rhodes, Greece, p. 84., September 2005. |

